

Chin J Plan Ecolo ›› 2018, Vol. 42 ›› Issue (2): 220-228.DOI: 10.17521/cjpe.2017.0258
Special Issue: 植物功能性状
• Research Articles • Previous Articles Next Articles
ZHAO Le-Wen1,CHEN Zi-Yi1,ZOU Ying1,FU Zi-Zhao1,WU Gui-Lin2,3,LIU Xiao-Rong2,3,LUO Qi2,3,LIN Yi-Xue4,2,LI Xiong-Ju1,LIU Zhi-Tong 1,LIU Hui2,*( )
)
Online:2018-02-20
Published:2018-04-16
Contact:
Hui LIU
Supported by:ZHAOLe-Wen, CHEN Zi-Yi, ZOU Ying, FU Zi-Zhao, WU Gui-Lin, LIU Xiao-Rong, LUO Qi, LIN Yi-Xue, LI Xiong-Ju, LIU Zhi-Tong, LIU Hui. Changes in hydraulic traits of nine vascular plants from different evolutionary lineages[J]. Chin J Plan Ecolo, 2018, 42(2): 220-228.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2017.0258
| 物种 Species | 缩略词 Abbreviation | 科 Family | |
|---|---|---|---|
| 蕨类植物 Ferns | 芒萁 Dicranopteris pedata | Dp | 里白科 Gleicheniaceae |
| 华南毛蕨 Cyclosorus parasiticus | Cp | 金星蕨科 Thelypteridaceae | |
| 乌毛蕨 Blechnum orientale | Bo | 乌毛蕨科 Blechnaceae | |
| 裸子植物 Gymnosperms | 罗汉松 Podocarpus macrophyllus | Pm | 罗汉松科 Podocarpaceae |
| 竹柏 Podocarpus nagi | Pn | 罗汉松科 Podocarpaceae | |
| 落羽杉 Taxodium distichum | Td | 杉科 Taxodiaceae | |
| 被子植物 Angiosperms | 海南木莲 Manglietia fordiana var. hainanensis | Mh | 木兰科 Magnoliaceae |
| 东京油楠 Sindora tonkinensis | St | 豆科 Fabaceae | |
| 羊蹄甲 Bauhinia purpurea | Bp | 豆科 Fabaceae |
Table 1 Summary of the nine species from three evolutionary lineages in this study
| 物种 Species | 缩略词 Abbreviation | 科 Family | |
|---|---|---|---|
| 蕨类植物 Ferns | 芒萁 Dicranopteris pedata | Dp | 里白科 Gleicheniaceae |
| 华南毛蕨 Cyclosorus parasiticus | Cp | 金星蕨科 Thelypteridaceae | |
| 乌毛蕨 Blechnum orientale | Bo | 乌毛蕨科 Blechnaceae | |
| 裸子植物 Gymnosperms | 罗汉松 Podocarpus macrophyllus | Pm | 罗汉松科 Podocarpaceae |
| 竹柏 Podocarpus nagi | Pn | 罗汉松科 Podocarpaceae | |
| 落羽杉 Taxodium distichum | Td | 杉科 Taxodiaceae | |
| 被子植物 Angiosperms | 海南木莲 Manglietia fordiana var. hainanensis | Mh | 木兰科 Magnoliaceae |
| 东京油楠 Sindora tonkinensis | St | 豆科 Fabaceae | |
| 羊蹄甲 Bauhinia purpurea | Bp | 豆科 Fabaceae |

Fig. 1 Comparison of sapwood-specific hydraulic conductivity (KS), leaf-specific hydraulic conductivity (KL) and leaf hydraulic conductance (Kleaf) among the nine species from three evolutionary lineages (mean ± SE, n = 3-5). Letters on top of each bar in the right column are HSD multiple comparison results, the phylogenetic tree of the nine species is drawn at the bottom. See species abbrivations in Table 1. Fern, ferns; Gym, gynosperms; Ang, angiosperms.
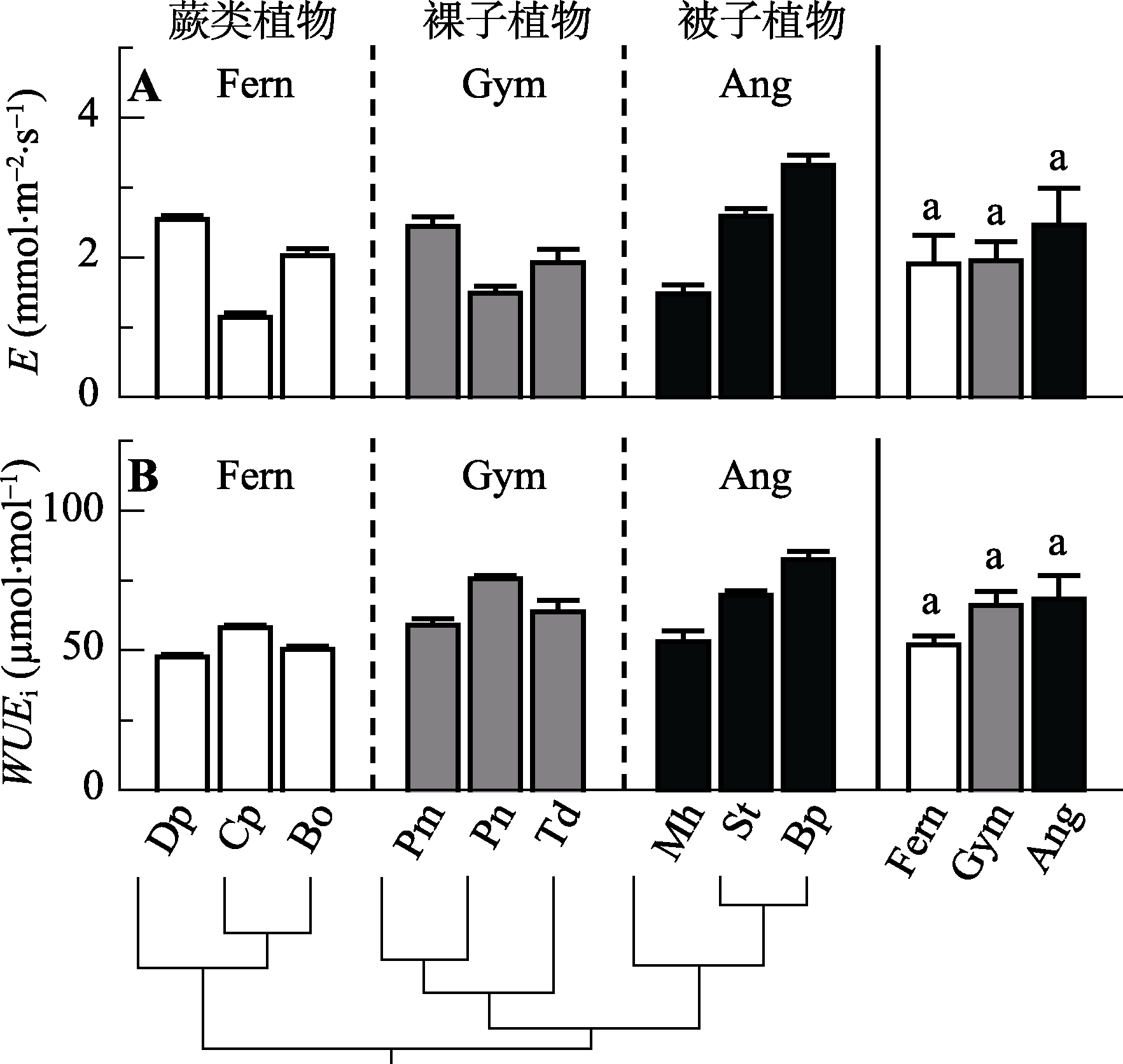
Fig. 2 Comparison of leaf transpiration rate (E) and intrinsic water use efficiency (WUEi) among the nine species from three evolutionary lineages (mean ± SE, n = 3-5). Notes are the same with Fig. 1.
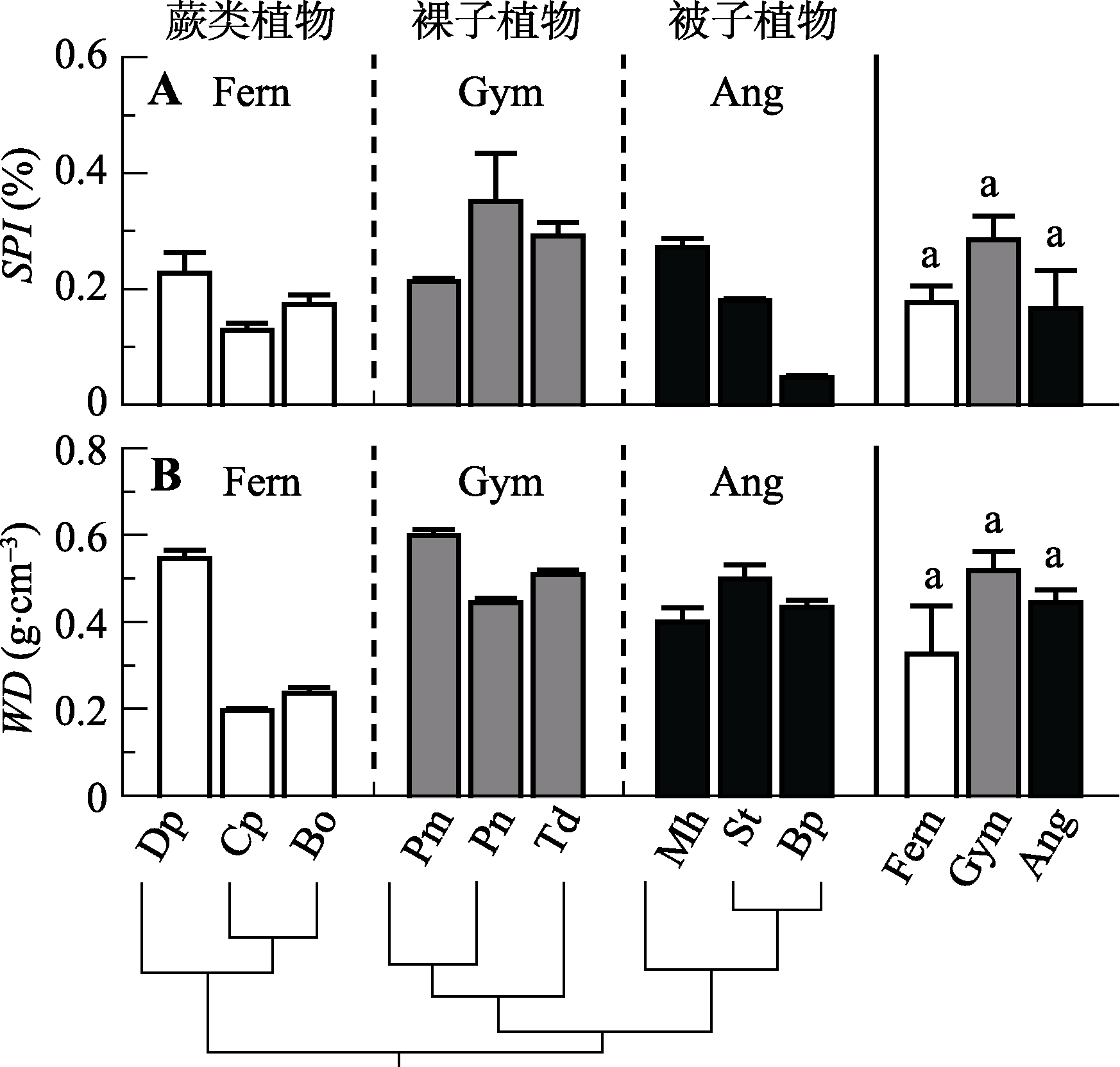
Fig. 3 Comparison of stomatal area index (SPI) and wood density (WD) among the nine species from three evolutionary lineages (mean ± SE, n = 3-5). Notes are the same with Fig. 1.
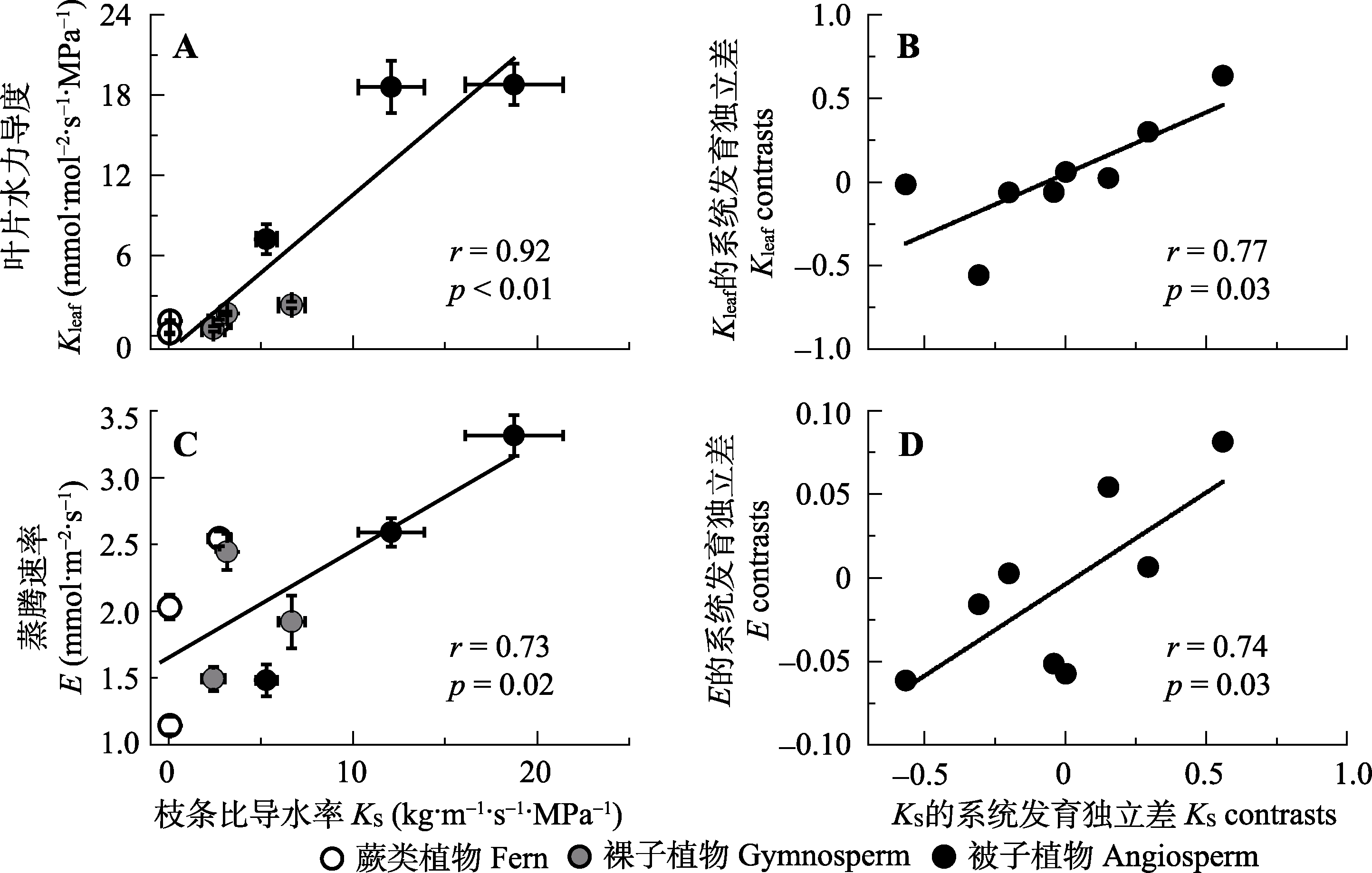
Fig. 4 Correlations between sapwood-specific hydraulic conductivity (KS) and leaf hydraulic conductance (Kleaf)(A, B), and leaf transpiration rate (E)(C, D) among the nine species from three evolutionary lineages. A, C, traditional cross-species correlations; B, D, correlations among phylogenetically independent contrasts. In A and C, data are mean ± SE (n = 3-5), Pearson correlation coefficients (r) and p values are reported.
| [1] |
Azani N, Babineau M, Bailey CD, Banks H, Barbosa AR, Pinto RB, Boatwright JS, Borges LM, Brown GK, Bruneau A, Candido E, Cardoso D, Chung K-F, Clark RP, Concei??o AdS, Crisp M, Cubas P, Delgado-Salinas A, Dexter KG, Doyle JJ, Duminil J, Egan AN, De La Estrella M, Falc?o MJ, Filatov DA, Fortuna-Perez AP, Fortunato RH, Gagnon E, Gasson P, Rando JG, Azevedo Tozzi AMGd, Gunn B, Harris D, Haston E, Hawkins JA, Herendeen PS, Hughes CE, Iganci JRV, Javadi F, Kanu SA, Kazempour- Osaloo S, Kite GC, Klitgaard BB, Kochanovski FJ, Koenen EJM, Kovar L, Lavin M, Roux Ml, Lewis GP, de Lima HC, López Roberts MC, Mackinder B, Maia VH, Malécot V, Mansano VF, Marazzi B, Mattapha S, Miller JT, Mitsuyuki C, Moura T, Murphy DJ, Nageswara-Rao M, Nevado B, Neves D, Ojeda DI, Pennington RT, Prado DE, Prenner G, de Queiroz LP, Ramos G, Ranzato Filardi FL, Ribeiro PG, Rico-Arce MdL, Sanderson MJ, Santos- Silva J, S?o-Mateus WMB, Silva MJS, Simon MF, Sinou C, Snak C, de Souza éR, Sprent J, Steele KP, Steier JE, Steeves R, Stirton CH, Tagane S, Torke BM, Toyama H, Cruz DTd, Vatanparast M, Wieringa JJ, Wink M, Wojciechowski MF, Yahara T, Yi T, Zimmerman E ( 2017). A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny, The Legume Phylogeny Working Group (LPWG). Taxon, 66, 44-77.
DOI URL |
| [2] |
Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA ( 2009). Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proceedings of the Royal Society B, 276, 1771-1776.
DOI URL |
| [3] |
Brodribb TJ, Bienaimé D, Marmottant P ( 2016). Revealing catastrophic failure of leaf networks under stress. Proceedings of the National Academy of Sciences of the United States of America, 113, 4865-4869.
DOI URL PMID |
| [4] |
Brodribb TJ, Feild TS ( 2010). Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecology Letters, 13, 175-183.
DOI URL PMID |
| [5] |
Brodribb TJ, Feild TS, Jordan GJ ( 2007). Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology, 144, 1890-1898.
DOI URL |
| [6] |
Brodribb TJ, Holbrook NM ( 2003 a). Changes in leaf hydraulic conductance during leaf shedding in seasonally dry tropical forest. New Phytologist, 158, 295-303.
DOI URL |
| [7] |
Brodribb TJ, Holbrook NM ( 2003 b). Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology, 132, 2166-2173.
DOI URL |
| [8] |
Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B ( 2005). Leaf hydraulic capacity in ferns, conifers and angiosperms, impacts on photosynthetic maxima. New Phytologist, 165, 839-846.
DOI URL PMID |
| [9] |
Buckley LB, Kingsolver JG ( 2012). Functional and phylogenetic approaches to forecasting species responses to climate change. Annual Review of Ecology, Evolution, and Systematics, 43, 205-264.
DOI URL |
| [10] | Darwin C ( 1859). On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. John Murray, London. |
| [11] |
Escalona JM, Flexas J, Medrano H ( 1999). Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Functional Plant Biology, 26, 421-433.
DOI URL |
| [12] |
Fan ZX, Zhang SB, Hao GY, Ferry Slik JW, Cao KF ( 2012). Hydraulic conductivity traits predict growth rates and adult stature of 40 Asian tropical tree species better than wood density. Journal of Ecology, 100, 732-741.
DOI URL |
| [13] |
Franks PJ, Beerling DJ ( 2009). Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences of the United States of America, 106, 10343-10347.
DOI URL |
| [14] |
Franks PJ, Drake PL, Beerling DJ ( 2009). Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density, an analysis using Eucalyptus globulus. Plant, Cell & Environment, 32, 1737-1748.
DOI URL PMID |
| [15] |
Freckleton RP, Harvey PH, Pagel M ( 2002). Phylogenetic analysis and comparative data, a test and review of evidence. The American Naturalist, 160, 712-726.
DOI URL PMID |
| [16] | Givnish TJ ( 2002). Adaptive significance of evergreen vs. deciduous leaves, solving the triple paradox. Silva Fennica, 36, 703-743. |
| [17] | Guyot G, Scoffoni C, Sack L ( 2012). Combined impacts of irradiance and dehydration on leaf hydraulic conductance, insights into vulnerability and stomatal control. Plant, Cell & Environment, 35, 857-871. |
| [18] |
Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA ( 2001). Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia, 126, 457-461.
DOI URL |
| [19] |
Ishida A, Nakano T, Yazaki K, Matsuki S, Koike N, Lauenstein D, Shimizu M, Yamashita N ( 2008). Coordination between leaf and stem traits related to leaf carbon gain and hydraulics across 32 drought-tolerant angiosperms. Oecologia, 156, 193-202.
DOI URL PMID |
| [20] |
Liu H, Xu QY, He PC, Santiago LS, Yang KM, Ye Q ( 2015 a). Strong phylogenetic signals and phylogenetic niche conservatism in ecophysiological traits across divergent lineages of Magnoliaceae. Scientific Reports, 5, 12246. DOI: 10.1038/srep12246.
DOI URL PMID |
| [21] |
Liu YY, Song J, Wang M, Li N, Niu CY, Hao GY ( 2015 b). Coordination of xylem hydraulics and stomatal regulation in keeping the integrity of xylem water transport in shoots of two compound-leaved tree species. Tree Physiology, 35, 1333-1342.
DOI URL PMID |
| [22] |
Nicotra AB, Davidson A ( 2010). Adaptive phenotypic plasticity and plant water use. Functional Plant Biology, 37, 117-127.
DOI URL |
| [23] |
Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, Tsukaya H ( 2011). The evolution and functional significance of leaf shape in the angiosperms. Functional Plant Biology, 38, 535-552.
DOI URL |
| [24] |
Oren R, Sperry JS, Ewers BE, Pataki DE, Philips N, Megonigal JP ( 2001). Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in a flooded Taxodium distichum L. forest: Hydraulic and non-hydraulic effects. Oecologia, 126, 21-29.
DOI URL PMID |
| [25] |
Paradis E, Claude J, Strimmer K ( 2004). APE, analyses of phylogenetics and evolution in R language. Bioinformatics, 20, 289-290.
DOI URL PMID |
| [26] | Quinn CJ, Price RA, Gadek PA ( 2002). Familial concepts and relationships in the conifer based on rbcL and matK sequence comparisons. Kew Bulletin, 57, 513-531. |
| [27] |
Sack L, Cowan PD, Jaikumar N, Holbrook NM ( 2003). The ‘hydrology’ of leaves, co-ordination of structure and function in temperature woody species. Plant, Cell & Environment, 26, 1343-1356.
DOI URL |
| [28] |
Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T ( 2004). Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia, 140, 543-550.
DOI URL PMID |
| [29] |
Scoffoni C, McKown AD, Rawls M, Sack L ( 2012). Dynamics of leaf hydraulic conductance with water status, quantification and analysis of species differences under steady state. Journal of Experimental Botany, 63, 643-658.
DOI URL PMID |
| [30] | Song J, Li RH, Zhu SD, Ye Q ( 2013). Leaf functional traits of ferns from different habitats in monsoon evergreen broad- leaved forest in Dinghushan Mountain . Journal of Tropical and Subtropical Botany, 21, 489-495. |
| 宋娟, 李荣华, 朱师丹 鼎湖山季风常绿阔叶林不同生境蕨类植物的叶片功能性状研究. 热带亚热带植物学报, 21, 489-495.] | |
| [31] |
Sperry JS, Donnelly JR, Tyree MT ( 1988). A method for measuring hydraulic conductivity and embolism in xylem. Plant, Cell & Environment, 11, 35-40.
DOI URL |
| [32] |
Testo W, Sundue M ( 2016). A 4000-species dataset provides new insight into the evolution of ferns. Molecular Phylogenetics and Evolution, 105, 200-211.
DOI URL PMID |
| [33] |
Trifiló P, Raimondo F, Savi T, Lo Gullo MA, Nardini A ( 2016). The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. Journal of Experimental Botany, 67, erw268. DOI: 10.1093/jxb/erw268.
DOI URL PMID |
| [34] |
Tuzet A, Perrier A, Leuning R ( 2003). A coupled model of stomatal conductance, photosynthesis and transpiration. Plant, Cell & Environment, 26, 1097-1116.
DOI URL |
| [35] |
Webb CO, Ackerly DD, Kembel SW ( 2008). Phylocom, software for the analysis of phylogenetic community structure trait evolution. Bioinformatics, 24, 2098-2100.
DOI URL |
| [36] | Wu DL ( 2006). loral of Guangdong . Guangdong Science & Technology Press, Guangzhou. |
| [ 吴德邻 ( 2006). 广东植物志 .广东科技出版社, 广州.] | |
| [37] | Zhan XY, Yu GR, Sheng WP, Fang HJ ( 2012). Foliar water use efficiency and nitrogen use efficiency of dominant plant species in main forests along the North-South Transect of East China. Chinese Journal of Applied Ecology, 23, 587-594. |
| [ 展小云, 于贵瑞, 盛文萍, 方华军 ( 2012). 中国东部南北样带森林优势植物叶片的水分利用效率和氮素利用效率. 应用生态学报 , 23, 587-594.] | |
| [38] |
Zhang Y, Yang SJ, Sun M, Cao KF ( 2014). Stomatal traits are evolutionarily associated with vein density in basal angiosperms. Plant Science Journal, 32, 320-328.
DOI URL |
|
[ 张亚, 杨石建, 孙梅, 曹坤芳 ( 2014). 基部被子植物气孔性状与叶脉密度的关联演化.植物科学学报 , 32, 320-328.]
DOI URL |
| [1] | Xu Zi-Yi Guang-Ze JIN. Variation and trade-offs in fine root functional traits of seedlings of different mycorrhizal types in mixed broadleaved-Korean pine forests [J]. Chin J Plant Ecol, 2024, 48(5): 612-622. |
| [2] | FU Liang-Chen, DING Zong-Ju, TANG Mao, ZENG Hui, ZHU Biao. Rhizosphere effects of Betula platyphylla and Quercus mongolica and their seasonal dynamics in Dongling Mountain, Beijing [J]. Chin J Plant Ecol, 2024, 48(4): 508-522. |
| [3] | LIU Cong-Cong, HE Nian-Peng, LI Ying, ZHANG Jia-Hui, YAN Pu, WANG Ruo-Meng, WANG Rui-Li. Current and future trends of plant functional traits in macro-ecology [J]. Chin J Plant Ecol, 2024, 48(1): 21-40. |
| [4] | ZHANG Min, SANG Ying, SONG Jin-Feng. Root pressure of hydroponic Dracaena sanderiana and its determinants [J]. Chin J Plant Ecol, 2023, 47(7): 1010-1019. |
| [5] | HE Lu-Lu, ZHANG Xuan, ZHANG Yu-Wen, WANG Xiao-Xia, LIU Ya-Dong, LIU Yan, FAN Zi-Ying, HE Yuan-Yang, XI Ben-Ye, DUAN Jie. Crown characteristics and its relationship with tree growth on different slope aspects for Larix olgensis var. changbaiensis plantation in eastern Liaoning mountainous area, China [J]. Chin J Plant Ecol, 2023, 47(11): 1523-1539. |
| [6] | YU Jun-Rui, WAN Chun-Yan, ZHU Shi-Dan. Hydraulic vulnerability segmentation in woody plant species from tropical and subtropical karst forests [J]. Chin J Plant Ecol, 2023, 47(11): 1576-1584. |
| [7] | LEI Zi-Ran, JIA Guo-Dong, YU Xin-Xiao, LIU Zi-He. A review of stable hydrogen and oxygen isotopic offset in plant water source research [J]. Chin J Plant Ecol, 2023, 47(1): 25-40. |
| [8] | ZHANG Hong-Xiang, WEN Zhi-Bin, WANG Qian. Population genetic structure of Malus sieversii and environmental adaptations [J]. Chin J Plant Ecol, 2022, 46(9): 1098-1108. |
| [9] | DONG Liu-Wen, REN Zheng-Wei, ZHANG Rui, XIE Chen-Di, ZHOU Xiao-Long. Functional diversity rather than species diversity can explain community biomass variation following short-term nitrogen addition in an alpine grassland [J]. Chin J Plant Ecol, 2022, 46(8): 871-881. |
| [10] | ZENG Kai-Na, SUN Hao-Ran, SHEN Yi-Chun, REN Ming-Xun. Pollination network and seasonal dynamics of Yangshan Wetland in Hainan Island, China [J]. Chin J Plant Ecol, 2022, 46(7): 775-784. |
| [11] | Min FAN, Yi-Tong LU, Zhao-Hua WANG, Ying-Qi HUANG, Yu PENG, Jia-Xin SHANG, Yang ZHANG. Effects of patch pattern on plant diversity and functional traits in center Hunshandak Sandland [J]. Chin J Plant Ecol, 2022, 46(1): 51-61. |
| [12] | ZHANG Jing-Hui, WANG Zheng, HUANG Yong-Mei, CHEN Hui-Ying, LI Zhi-Yong, LIANG Cun-Zhu. Effects of grassland utilization on the functional traits of dominant plants in a temperate typical steppe [J]. Chin J Plant Ecol, 2021, 45(8): 818-833. |
| [13] | SUN Hao-Zhe, WANG Xiang-Ping, ZHANG Shu-Bin, WU Peng, YANG Lei. Abiotic and biotic modulators of litterfall production and its temporal stability during the succession of broad-leaf and Korean pine mixed forest [J]. Chin J Plant Ecol, 2021, 45(6): 594-605. |
| [14] | WANG Zhao-Ying, CHEN Xiao-Ping, CHENG Ying, WANG Man-Tang, ZHONG Quan-Lin, LI Man, CHENG Dong-Liang. Leaf and fine root economics spectrum across 49 woody plant species in Wuyi Mountains [J]. Chin J Plant Ecol, 2021, 45(3): 242-252. |
| [15] | DAI Jing-Zhong, BAI Yu-Ting, WEI Zhi-Jun, ZHANG Chu, YAN Rui-Rui. Effects of root-cutting in the vegetative phase on plant functional traits of Leymus chinensis [J]. Chin J Plant Ecol, 2021, 45(12): 1292-1302. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn