

Chin J Plant Ecol ›› 2018, Vol. 42 ›› Issue (11): 1055-1070.DOI: 10.17521/cjpe.2018.0156
• Review • Next Articles
Hua-Jun YIN1,*( ),Zi-Liang ZHANG1,2,Qing LIU1
),Zi-Liang ZHANG1,2,Qing LIU1
Received:2018-07-05
Accepted:2018-11-04
Online:2018-11-20
Published:2019-03-13
Contact:
Hua-Jun YIN
Supported by:Hua-Jun YIN, Zi-Liang ZHANG, Qing LIU. Root exudates and their ecological consequences in forest ecosystems: Problems and perspective[J]. Chin J Plant Ecol, 2018, 42(11): 1055-1070.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2018.0156
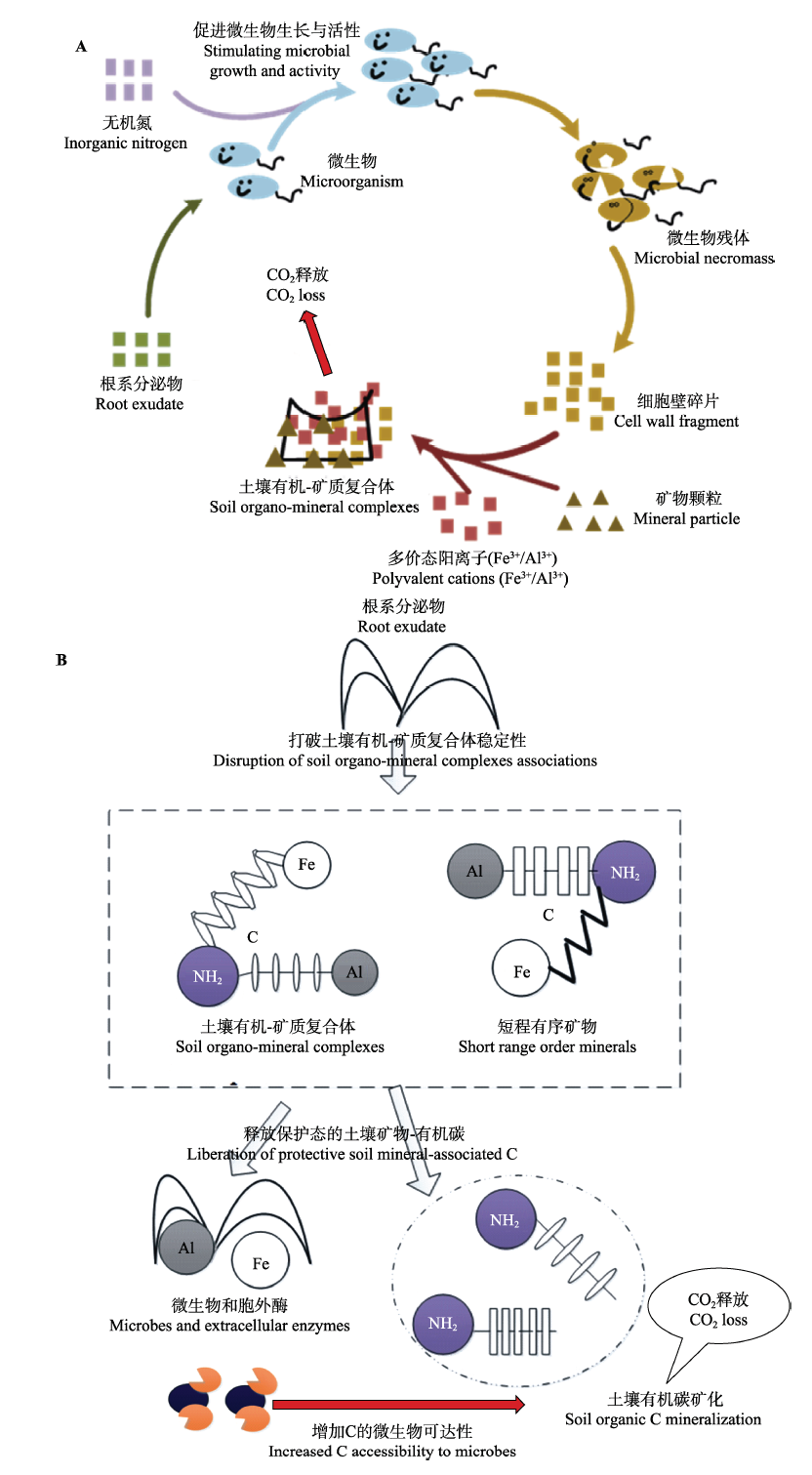
Fig. 2 Proposed mechanisms for the exudate-induced acceleration of the microbial mineralization of soil organic carbon in the rhizosphere (i.e., rhizosphere priming effects). A, The traditional view is that root exudate compounds stimulate microbial growth and activity via co-metabolism, and so increase the overall physiological potential of the decomposer community for carbon mineralization. B, The alternative mechanism proposed here takes into account that large quantities of soil C are inaccessible to microbes owing to associations with mineral phases. Root exudates that can act as ligands effectively liberate C through complexation and dissolution reactions with protective mineral phases, thereby promoting its accessibility to microbes and accelerating its loss from the system through microbial mineralization.
