

植物生态学报 ›› 2019, Vol. 43 ›› Issue (6): 512-520.DOI: 10.17521/cjpe.2019.0082
冯璐1,3,*( ),卜兆君2,3,吴玉环4,刘莎莎2,3,刘超2,3
),卜兆君2,3,吴玉环4,刘莎莎2,3,刘超2,3
收稿日期:2019-04-15
修回日期:2019-06-03
出版日期:2019-06-20
发布日期:2019-09-30
通讯作者:
冯璐
基金资助:
FENG Lu1,3,*( ),BU Zhao-Jun2,3,WU Yu-Huan4,LIU Sha-Sha2,3,LIU Chao2,3
),BU Zhao-Jun2,3,WU Yu-Huan4,LIU Sha-Sha2,3,LIU Chao2,3
Received:2019-04-15
Revised:2019-06-03
Online:2019-06-20
Published:2019-09-30
Contact:
FENG Lu
Supported by:摘要:
研究泥炭地特征性环境因子——淹水、少氧和化感物质对泥炭藓孢子持久性的影响, 可深入理解泥炭地泥炭藓持久孢子库的形成机制, 为退化泥炭地泥炭藓地被恢复研究提供参考。该研究以藓丘种和丘间种两种泥炭藓的孢子为试验材料, 通过室内模拟控制实验的方法, 研究泥炭藓孢子在空气、超纯水、泥炭地地表水和泥炭藓沥出液中, 及3种速率充气下, 孢子萌发力持久性的变化。经充气处理后, 泥炭藓孢子持久性显著低于不充气处理。不充气时, 泥炭藓孢子在含有化感物质的泥炭地地表水和泥炭藓沥出液中保存, 持久性显著高于在超纯水中保存。通径分析结果显示, 溶解氧是影响泥炭地泥炭藓孢子持久性的主要因子和限制因子, 养分元素氮(TN)和磷(TP)的浓度为孢子持久性的负作用因子。研究结果表明, 泥炭藓孢子散布于苔藓地被基质或淹水的丘间生境中, 比暴露于空气或在无化感物质的水中, 能更好地维持萌发力。泥炭地中, 泥炭藓孢子和其他植物的繁殖体的超长寿命可能归因于少氧、养分贫乏和丰富的化感物质等泥炭地特征性环境因子。
冯璐, 卜兆君, 吴玉环, 刘莎莎, 刘超. 泥炭地特征性环境因子促进泥炭藓持久孢子库的形成. 植物生态学报, 2019, 43(6): 512-520. DOI: 10.17521/cjpe.2019.0082
FENG Lu, BU Zhao-Jun, WU Yu-Huan, LIU Sha-Sha, LIU Chao. Characteristic environmental factors in peatlands facilitate the formation of persistent Sphagnum spore banks. Chinese Journal of Plant Ecology, 2019, 43(6): 512-520. DOI: 10.17521/cjpe.2019.0082
| DO (mg·L-1) | pH | Eh (mV) | ||
|---|---|---|---|---|
| 超纯水 Ultrapure water | 不充气 Control | 8.91 ± 0.02Ab | 5.36 ± 0.04Bb | 196.7 ± 3.9Bb |
| 低速率充气 Low | 8.84 ± 0.02c | 5.24 ± 0.06b | 191.0 ± 5.0b | |
| 高速率充气 High | 9.09 ± 0.01a | 5.55 ± 0.07a | 206.2 ± 1.0a | |
| 泥炭地地表水 Peatland surface water | 不充气 Control | 6.91 ± 0.02Cc | 5.80 ± 0.02A | 181.2 ± 3.7Cc |
| 低速率充气 Low | 9.60 ± 0.04b | 7.47 ± 0.06a | 192.8 ± 1.4b | |
| 高速率充气 High | 10.08 ± 0.03a | 7.19 ± 0.01b | 198.6 ± 1.6a | |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 不充气 Control | 7.52 ± 0.17Bc | 4.96 ± 0.06Cc | 247.1 ± 2.5Aa |
| 低速率充气 Low | 9.70 ± 0.02b | 5.91 ± 0.09b | 196.6 ± 5.8b | |
| 高速率充气 High | 10.25 ± 0.02a | 6.14 ± 0.01b | 183.9 ± 0.4c |
表1 不同保存液和不同充气速率处理中的溶解氧浓度(DO)、pH值和氧化还原电位(Eh)(平均值±标准误差, n = 3)
Table 1 Dissolved oxygen concentration (DO), pH value and redox potential (Eh) in different water storage solutions with or without air injection (mean ± SE, n = 3)
| DO (mg·L-1) | pH | Eh (mV) | ||
|---|---|---|---|---|
| 超纯水 Ultrapure water | 不充气 Control | 8.91 ± 0.02Ab | 5.36 ± 0.04Bb | 196.7 ± 3.9Bb |
| 低速率充气 Low | 8.84 ± 0.02c | 5.24 ± 0.06b | 191.0 ± 5.0b | |
| 高速率充气 High | 9.09 ± 0.01a | 5.55 ± 0.07a | 206.2 ± 1.0a | |
| 泥炭地地表水 Peatland surface water | 不充气 Control | 6.91 ± 0.02Cc | 5.80 ± 0.02A | 181.2 ± 3.7Cc |
| 低速率充气 Low | 9.60 ± 0.04b | 7.47 ± 0.06a | 192.8 ± 1.4b | |
| 高速率充气 High | 10.08 ± 0.03a | 7.19 ± 0.01b | 198.6 ± 1.6a | |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 不充气 Control | 7.52 ± 0.17Bc | 4.96 ± 0.06Cc | 247.1 ± 2.5Aa |
| 低速率充气 Low | 9.70 ± 0.02b | 5.91 ± 0.09b | 196.6 ± 5.8b | |
| 高速率充气 High | 10.25 ± 0.02a | 6.14 ± 0.01b | 183.9 ± 0.4c |
| TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚 Phenolics | |
|---|---|---|---|---|---|---|---|
| 超纯水 Ultrapure water | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.05 ± 0.01c | 0.00 ± 0.00c | 0.02 ± 0.00c | 0.00 ± 0.00c |
| 泥炭地地表水 Peatland surface water | 0.52 ± 0.05b | 0.03 ± 0.01b | 2.90 ± 0.33b | 5.22 ± 0.70a | 1.64 ± 0.16b | 1.43 ± 0.12a | 6.51 ± 0.05a |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 5.03 ± 0.31a | 0.26 ± 0.04a | 8.89 ± 1.13a | 0.71 ± 0.49b | 4.33 ± 0.18a | 0.39 ± 0.06b | 3.23 ± 0.09b |
表2 三种保存液的主要化学元素和总酚浓度(mg·L-1)(平均值±标准误差, n = 3)
Table 2 Main chemical elements and total phenolics concentration (mg·L-1) in three solutions (mean ± SE, n = 3)
| TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚 Phenolics | |
|---|---|---|---|---|---|---|---|
| 超纯水 Ultrapure water | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.00 ± 0.00c | 0.05 ± 0.01c | 0.00 ± 0.00c | 0.02 ± 0.00c | 0.00 ± 0.00c |
| 泥炭地地表水 Peatland surface water | 0.52 ± 0.05b | 0.03 ± 0.01b | 2.90 ± 0.33b | 5.22 ± 0.70a | 1.64 ± 0.16b | 1.43 ± 0.12a | 6.51 ± 0.05a |
| 中位泥炭藓沥出液 Leachate water of Sphagnum magellanicum | 5.03 ± 0.31a | 0.26 ± 0.04a | 8.89 ± 1.13a | 0.71 ± 0.49b | 4.33 ± 0.18a | 0.39 ± 0.06b | 3.23 ± 0.09b |
| 来源 Source | d.f. | 尖叶泥炭藓 Sphagnum capillifolium | 喙叶泥炭藓 Sphagnum flexuosum | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| 保存液类型 Water type | 2 | 3.74 | 0.044 | 3.33 | 0.059 |
| 充气速率 Air injection | 2 | 16.31 | 0.000 | 2.90 | 0.081 |
| 保存液类型 × 充气速率 Water type × Air injection | 4 | 5.57 | 0.004 | 3.73 | 0.022 |
表3 保存液类型和充气速率及二者的交互作用对泥炭藓孢子持久性影响的双因素方差分析
Table 3 Two-way ANOVA on the effect of water type, air injection and the interaction between water type and air injection on spore persistence
| 来源 Source | d.f. | 尖叶泥炭藓 Sphagnum capillifolium | 喙叶泥炭藓 Sphagnum flexuosum | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| 保存液类型 Water type | 2 | 3.74 | 0.044 | 3.33 | 0.059 |
| 充气速率 Air injection | 2 | 16.31 | 0.000 | 2.90 | 0.081 |
| 保存液类型 × 充气速率 Water type × Air injection | 4 | 5.57 | 0.004 | 3.73 | 0.022 |
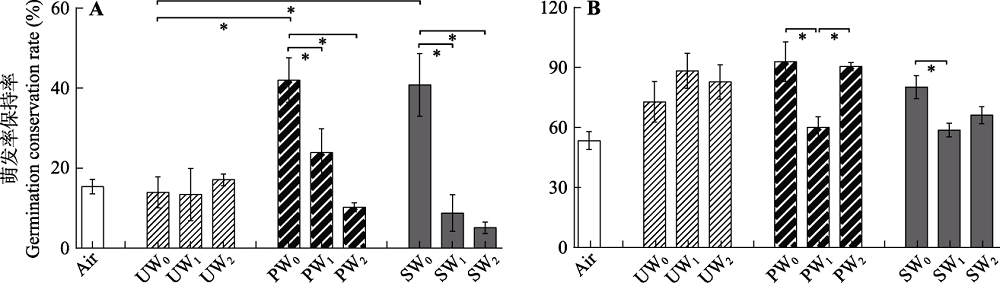
图1 经3种保存液和3种程度充气处理后泥炭藓孢子的萌发率保持率(平均值±标准误差)。A, 尖叶泥炭藓(Sphagnum capillifolium)。B, 喙叶泥炭藓(Sphagnum flexuosum); PW, 泥炭地表水; SW, 泥炭藓沥出液; UW, 超纯水。0, 不充气; 1, 低速率充气; 2, 高速率充气。*表示不同分组内(UW0、PW0和SW0; UW0、UW1和UW2; PW0、PW1和PW2; SW0、SW1和SW2; Air和UW0)的处理间有显著差异(p < 0.05)。
Fig. 1 Germination conservation rate of Sphagnum spores after three types of storage solutions and three levels of air injection. (mean ± SE) A, S. capillifolium. B, S. flexuosum PW, peatland surface water; SW, Sphagnum leachate water; UW, ultrapurewater. 0, no air injection; 1, low air injection; 2, high air injection. * in each group (UW0, PW0 and SW0; UW0, UW1 and UW2; PW0, PW1 and PW2; SW0, SW1 and SW2; Air and UW0) indicated significant differences in one-way ANOVA (p < 0.05).
| 因子 Factor | DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics |
|---|---|---|---|---|---|---|---|---|---|---|
| DO | ||||||||||
| pH | 0.562 | |||||||||
| Eh | -0.322 | -0.401 | ||||||||
| TN | 0.108 | -0.183 | 0.364 | |||||||
| TP | 0.108 | -0.175 | 0.362 | 1.000 | ||||||
| K+ | 0.096 | -0.008 | 0.310 | 0.973 | 0.976 | |||||
| Ca2+ | -0.074 | 0.765 | -0.296 | -0.307 | -0.296 | -0.080 | ||||
| Na+ | 0.091 | 0.038 | 0.293 | 0.958 | 0.961 | 0.998 | -0.022 | |||
| Mg2+ | -0.061 | 0.766 | -0.254 | -0.173 | -0.161 | 0.058 | 0.990 | 0.116 | ||
| 总酚 Phenolics | 0.047 | 0.767 | -0.156 | 0.051 | 0.063 | 0.277 | 0.925 | 0.332 | 0.965 | |
| 尖叶泥炭藓孢子持久性 Sphagnum capillifolium GCR | -0.777 | -0.187 | 0.375 | -0.126 | -0.123 | -0.057 | 0.310 | -0.039 | 0.303 | 0.206 |
| 喙叶泥炭藓孢子持久性 Sphagnum flexuosum GCR | -0.402 | -0.207 | 0.052 | -0.382 | -0.382 | -0.365 | 0.146 | -0.357 | 0.096 | 0.031 |
表4 保存液理化指标间及其与两种泥炭藓孢子持久性的相关关系
Table 4 Correlation analysis among water physicochemical indicators and between those indicators with sphagnum spore persistence
| 因子 Factor | DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics |
|---|---|---|---|---|---|---|---|---|---|---|
| DO | ||||||||||
| pH | 0.562 | |||||||||
| Eh | -0.322 | -0.401 | ||||||||
| TN | 0.108 | -0.183 | 0.364 | |||||||
| TP | 0.108 | -0.175 | 0.362 | 1.000 | ||||||
| K+ | 0.096 | -0.008 | 0.310 | 0.973 | 0.976 | |||||
| Ca2+ | -0.074 | 0.765 | -0.296 | -0.307 | -0.296 | -0.080 | ||||
| Na+ | 0.091 | 0.038 | 0.293 | 0.958 | 0.961 | 0.998 | -0.022 | |||
| Mg2+ | -0.061 | 0.766 | -0.254 | -0.173 | -0.161 | 0.058 | 0.990 | 0.116 | ||
| 总酚 Phenolics | 0.047 | 0.767 | -0.156 | 0.051 | 0.063 | 0.277 | 0.925 | 0.332 | 0.965 | |
| 尖叶泥炭藓孢子持久性 Sphagnum capillifolium GCR | -0.777 | -0.187 | 0.375 | -0.126 | -0.123 | -0.057 | 0.310 | -0.039 | 0.303 | 0.206 |
| 喙叶泥炭藓孢子持久性 Sphagnum flexuosum GCR | -0.402 | -0.207 | 0.052 | -0.382 | -0.382 | -0.365 | 0.146 | -0.357 | 0.096 | 0.031 |
| 物种 Species | 因子 Factor | 直接作用 Direct effect | 间接作用 Indirect effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics | |||
| 尖叶泥炭藓 Sphagnum capillifolium | DO | -0.777 | 0.270 | -0.068 | -0.007 | -0.007 | 0.003 | -0.030 | 0.005 | -0.025 | 0.018 | |
| pH | 0.480 | -0.437 | -0.084 | 0.012 | 0.011 | 0.000 | 0.308 | 0.002 | 0.312 | 0.297 | ||
| Eh | 0.210 | 0.250 | -0.192 | -0.024 | -0.022 | 0.008 | -0.119 | 0.015 | -0.103 | -0.061 | ||
| TN | -0.067 | -0.103 | -0.088 | 0.076 | -0.062 | 0.026 | -0.124 | 0.049 | -0.070 | 0.020 | ||
| TP | -0.062 | -0.102 | -0.084 | 0.076 | -0.067 | 0.026 | -0.119 | 0.049 | -0.066 | 0.024 | ||
| K+ | 0.027 | -0.091 | -0.004 | 0.065 | -0.065 | -0.061 | -0.032 | 0.051 | 0.024 | 0.107 | ||
| Ca2+ | 0.403 | 0.070 | 0.367 | -0.062 | 0.021 | 0.018 | -0.002 | -0.001 | 0.403 | 0.358 | ||
| Na+ | 0.051 | -0.087 | 0.018 | 0.062 | -0.064 | -0.060 | 0.027 | -0.009 | 0.047 | 0.129 | ||
| Mg2+ | 0.407 | 0.058 | 0.367 | -0.053 | 0.012 | 0.010 | 0.002 | 0.399 | 0.006 | 0.374 | ||
| 总酚Phenolics | 0.387 | -0.045 | 0.368 | -0.033 | -0.003 | -0.004 | 0.007 | 0.373 | 0.017 | 0.393 | ||
| 喙叶泥炭藓 Sphagnum flexuosum | DO | -0.402 | 0.014 | 0.029 | -0.040 | -0.040 | -0.034 | -0.009 | -0.032 | -0.005 | 0.003 | |
| pH | 0.025 | -0.226 | 0.036 | 0.068 | 0.065 | 0.003 | 0.098 | -0.013 | 0.060 | 0.042 | ||
| Eh | -0.089 | 0.129 | -0.010 | -0.135 | -0.135 | -0.111 | -0.038 | -0.103 | -0.020 | -0.009 | ||
| TN | -0.372 | -0.044 | -0.005 | -0.032 | -0.372 | -0.348 | -0.039 | -0.336 | -0.013 | 0.003 | ||
| TP | -0.372 | -0.043 | -0.004 | -0.032 | -0.372 | -0.349 | -0.038 | -0.337 | -0.013 | 0.003 | ||
| K+ | -0.358 | -0.038 | 0.000 | -0.028 | -0.362 | -0.363 | -0.010 | -0.350 | 0.005 | 0.015 | ||
| Ca2+ | 0.128 | 0.030 | 0.019 | 0.026 | 0.114 | 0.110 | 0.029 | 0.008 | 0.077 | 0.051 | ||
| Na+ | -0.351 | -0.037 | 0.001 | -0.026 | -0.356 | -0.358 | -0.357 | -0.003 | 0.009 | 0.018 | ||
| Mg2+ | 0.078 | 0.024 | 0.019 | 0.023 | 0.064 | 0.060 | -0.021 | 0.127 | -0.041 | 0.053 | ||
| 总酚Phenolics | 0.055 | -0.019 | 0.019 | 0.014 | -0.019 | -0.023 | -0.099 | 0.118 | -0.117 | 0.075 | ||
表5 两种泥炭藓孢子持久性与保存液理化指标的通径分析
Table 5 Path analysis of spore persistence and water physicochemical indicators
| 物种 Species | 因子 Factor | 直接作用 Direct effect | 间接作用 Indirect effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DO | pH | Eh | TN | TP | K+ | Ca2+ | Na+ | Mg2+ | 总酚Phenolics | |||
| 尖叶泥炭藓 Sphagnum capillifolium | DO | -0.777 | 0.270 | -0.068 | -0.007 | -0.007 | 0.003 | -0.030 | 0.005 | -0.025 | 0.018 | |
| pH | 0.480 | -0.437 | -0.084 | 0.012 | 0.011 | 0.000 | 0.308 | 0.002 | 0.312 | 0.297 | ||
| Eh | 0.210 | 0.250 | -0.192 | -0.024 | -0.022 | 0.008 | -0.119 | 0.015 | -0.103 | -0.061 | ||
| TN | -0.067 | -0.103 | -0.088 | 0.076 | -0.062 | 0.026 | -0.124 | 0.049 | -0.070 | 0.020 | ||
| TP | -0.062 | -0.102 | -0.084 | 0.076 | -0.067 | 0.026 | -0.119 | 0.049 | -0.066 | 0.024 | ||
| K+ | 0.027 | -0.091 | -0.004 | 0.065 | -0.065 | -0.061 | -0.032 | 0.051 | 0.024 | 0.107 | ||
| Ca2+ | 0.403 | 0.070 | 0.367 | -0.062 | 0.021 | 0.018 | -0.002 | -0.001 | 0.403 | 0.358 | ||
| Na+ | 0.051 | -0.087 | 0.018 | 0.062 | -0.064 | -0.060 | 0.027 | -0.009 | 0.047 | 0.129 | ||
| Mg2+ | 0.407 | 0.058 | 0.367 | -0.053 | 0.012 | 0.010 | 0.002 | 0.399 | 0.006 | 0.374 | ||
| 总酚Phenolics | 0.387 | -0.045 | 0.368 | -0.033 | -0.003 | -0.004 | 0.007 | 0.373 | 0.017 | 0.393 | ||
| 喙叶泥炭藓 Sphagnum flexuosum | DO | -0.402 | 0.014 | 0.029 | -0.040 | -0.040 | -0.034 | -0.009 | -0.032 | -0.005 | 0.003 | |
| pH | 0.025 | -0.226 | 0.036 | 0.068 | 0.065 | 0.003 | 0.098 | -0.013 | 0.060 | 0.042 | ||
| Eh | -0.089 | 0.129 | -0.010 | -0.135 | -0.135 | -0.111 | -0.038 | -0.103 | -0.020 | -0.009 | ||
| TN | -0.372 | -0.044 | -0.005 | -0.032 | -0.372 | -0.348 | -0.039 | -0.336 | -0.013 | 0.003 | ||
| TP | -0.372 | -0.043 | -0.004 | -0.032 | -0.372 | -0.349 | -0.038 | -0.337 | -0.013 | 0.003 | ||
| K+ | -0.358 | -0.038 | 0.000 | -0.028 | -0.362 | -0.363 | -0.010 | -0.350 | 0.005 | 0.015 | ||
| Ca2+ | 0.128 | 0.030 | 0.019 | 0.026 | 0.114 | 0.110 | 0.029 | 0.008 | 0.077 | 0.051 | ||
| Na+ | -0.351 | -0.037 | 0.001 | -0.026 | -0.356 | -0.358 | -0.357 | -0.003 | 0.009 | 0.018 | ||
| Mg2+ | 0.078 | 0.024 | 0.019 | 0.023 | 0.064 | 0.060 | -0.021 | 0.127 | -0.041 | 0.053 | ||
| 总酚Phenolics | 0.055 | -0.019 | 0.019 | 0.014 | -0.019 | -0.023 | -0.099 | 0.118 | -0.117 | 0.075 | ||
| [1] | Abbott GD, Swain EY, Muhammad AB, Allton K, Belyea LR, Laing CG, Cowie GL (2013). Effect of water-table fluctuations on the degradation of Sphagnum phenols in surficial peats. Geochimica et Cosmochimica Acta, 106, 177-191. |
| [2] | Aerts R, Wallen B, Malmer N (1992). Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. Journal of Ecology, 80, 131-140. |
| [3] | Boatman DJ, Lark PM (1971). Inorganic nutrition of the protonemata of Sphagnum papillosum Lindb., S. magellanicum Brid. and S. cuspidatum Ehrh. New Phytologist, 70, 1053-1059. |
| [4] | Bu ZJ, Li Z, Liu LJ, Sundberg S, Feng YM, Yang YH, Liu S, Song X, Zhang XL (2017a). Bryophyte spore germinability is inhibited by peatland substrates. Acta Oecologica, 78, 34-40. |
| [5] | Bu ZJ, Sundberg S, Feng L, Li HK, Zhao HY, Li HC (2017b). The Methuselah of plant diaspores: Sphagnum spores can survive in nature for centuries. New Phytologist, 214, 1398-1402. |
| [6] | Clymo RS, Duckett JG (1986). Regeneration of Sphagnum. New Phytologist, 102, 589-614. |
| [7] |
Du JJ, Chen ZW (2010). The methods of path analysis by SPSS linear regression. Bulletin of Biology, 45(2), 4-6.
DOI URL |
|
[ 杜家菊, 陈志伟 (2010). 使用SPSS线性回归实现通径分析的方法. 生物学通报, 45(2), 4-6.]
DOI URL |
|
| [8] | Feng L, Bu ZJ, Mallik A, Wang ZC, Liu SS, Wu YH (2017). Continuous waterlogging may not facilitate germinability maintenance of Sphagnum spores. Wetlands, 37, 1015-1022. |
| [9] | Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC (2009). Global patterns in belowground communities. Ecology Letters, 12, 1238-1249. |
| [10] | González-Benito ME, Pérez-García F, Tejeda G, Gómez- Campo C (2011). Effect of the gaseous environment and water content on seed viability of four Brassicaceae species after 36 years storage. Seed Science and Technology, 39, 443-451. |
| [11] | Hättenschwiler S, Vitousek PM (2000). The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends in Ecology & Evolution, 15, 238-243. |
| [12] | Jauhiainen S (1998). Seed and spore banks of two boreal mires. Annales Botanici Fennici, 35, 197-201. |
| [13] | Ke X, Lu W, Conrad R (2015). High oxygen concentration increases the abundance and activity of bacterial rather than archaeal nitrifiers in rice field soil. Microbial Ecology, 70, 961-970. |
| [14] | Li J, He Y, Ma D, He B, Wang Y, Chen B (2018a). Volatile allelochemicals of Chenopodium ambrosioides L. induced mitochondrion-mediated Ca 2+-dependent and caspase-dependent apoptosis signaling pathways in receptor plant cells. Plant and Soil, 425, 297-308. |
| [15] | Li Y, Rashid A, Wang HJ, Hu AY, Lin LF, Yu CP, Chen M, Sun Q (2018b). Contribution of biotic and abiotic factors in the natural attenuation of sulfamethoxazole: A path analysis approach. Science of the Total Environment, 633, 1217-1226. |
| [16] | Liu LJ, Bu ZJ, Liu S, Chen YD, Feng L, Fu B, Yang YH, Wang SZ (2019). Sand and dust deposition may retard the autogenic vegetation succession of peatlands. Scientia Geographica Sinica, 39, 351-358. |
| [ 刘礼洁, 卜兆君, 刘霜, 陈永达, 冯璐, 付彪, 杨云荷, 王升忠 (2019). 沙尘沉降可能阻滞泥炭地植被的自发演替. 地理科学, 39, 351-358.] | |
| [17] | McLetchie DN (1999). Dormancy/Nondormancy cycles in spores of the liverwort Sphaerocarpos texanus. The Bryologist, 102, 15-21. |
| [18] | Michel P, Burritt DJ, Lee WG (2011). Bryophytes display allelopathic interactions with tree species in native forest ecosystems. Oikos, 120, 1272-1280. |
| [19] | Mishler BD, Newton AE (1988). Influences of mature plants and desiccation on germination of spores and gametophyticfragments of Tortula. Journal of Bryology, 15, 327-342. |
| [20] | Montenegro G, Portaluppi MC, Salas FA, Díaz MF (2009). Biological properties of the Chilean native moss Sphagnum magellanicum. Biological Research, 42, 233-237. |
| [21] | Ooi MKJ, Auld TD, Denham AJ (2009). Climate change and bet-hedging: Interactions between increased soil temperatures and seed bank persistence. Global Change Biology, 15, 2375-2386. |
| [22] | Pinsonneault AJ, Moore TR, Roulet NT (2016). Effects of long-term fertilization on peat stoichiometry and associated microbial enzyme activity in an ombrotrophic bog. Biogeochemistry, 129, 149-164. |
| [23] | Proctor MCF, Oliver MJ, Wood AJ, Alpert P, Stark LR, Cleavitt NL, Mishler BD (2007). Desiccation-tolerance in bryophytes: A review. The Bryologist, 110, 595-621. |
| [24] | Rudolph H, Kirchhoff M, Gliesmann S (1988). Sphagnum culture techniques. In: Glime JM ed. Methods in Bryology. Proceedings of the Bryological Methods Workshop, Mainz, Hattori Botanical Laboratory, Nichinan, Japan. |
| [25] | Saatkamp A, Poschlod P, Venable DL (2014). The functional role of soil seed banks in natural communities. In: Gallagher RS ed. Seeds: The Ecology of Regeneration in Plant Communities. CABI, Wallingford. 263-295. |
| [26] | Shen-Miller J, Mudgett MB, Schopf JW, Clarke S, Berger R (1995). Exceptional seed longevity and robust growth: Ancient Sacred Lotus from China. American Journal of Botany, 82, 1367-1380. |
| [27] | Song YY, Song CC, Meng HN, Swarzenski CM, Wang XW, Tan WW (2017). Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecological Engineering, 100, 175-185. |
| [28] | Sundberg S, Rydin H (2000). Experimental evidence for a persistent spore bank in Sphagnum. New Phytologist, 148, 105-116. |
| [29] | Sundberg S, Rydin H (2002). Habitat requirements for establishment of Sphagnum from spores. Journal of Ecology, 90, 268-278. |
| [30] | Taârit MB, Msaada K, Hosni K, Marzouk B (2012). Physiological changes, phenolic content and antioxidant activity of Salvia officinalis L. grown under saline conditions. Journal of the Science of Food and Agriculture, 92, 1614-1619. |
| [31] | Tellier A (2019). Persistent seed banking as eco-evolutionary determinant of plant nucleotide diversity: Novel population genetics insights. New Phytologist, 221, 725-730. |
| [32] | Turetsky MR (2003). The role of bryophytes in carbon and nitrogen cycling. The Bryologist, 106, 395-409. |
| [33] | van Zanten BO (1978). Experimental studies on trans-oceanic long-range dispersal of moss spores in the Southern Hemisphere. Journal of the Hattori Botanical Laboratory, 44, 445-482. |
| [34] | Verhoeven JTA, Liefveld WM (1997). The ecological significance of organochemical compounds in Sphagnum. Acta Botanica Neerlandica, 46, 117-130. |
| [35] | Wheeler BD, Proctor MCF (2000). Ecological gradients, subdivisions and terminology of north-west European mires. Journal of Ecology, 88, 187-203. |
| [36] | Whitehead J, Wittemann M, Cronberg N (2018). Allelopathy in bryophytes—A review. Lindbergia, 41, 01097. DOI: 10.25227/linbg.01097. |
| [37] | Yu Z, Dahlgren RA (2000). Evaluation of methods for measuring polyphenols in conifer foliage. Journal of Chemical Ecology, 26, 2119-2140. |
| [38] | Yuan M, Bu ZJ, Liu C, Ma JZ, Wang SZ (2015). Effects of water level and light intensity on capsule production dynamics of Sphagnum capillifolium. Chinese Journal of Plant Ecology, 39, 501-507. |
| [ 袁敏, 卜兆君, 刘超, 马进泽, 王升忠 (2015). 水位与光强变化对尖叶泥炭藓孢蒴生产动态的影响. 植物生态学报, 39, 501-507.] |
| [1] | 李小玲, 朱道明, 余玉蓉, 吴浩, 牟利, 洪柳, 刘雪飞, 卜贵军, 薛丹, 吴林. 模拟氮沉降对鄂西南贫营养泥炭地两种藓类植物生长与分解的影响[J]. 植物生态学报, 2023, 47(5): 644-659. |
| [2] | 余玉蓉, 吴浩, 高娅菲, 赵媛博, 李小玲, 卜贵军, 薛丹, 刘正祥, 武海雯, 吴林. 模拟氮沉降对鄂西南湿地泥炭藓生理及形态特征的影响[J]. 植物生态学报, 2023, 47(11): 1493-1506. |
| [3] | 罗明没, 陈悦, 杨刚, 胡斌, 李玮, 陈槐. 若尔盖退化泥炭地土壤原核微生物群落结构对水位恢复的短期响应[J]. 植物生态学报, 2021, 45(5): 552-561. |
| [4] | 刘雪飞, 吴林, 王涵, 洪柳, 熊莉军. 鄂西南亚高山湿地泥炭藓的生长与分解[J]. 植物生态学报, 2020, 44(3): 228-235. |
| [5] | 刘媛媛, 马进泽, 卜兆君, 王升忠, 张雪冰, 张婷玉, 刘莎莎, 付彪, 康媛. 地理来源与生物化学属性对泥炭地植物残体分解的影响[J]. 植物生态学报, 2018, 42(7): 713-722. |
| [6] | 李瑞, 胡朝臣, 许士麒, 吴迪, 董玉平, 孙新超, 毛瑢, 王宪伟, 刘学炎. 大兴安岭泥炭地植物叶片碳氮磷含量及其化学计量学特征[J]. 植物生态学报, 2018, 42(12): 1154-1167. |
| [7] | 袁敏, 卜兆君, 刘超, 马进泽, 王升忠. 水位与光强变化对尖叶泥炭藓孢蒴生产动态的影响[J]. 植物生态学报, 2015, 39(5): 501-507. |
| [8] | 王明道, 陈红歌, 刘新育, 高玉千, 吴坤, 贾新成. 地黄对芝麻的化感作用及其化感物质的分离鉴定[J]. 植物生态学报, 2009, 33(6): 1191-1198. |
| [9] | 孔垂华, 徐涛, 胡飞. 胜红蓟化感物质之间相互作用的研究[J]. 植物生态学报, 1998, 22(5): 403-408. |
| [10] | 王大力, 祝心如. 三裂叶豚草的化感作用研究[J]. 植物生态学报, 1996, 20(4): 330-337. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19