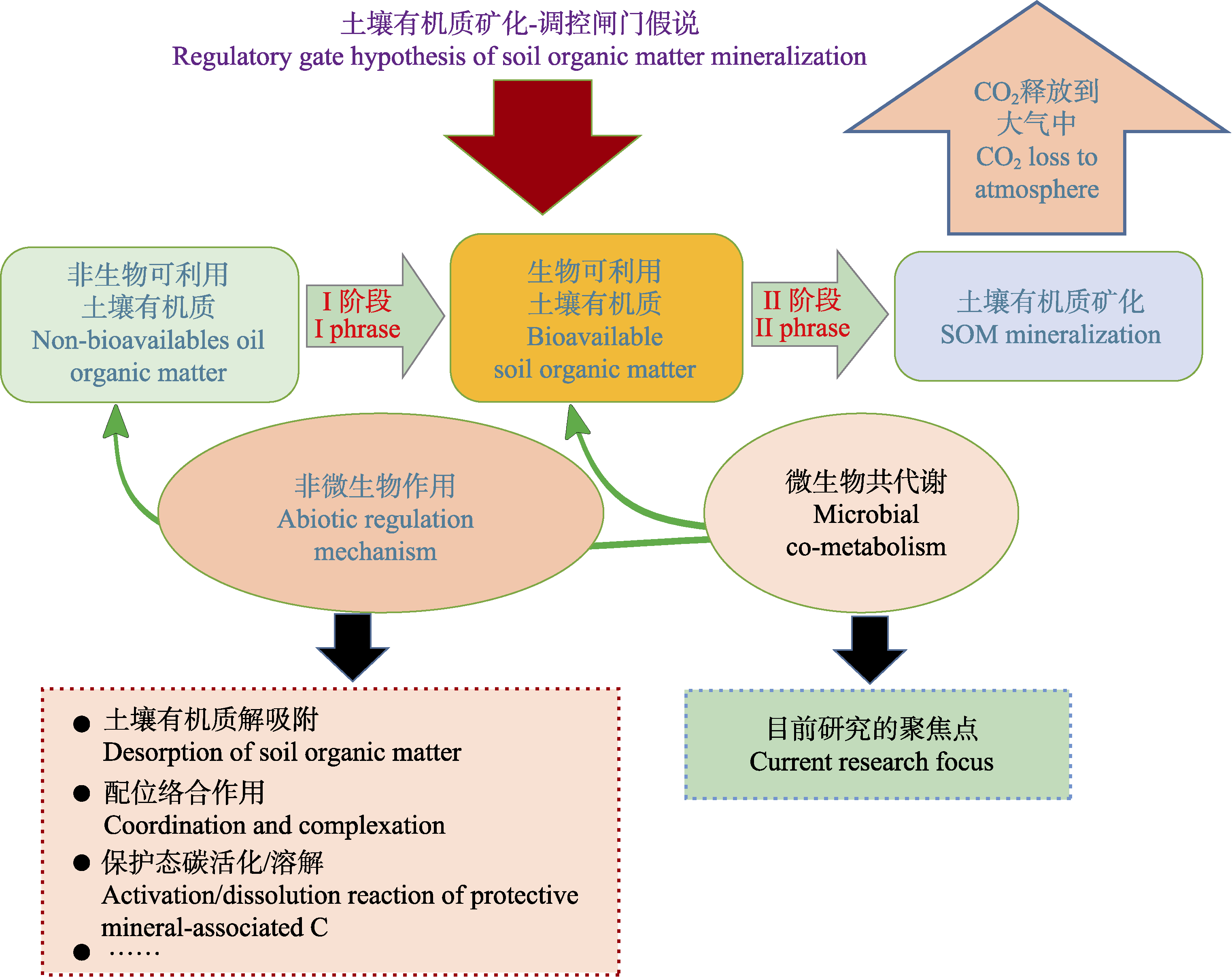
Fig. 3 Diagrammatic representation of the Regulatory Gate Hypothesis. I phrase is the abiological transformation of non-bioavailable soil organic matter (SOM). II phrase is the biological mineralization of bioavailable SOM.
| [1] | Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM ( 2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology, 57, 233-266. |
| [2] |
Bardgett RD, Mommer L, de Vries FT ( 2014). Going underground: Root traits as drivers of ecosystem processes. Trends in Ecology & Evolution, 29, 692-699.
DOI URL PMID |
| [3] |
Cairney JWG ( 2012). Extramatrical mycelia of ectomycorrhizal fungi as moderators of carbon dynamics in forest soil. Soil Biology & Biochemistry, 47, 198-208.
DOI URL |
| [4] |
Cheng WX, Parton WJ, Gonzalez-Meler MA, Phillips R ( 2014). Synthesis and modeling perspectives of rhizosphere priming. New Phytologist, 201, 31-44.
DOI URL PMID |
| [5] |
Cleveland CC, Houlton BZ, Smith WK, Marklein AR, Reed SC, Parton W, Del Grosso SJ, Running SW ( 2013). Patterns of new versus recycled primary production in the terrestrial biosphere. Proceedings of the National Academy of Sciences of the United States of America, 110, 12733-12737.
DOI URL PMID |
| [6] | Cleveland CC, Liptzin D ( 2007). C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry, 85, 235-252. |
| [7] |
Darrah PR ( 1991). Measuring the diffusion coefficient of rhizosphere exudates in soil. I. The diffusion of non-sorbing compounds. Journal of Soil Science, 42, 413-420.
DOI URL |
| [8] |
Dessaux Y, Hinsinger P, Lemanceau P ( 2009). Rhizosphere: So many achievements and even more challenges. Plant and Soil, 321, 1-3.
DOI URL |
| [9] |
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA ( 2013). Rhizosphere priming: A nutrient perspective. Frontiers in Microbiology, 4, 1-4.
DOI URL PMID |
| [10] |
Drake JE, Darby BA, Giasson MA, Kramer MA, Phillips RP, Finzi AC ( 2013). Stoichiometry constrains microbial response to root exudation-insights from a model and a field experiment in a temperate forest. Biogeosciences, 10, 821-838.
DOI URL |
| [11] | Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Tresder KK, Schlesinger WH, DeLucia EH, Finzi AC ( 2011). Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecology Letters, 14, 349-357. |
| [12] |
Erktan A, McCormack ML, Roumet C ( 2018). Frontiers in root ecology: Recent advances and future challenges. Plant and Soil, 424, 1-9.
DOI URL |
| [13] |
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP ( 2015). Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Global Change Biology, 21, 2082-2094.
DOI URL PMID |
| [14] | Fuhrer T, Zamboni N ( 2015). High-throughput discovery metabolomics. Current Opinion in Biotechnology, 31, 73-78. |
| [15] |
Guo DL, Li H, Mitchell RJ, Han WX, Hendricks JJ, Fahey TJ, Hendrick RL ( 2008). Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytologist, 177, 443-456.
DOI URL PMID |
| [16] |
Guyonnet JP, Cantarel AAM, Simon L, Haichar FZ ( 2018). Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecology and Evolution, 8, 8573-8581.
DOI URL |
| [17] |
Haichar FE, Santaella C, Heulin T, Achouak W ( 2014). Root exudates mediated interactions belowground. Soil Biology & Biochemistry, 77, 69-80.
DOI URL |
| [18] |
Hinsinger P, Gobran GR, Gregory PJ, Wenzel WW ( 2005). Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytologist, 168, 293-303.
DOI URL PMID |
| [19] | Högberg P, Read DJ ( 2006). Towards a more plant physiological perspective on soil ecology. Trends in Ecology and Evolution, 21, 548-554. |
| [20] |
Holz M, Zarebanadkouki M, Kuzyakov Y, Pausch J, Carminati A ( 2018). Root hairs increase rhizosphere extension and carbon input to soil. Annals of Botany, 121, 61-69.
DOI URL PMID |
| [21] |
Hu LF, Robert CAM, Cadot S, Zhang X, Ye M, Li B, Manzo D, Chervet N, Steinger T, van der Heijden MGA, Schlaeppi K, Erb M ( 2018). Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nature Communications, 9, 1-13.
DOI URL |
| [22] |
Jilling A, Keiluweit M, Contosta AR, Frey S, Schimel J, Schnecker J, Smith RG, Tiemann L, Grandy AS ( 2018). Minerals in the rhizosphere: Overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry, 139, 103-122.
DOI URL |
| [23] |
Keiluweit M, Bougoure JJ, Nico PS, Pett-Ridge J, Weber PK, Kleber M ( 2015). Mineral protection of soil carbon counteracted by root exudates. Nature Climate Change, 5, 588-595.
DOI URL |
| [24] |
Kemmitt SJ, Lanyon CV, Waite IS, Wen Q, Addiscott TM, Bird NRA, O’Donnell AG, Brookes PC ( 2008). Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass a new perspective. Soil Biology & Biochemistry, 40, 61-73.
DOI URL |
| [25] |
Klein T, Siegwolf RT, Körner C ( 2016). Belowground carbon trade among tall trees in a temperate forest. Science, 352, 342-344.
DOI URL PMID |
| [26] | Kong CH, Li HB, Hu F ( 2006). Allelochemicals released by rice roots and residues in soil. Plant and Soil, 288, 47-56. |
| [27] |
Kuzyakov Y ( 2002). Review: Factors affecting rhizosphere priming effects. Journal of Plant Nutrition and Soil Science, 165, 382-396.
DOI URL |
| [28] |
Laliberté E ( 2017). Below-ground frontiers in trait-based plant ecology. New Phytologist, 213, 1597-1603.
DOI URL PMID |
| [29] |
Li X, Dong JL, Chu WY, Chen YJ, Duan ZQ ( 2018). The relationship between root exudation properties and root morphological traits of cucumber grown under different nitrogen supplies and atmospheric CO2 concentrations. Plant and Soil, 425, 415-432.
DOI URL |
| [30] |
Luginbuehl LH, Menard GN, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Eastmond PJ ( 2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science, 356, 1175-1178.
DOI URL PMID |
| [31] |
Ma ZQ, Guo DL, Xu XL, Lu MZ, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO ( 2018). Evolutionary history resolves global organization of root functional traits. Nature, 555, 94-97.
DOI URL PMID |
| [32] |
Martinière A, Gibrata R, Sentenaca H, Dumonta X, Gaillarda I, Parisa N ( 2018). Uncovering pH at both sides of the root plasma membrane interface using noninvasive imaging. Proceedings of the National Academy of Sciences of the United States of America, 115, 6488-6493.
DOI URL |
| [33] |
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo DL, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppälammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M ( 2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytologist, 207, 505-518.
DOI URL PMID |
| [34] |
Meier IC, Pritchard SG, Brzostek ER, McCormack ML, Phillips RP ( 2015). The rhizosphere and hyphosphere differ in their impacts on carbon and nitrogen cycling in forests exposed to elevated CO2. New Phytologist, 205, 1164-1174.
DOI URL PMID |
| [35] |
Moore JAM, Jiang J, Patterson CM, Mayes MA, Wang G, Classen AT ( 2015). Interactions among roots, mycorrhizas and free-living microbial communities differentially impact soil carbon processes. Journal of Ecology, 103, 1442-1453.
DOI URL |
| [36] |
Nakayama M, Tateno R ( 2018). Solar radiation strongly influences the quantity of forest tree root exudates. Trees, 32, 871-879.
DOI URL |
| [37] |
Neumann G, George TS, Plassard C ( 2009). Strategies and methods for studying the rhizosphere—The plant science toolbox. Plant and Soil, 321, 431-456.
DOI URL |
| [38] |
Oburge E, Jones DJ ( 2018). Sampling root exudates—Mission impossible? Rhizosphere, 6, 116-133.
DOI URL |
| [39] |
Pérez-Hargunideguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson CJG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC ( 2013). New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany, 61, 167-234.
DOI URL |
| [40] |
Phillips RP, Erlitz Y, Bier R, Bernhardt ES ( 2008). A new approach for capturing soluble root exudates in forest soils. Functional Ecology, 22, 990-999.
DOI URL |
| [41] |
Phillips RP, Finzi AC, Bernhardt ES ( 2011). Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecology Letters, 14, 187-194.
DOI URL PMID |
| [42] |
Preece C, Farré-Armengol G, Llusià J, Peñuelas J ( 2018). Thirsty tree roots exude more carbon. Tree Physiology, 38, 690-695.
DOI URL PMID |
| [43] |
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL ( 2002). Fine root architecture of nine North American trees. Ecological Monographs, 72, 293-309.
DOI URL |
| [44] | Proctor C, He YH ( 2017). Quantifying root extracts and exudates of sedge and shrub in relation to root morphology. Soil Biology & Biochemistry, 114, 168-180. |
| [45] |
Roumet C, Birouste M, Picon-Cochard C, Ghestem M, Osman N, Vrignon-Brenas S, Cao K, Stokes A ( 2016). Root structure-function relationships in 74 species: Evidence of a root economics spectrum related to carbon economy. New Phytologist, 210, 815-826.
DOI URL PMID |
| [46] |
Sandnes A, Eldhuset TD, Wollebaek G ( 2005). Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biology & Biochemistry, 37, 259-269.
DOI URL |
| [47] |
Shahzad T, Rashid MI, Maire V, Barot S, Perveen N, Alvarez G, Mougin C, Fontaine S ( 2018). Root penetration in deep soil layers stimulates mineralization of millennia old organic carbon. Soil Biology & Biochemistry, 124, 150-160.
DOI URL |
| [48] |
Shipley B, Bello FD, Cornelissen JHC, Lalibert E, Laughlin DC, Reich PB ( 2016). Reinforcing loose foundation stones in trait-based plant ecology. Oecologia, 180, 923-931.
DOI URL |
| [49] |
Singh BK, Millard P, Whiteley AS, Murrell JC ( 2004). Unravelling rhizosphere-microbial interactions: Opportunities and limitations. TRENDS in Microbiology, 12, 386-393.
DOI URL PMID |
| [50] | Smith SE, Read D ( 2008). Mycorrhizal Symbiosis. 3rd edn. Academic Press, London. |
| [51] | Strehmel N, Bottcher C, Schmidt S, Scheel D ( 2014). Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry, 108, 35-46. |
| [52] |
Sun Y, Xu XL, Kuzyakov Y ( 2014). Mechanisms of rhizosphere priming effects and their ecological significance. Chinese Journal of Plant Ecology, 38, 62-75.
DOI URL |
|
[ 孙悦, 徐兴良 , Kuzyakov Y ( 2014). 根际激发效应的发生机制及其生态重要性. 植物生态学报, 38, 62-75.]
DOI URL |
|
| [53] |
Sun L, Lu YF, Yu FW, Kronzucker HJ, Shi WM ( 2016). Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytologist, 212, 646-656.
DOI URL PMID |
| [54] |
Tan WB, Wang GA, Huang CH, Gao RT, Xi BD, Zhu B ( 2017). Physico-chemical protection, rather than biochemical composition, governs the responses of soil organic carbon decomposition to nitrogen addition in a temperate agroecosystem. Science of the Total Environment, 598, 282-288.
DOI URL PMID |
| [55] |
Terrer C, Vicca S, Stocker BD, Hungate BA, Phillips RP, Reich PB, Prentice IC ( 2018). Ecosystem responses to elevated CO2 governed by plant-soil interactions and the cost of nitrogen acquisition. New Phytologist, 217, 507-522.
DOI URL PMID |
| [56] | Treseder KK, Holden SR ( 2013). Fungal carbon sequestration. Science, 339, 1528-1529. |
| [57] |
Tückmantel T, Leuschner C, Preusser S, Kandeler E, Angst G, Mueller CW, Meier IC ( 2017). Root exudation patterns in a beech forest: Dependence on soil depth, root morphology, and environment. Soil Biology & Biochemistry, 107, 188-197.
DOI URL |
| [58] | Uren NC ( 2000). Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinto R, Varanini Z, Nannipieri P eds. The Rhizosphere, Biochemistry and Organic Substances at the Soil-plant Interface. Marcel Dekker, New York. 19-40. |
| [59] |
van Dam NM, Bouwmeester HJ ( 2016). Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends in Plant Science, 21, 256-265.
DOI URL PMID |
| [60] |
Wallander H, Ekblad A, Bergh J ( 2011). Growth and carbon sequestration by ectomycorrhizal fungi in intensively fertilized Norway spruce forests. Forest Ecology and Management, 262, 999-1007.
DOI URL |
| [61] |
Wallander H, Ekblad A, Godbold DL, Johnson D, Bahr A, Baldrian P, Bjork RG, Kieliszewska-Rokicka B, Kjoller R, Kraigher H, Plassard C, Rudawska M ( 2013). Evaluation of methods to estimate production, biomass and turnover of ectomycorrhizal mycelium in forests soils—A review. Soil Biology & Biochemistry, 57, 1034-1047.
DOI URL |
| [62] |
Warren CR ( 2016). Simultaneous efflux and uptake of metabolites by roots of wheat. Plant and Soil, 406, 359-374.
DOI URL |
| [63] |
Wu LK, Lin XM, Lin WX ( 2014). Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chinese Journal of Plant Ecology, 38, 298-310.
DOI URL |
|
[ 吴林坤, 林向民, 林文雄 ( 2014). 根系分泌物介导下植物-土壤-微生物互作关系研究进展与展望. 植物生态学报, 38, 298-310.]
DOI URL |
|
| [64] |
Wutzler T, Reichstein M ( 2013). Priming and substrate quality interactions in soil organic matter models. Biogeosciences, 10, 2089-2103.
DOI URL |
| [65] |
Xia B, Zhou Y, Liu X, Xiao J, Liu Q, Gu YC, Ding LS ( 2012). Use of electrospray ionization ion-trap tandem mass spectrometry and principal component analysis to directly distinguish monosaccharides. Rapid Communications in Mass Spectrometry, 26, 1259-1264.
DOI URL PMID |
| [66] |
Yin LM, Dijkstra FA, Wang P, Zhu B, Cheng WX ( 2018). Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytologist, 218, 1036-1048.
DOI URL PMID |
| [67] |
Yin HJ, Li YF, Xiao J, Cheng XY, Xu ZF, Liu Q ( 2013). Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Global Change Biology, 19, 2158-2167.
DOI URL PMID |
| [68] |
Yin HJ, Phillips RP, Liang RB, Xu ZF, Liu Q ( 2016). Resource stoichiometry mediates soil C loss and nutrient transformations in forest soils. Applied Soil Ecology, 108, 248-257.
DOI URL |
| [69] |
Yin HJ, Wheeler E, Phillips RP ( 2014). Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biology & Biochemistry, 78, 213-221.
DOI URL |
| [70] |
Yuan YS, Zhao WQ, Zhang ZL, Xiao J, Li DD, Liu Q, Yin HJ ( 2018). Impacts of oxalic acid and glucose additions on N transformation in microcosms via artificial roots. Soil Biology & Biochemistry, 121, 16-23.
DOI URL |
| [71] |
Zhang DD, Liang XH, Wang J ( 2014). A review of plant root exudates. Agricultural Science Bulletin, 30, 314-320.
DOI URL |
|
[ 张豆豆, 梁新华, 王俊 ( 2014). 植物根系分泌物研究综述. 中国农学通报, 30, 314-320.]
DOI URL |
|
| [72] |
Zhang FS, Shen JB ( 1999). Preliminary development of the theoretical concept on rhizosphere micro-ecosystem. Review of China Agriculture Science and Technology, (4), 15-20.
DOI URL |
|
[ 张福锁, 申建波 ( 1999). 根际微生态系统理论框架的初步构建. 中国农业科技导报,( 4), 15-20.]
DOI URL |
|
| [73] |
Zhang ZL, Phillips RP, Zhao WQ, Yuan YS, Liu Q, Yin HJ ( 2018 a). Mycelia-derived C contributes more to nitrogen cycling than root-derived C in ectomycorrhizal alpine forests. Functional Ecology, DOI: 10.1111/1365-2435.13236.
DOI URL |
| [74] |
Zhang ZL, Xiao J, Yuan YS, Zhao CZ, Liu Q, Yin HJ ( 2018 b). Mycelium- and root-derived C inputs differ in their impacts on soil organic C pools and decomposition in forests. Soil Biology & Biochemistry, 123, 257-265.
DOI URL |
| [75] |
Zhu B, Cheng WX ( 2012). Nodulated soybean enhances rhizosphere priming effects on soil organic matter decomposition more than non-nodulated soybean. Soil Biology & Biochemistry, 51, 56-65.
DOI URL |
| [76] |
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng WX ( 2014). Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biology & Biochemistry, 76, 183-192.
DOI URL |
| [1] | Qingshui Yu xiaofeng ni Jiangling Zhu Zhi-Yao TANG Jing-Yun FANG. Effects of 10 years of nitrogen and phosphorus additions on leaf non-structural carbohydrates of dominant plants in two tropical rainforests in the Jianfengling, Hainan [J]. Chin J Plant Ecol, 2024, 48(预发表): 0-0. |
| [2] | Yao Liu Quan-Lin ZHONG Chao-Bin XU Dong-Liang CHENG 芳 跃郑 Zou Yuxing Zhang Xue Xin-Jie Zheng Yun-Ruo Zhou. Relationship between fine root functional traits and rhizosphere microenvironment of Machilus pauhoi at different sizes [J]. Chin J Plant Ecol, 2024, 48(预发表): 0-0. |
| [3] | 建 周 Han Wang. A review of forest size structure studies: from statistical description to theoretical deduction [J]. Chin J Plant Ecol, 2024, 48(预发表): 0-0. |
| [4] | FU Liang-Chen, DING Zong-Ju, TANG Mao, ZENG Hui, ZHU Biao. Rhizosphere effects of Betula platyphylla and Quercus mongolica and their seasonal dynamics in Dongling Mountain, Beijing [J]. Chin J Plant Ecol, 2024, 48(4): 508-522. |
| [5] | ZHANG Yu-Jian, LIU Yan-Hong. Tree physiology and major influencing factors under forest fires [J]. Chin J Plant Ecol, 2024, 48(3): 269-286. |
| [6] | YANG An-Na, LI Zeng-Yan, MOU Ling, YANG Bai-Yu, SAI Bi-Le, ZHANG Li, ZHANG Zeng-Ke, WANG Wan-Sheng, DU Yun-Cai, YOU Wen-Hui, YAN En-Rong. Variation in soil bacterial community across vegetation types in Dajinshan Island, Shanghai [J]. Chin J Plant Ecol, 2024, 48(3): 377-389. |
| [7] | XUE Zhi-Fang, LIU Tong, WANG Li-Sheng, SONG Ji-Hu, CHEN Hong-Yang, XU Ling, YUAN Ye. Community structure and characteristics of plain valley forests in main tributaries of Ertix River Basin, China [J]. Chin J Plant Ecol, 2024, 48(3): 390-402. |
| [8] | CHEN Zhao-Quan, WANG Ming-Hui, HU Zi-Han, LANG Xue-Dong, HE Yun-Qiong, LIU Wan-De. Mechanisms of seedling community assembly in a monsoon evergreen broadleaf forest in Pu’er, Yunnan, China [J]. Chin J Plant Ecol, 2024, 48(1): 68-79. |
| [9] | YUAN Ya-Ni, ZHOU Zhe, CHEN Bin-Zhou, GUO Yao-Xin, YUE Ming. Differential ecological strategies in functional traits among coexisting tree species in a Quercus aliena var. acuteserrata forest [J]. Chin J Plant Ecol, 2023, 47(9): 1270-1277. |
| [10] | ZHANG Ying, ZHANG Chang-Hong, WANG Qi-Tong, ZHU Xiao-Min, YIN Hua-Jun. Difference of soil carbon sequestration between rhizosphere and bulk soil in a mountain coniferous forest in southwestern China under nitrogen deposition [J]. Chin J Plant Ecol, 2023, 47(9): 1234-1244. |
| [11] | ZHANG Hui-Ling, ZHANG Yao-Yi, PENG Qing-Qing, YANG Jing, NI Xiang-Yin, WU Fu-Zhong. Variations of trace-elements resorption efficiency in leaves of different tree species as affected by life forms in a mid-subtropical common garden [J]. Chin J Plant Ecol, 2023, 47(7): 978-987. |
| [12] | SHEN Jian, HE Zong-Ming, DONG Qiang, GAO Shi-Lei, LIN Yu. Effects of mild fire on soil respiration rate and abiotic factors in coastal sandy plantation [J]. Chin J Plant Ecol, 2023, 47(7): 1032-1042. |
| [13] | ZHANG Zhong-Yang, SONG Xi-Qiang, REN Ming-Xun, ZHANG Zhe. Ecological functions of vascular epiphytes in habitat construction [J]. Chin J Plant Ecol, 2023, 47(7): 895-911. |
| [14] | ZHANG Zhong-Fu, WANG Si-Hai, YANG Wei, CHEN Jian. Response of rhizosphere microbial community structure and functional characteristics to health status of Malania oleifera [J]. Chin J Plant Ecol, 2023, 47(7): 1020-1031. |
| [15] | LI Liu, LIU Qing-Hua, YIN Chun-Ying. Selenium biofortification in plants and application potential of microorganisms in selenium biofortification [J]. Chin J Plant Ecol, 2023, 47(6): 756-769. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn