

植物生态学报 ›› 2015, Vol. 39 ›› Issue (5): 501-507.DOI: 10.17521/cjpe.2015.0048
收稿日期:2015-02-02
接受日期:2015-03-31
出版日期:2015-05-01
发布日期:2015-05-26
通讯作者:
卜兆君
作者简介:*作者简介:E-mail:
基金资助:
YUAN Min, BU Zhao-Jun*( ), LIU Chao, MA Jin-Ze, WANG Sheng-Zhong
), LIU Chao, MA Jin-Ze, WANG Sheng-Zhong
Received:2015-02-02
Accepted:2015-03-31
Online:2015-05-01
Published:2015-05-26
Contact:
Zhao-Jun BU
About author:# Co-first authors
摘要:
选取尖叶泥炭藓(Sphagnum capillifolium)为试验材料, 在模拟水位与光强条件下, 对人工构建的苔藓植物群落进行室内培养, 每隔1-3天观察并记录植株高度、孢蒴变化过程及变化时间, 分析了不同水位与光强条件对孢蒴生产的植物功能属性动态的影响。水位上升促进了蒴柄伸长及植株高增长, 增加了孢蒴开裂率及遮蔽率。光强增加有助于孢蒴生长, 并提高了孢蒴开裂率。在孢蒴直径以及植株高增长性状上, 水位与光强存在交互作用。水位与光强对孢蒴增长率均没有影响。此外, 水位升高与光强增加使孢蒴成熟及蒴柄伸长时间提前, 总体上使孢子释放时间分别提前了4.0 d和4.8 d, 由此可能减小了孢子体因受夏季干旱影响而败育的风险。孢子释放后, 繁殖株高增长加速, 可为未来的再次繁殖奠定基础。
袁敏, 卜兆君, 刘超, 马进泽, 王升忠. 水位与光强变化对尖叶泥炭藓孢蒴生产动态的影响. 植物生态学报, 2015, 39(5): 501-507. DOI: 10.17521/cjpe.2015.0048
YUAN Min,BU Zhao-Jun,LIU Chao,MA Jin-Ze,WANG Sheng-Zhong. Effects of water level and light intensity on capsule production dynamics of Sphagnum capillifolium. Chinese Journal of Plant Ecology, 2015, 39(5): 501-507. DOI: 10.17521/cjpe.2015.0048

图1 尖叶泥炭藓孢蒴形态变化的时间动态——以低水位、弱光强处理中箭头所示的孢蒴为例。A, 7月21日, 新生球形孢蒴呈鹅黄色, 外被雌苞叶, 无蒴柄。B, 8月2日, 蒴柄形成并伸长, 孢蒴呈黑褐色。C, 8月6日, 蒴柄进一步伸展, 孢蒴呈红褐色的粗圆柱形。D, 8月8日, 蒴盖脱落, 孢子释放, 孢蒴呈细圆柱形。
Fig. 1 Morphological change in Sphagnum capillifolium capsules. The capsule highlighted by the arrow is an example under low water level and weak light conditions. A, On July 21, a newborn spherical capsule is yellow, wrapped by perichaetial leaves and no seta developed. B, On August 2, a seta gradually formed and elongated and the capsule was dark brown. C, On August 6, the seta further extended and the capsule became red brown in color and thick cylindrical in shape. D, On August 8, after operculum falling off, spores released and the capsule became thin cylindrical in shape.
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 繁殖株高增长 Height increment of reproductive shoots | 16.66 | < 0.001** | 0.21 | 0.651 | 7.26 | 0.008** | ||
| 营养株高增长 Height increment of vegetative shoots | 4.44 | 0.036* | 1.21 | 0.273 | 6.08 | 0.015* | ||
| 蒴柄长度 Seta length | 10.32 | 0.002** | 0.00 | 0.966 | 0.64 | 0.426 | ||
| 孢蒴直径 Capsule diameter | 0.04 | 0.838 | 14.73 | 0.000** | 13.77 | < 0.001** | ||
表1 水位与光强对植株高增长和孢子体形态影响的双因素方差分析
Table 1 Two-way ANOVA for effects of water level and light intensity on height increment and sporophyte morphology
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 繁殖株高增长 Height increment of reproductive shoots | 16.66 | < 0.001** | 0.21 | 0.651 | 7.26 | 0.008** | ||
| 营养株高增长 Height increment of vegetative shoots | 4.44 | 0.036* | 1.21 | 0.273 | 6.08 | 0.015* | ||
| 蒴柄长度 Seta length | 10.32 | 0.002** | 0.00 | 0.966 | 0.64 | 0.426 | ||
| 孢蒴直径 Capsule diameter | 0.04 | 0.838 | 14.73 | 0.000** | 13.77 | < 0.001** | ||
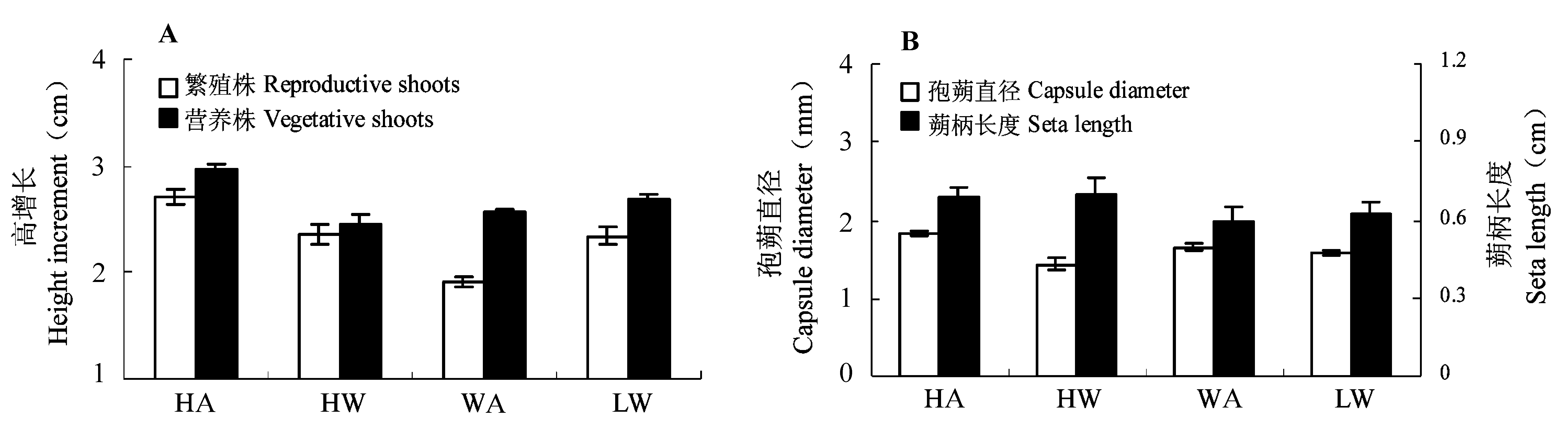
图2 水位与光强对植株高增长(A)和孢子体形态(B)的影响(平均值±标准误差)。A, 一般光强; H, 高水位; L, 低水位; W, 弱光强。
Fig. 2 Effect of water level and light intensity on shoot height increment (A) and sporophyte morphology (B) (mean ± SE). A, ambient light; H, high water level; L, low water level; W, weak light.
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 孢蒴增长率 Capsule growth rate | 0.74 | 0.403 | 0.62 | 0.443 | 0.23 | 0.636 | ||
| 孢蒴开裂率 Capsule cracking rate | 5.80 | 0.037* | 5.39 | 0.033* | 0.24 | 0.554 | ||
| 孢蒴遮蔽率 Rate of capsules being shaded | 11.82 | 0.003** | 0.07 | 0.792 | 2.67 | 0.122 | ||
表2 水位与光强对孢蒴生产动态影响的重复测量方差分析
Table 2 Repetitive measurement and analysis of variance (ANOVA) for effects of water level and light intensity on capsule production dynamics
| 指标 Index | 水位 Water level | 光强 Light intensity | 水位×光强 Water level × light intensity | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |||
| 孢蒴增长率 Capsule growth rate | 0.74 | 0.403 | 0.62 | 0.443 | 0.23 | 0.636 | ||
| 孢蒴开裂率 Capsule cracking rate | 5.80 | 0.037* | 5.39 | 0.033* | 0.24 | 0.554 | ||
| 孢蒴遮蔽率 Rate of capsules being shaded | 11.82 | 0.003** | 0.07 | 0.792 | 2.67 | 0.122 | ||
| 条件 Condition | 孢蒴形成→孢蒴成熟 Capsule formation→ Capsule maturation | 孢蒴成熟→蒴柄长成 Capsule maturation→ Seta maturation | 蒴柄长成→孢蒴开裂 Seta maturation→ Capsule dehiscing |
|---|---|---|---|
| 高水位 High water level | 11.0 ± 1.2 | 2.7 ± 1.2 | 2.3 ± 1.4 |
| 低水位 Low water level | 12.1 ± 2.4 | 3.8 ± 1.9 | 4.1 ± 2.4 |
| 一般光强 Ambient light | 10.4 ± 1.5 | 2.3 ± 1.4 | 2.5 ± 1.3 |
| 弱光强 Weak light | 12.7 ± 1.7 | 3.4 ± 1.9 | 3.9 ± 2.5 |
表3 不同水位与光强条件下孢蒴生产过程各阶段所需时间(平均值±标准偏差)
Table 3 Time needed for each stage of capsule production under different water level and light intensity (mean ± SD)
| 条件 Condition | 孢蒴形成→孢蒴成熟 Capsule formation→ Capsule maturation | 孢蒴成熟→蒴柄长成 Capsule maturation→ Seta maturation | 蒴柄长成→孢蒴开裂 Seta maturation→ Capsule dehiscing |
|---|---|---|---|
| 高水位 High water level | 11.0 ± 1.2 | 2.7 ± 1.2 | 2.3 ± 1.4 |
| 低水位 Low water level | 12.1 ± 2.4 | 3.8 ± 1.9 | 4.1 ± 2.4 |
| 一般光强 Ambient light | 10.4 ± 1.5 | 2.3 ± 1.4 | 2.5 ± 1.3 |
| 弱光强 Weak light | 12.7 ± 1.7 | 3.4 ± 1.9 | 3.9 ± 2.5 |
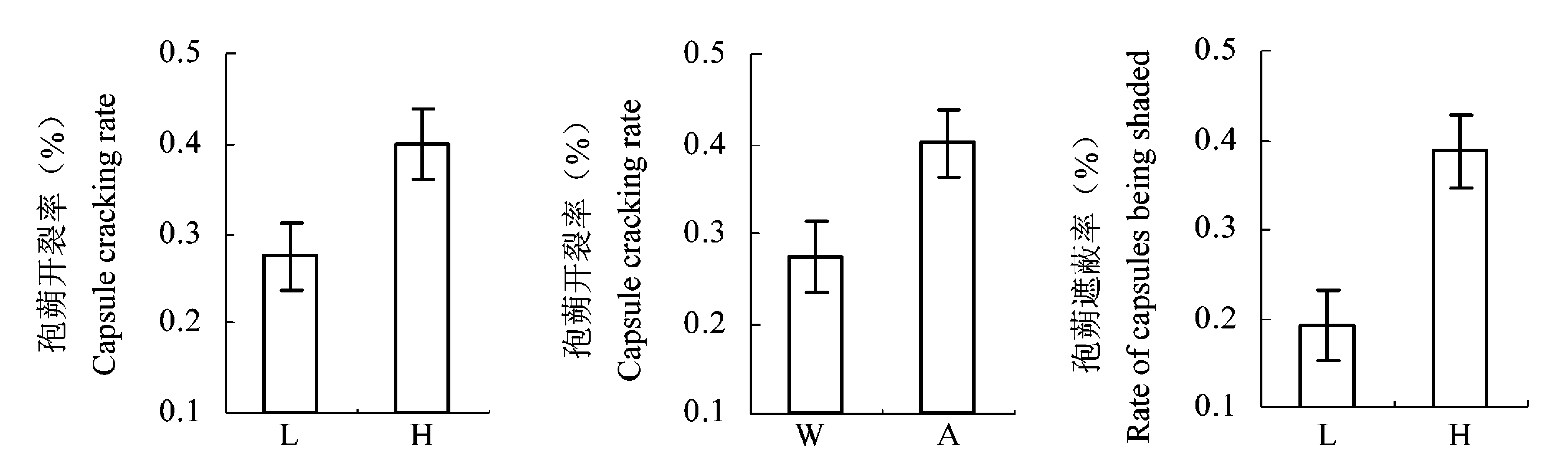
图3 水位与光强对孢蒴开裂率及遮蔽率的影响(平均值±标准误差)。A, 一般光强; H, 高水位; L, 低水位; W, 弱光强。
Fig. 3 Effect of water level and light intensity on capsule cracking rate and shaded rate (mean ± SE). A, ambient light; H, high water level; L, low water level; W, weak light.
| 1 | Bao WM, Cao JG (2001). Spore germination and sexual reproduction in Sphagnum.Bulletin of Biology, 36(1), 8-9(in Chinese). |
| [包文美, 曹建国 (2001). 泥炭藓及其孢子萌发和有性生殖. 生物学通报, 36(1), 8-9.] | |
| 2 | Bragazza L (2008). A climatic threshold triggers the die-off of peat mosses during an extreme heat wave.Global Change Biology, 14, 2688-2695. |
| 3 | Bragazza L, Parisod J, Buttler A, Bardgett RD (2013). Biogeochemical plant-soil microbe feedback in response to climate warming in peatlands.Nature Climate Change, 3, 273-277. |
| 4 | Bu ZJ, Rydin H, Chen X (2011). Direct and interaction- mediated effects of environmental changes on peatland bryophytes.Oecologia, 166, 555-563. |
| 5 | Bubier JL, Moore TR, Bledzki LA (2007). Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog.Global Change Biology, 13, 1168-1186. |
| 6 | Clymo RS (1998). Sphagnum, the peatland carbon economy, and climate change. In: Bates JW, Ashton NW, Duckett JG eds. Bryology for the Twenty-first Century. Maney Publishing and the British Bryological Society, Leeds, UK. 361-368. |
| 7 | Cronberg N (1993). Reproductive biology of Sphagnum.Lindbergia, 17, 69-82. |
| 8 | Crum H (1972). The geographic origins of the mosses of North America’s eastern deciduous forest.Journal of the Hattori Botanical Laboratory, 35, 269-298. |
| 9 | Ehrlén J, Bisang I, Hedenäs L (2000). Costs of sporophyte production in the moss, Dicranum polysetum.Plant Ecology, 149, 207-217. |
| 10 | Gao Q, Cao T, Fu X (2000). Types of spore dispersal of mosses in relation to evolution system.Acta Botanica Yunnanica, 22, 268-276(in Chinese with English abstract). |
| [高谦, 曹同, 付星 (2000). 藓类植物传孢类型及其系统演化关系. 云南植物研究, 22, 268-276. ] | |
| 11 | Glime JM (. Cited: Feb. 2015. |
| 12 | Gravobik SI (1986). Influence of some ecological factors on the spore productivity of Sphagnum mosses. Botanicheskii Zhurnal (in Russian), 71, 1652-1657. |
| 13 | Ingold CT (1965). Spore Liberation. Clarendon Press, Oxford. |
| 14 | Johansson V, Lönnell N, Sundberg S, Hylander K (2014). Release thresholds for moss spores: The importance of turbulence and sporophyte length.Journal of Ecology, 102, 721-729. |
| 15 | Jones EW (1986). Bryophytes in chawley brick pit, Oxford, 1948-1985.Journal of Bryology, 14, 347-358. |
| 16 | Longton RE (1997). Reproductive biology and life-history strategies.Advances of Bryology, 6, 65-101. |
| 17 | Moore TR, Roulet NT, Waddington JM (1998). Uncertainty in predicting the effect of climatic change on the carbon cycling of Candian peatlands.Climatic Change, 40, 229-245. |
| 18 | Nawaschin S (1897). Über die Sporenausschleuderung bei den Torfmoosen.Flora, 48, 151-159. |
| 19 | Rochefort L (2000). Sphagnum―A keystone genus in habitat restoration.The Bryologist, 103, 503-508. |
| 20 | Rudolph H, Kirchhoff M, Gliesmann S (1988). Sphagnum culture techniques. In: Glime JM ed. Methods in Bryology. Hattori Botanical Laboratory, Nichinan. 25-34. |
| 21 | Rydin H, Clymo RS (1989). Transport of carbon and phosphorus compounds about Sphagnum.Proceedings of the Royal Society of London Series B: Biological Sciences, 237, 63-84. |
| 22 | Söderström L, Herben T (1997). Dynamics of bryophyte metapopulations.Advances of Bryology, 6, 205-240. |
| 23 | Soro A, Sundberg S, Rydin H (1999). Species diversity, niche metrics and species associations in harvested and undisturbed bogs.Journal of Vegetation Science, 10, 549-560. |
| 24 | Stark LR, Mishler BD, McLetchie DN (2000). The cost of realized sexual reproduction: Assessing patterns of reproductive allocation and sporophyte abortion in a desert moss.American Journal of Botany, 87, 1599-1608. |
| 25 | Sundberg S (2000). The ecological significance of sexual reproduction in peat mosses (Sphagnum).Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology, 581, 1-37. |
| 26 | Sundberg S (2002). Sporophyte production and spore dispersal phenology in Sphagnum: The importance of summer moisture and patch characteristics.Canadian Journal of Botany, 80, 543-556. |
| 27 | Sundberg S (2005). Larger capsules enhance short-range spore dispersal in Sphagnum, but what happens further away?Oikos, 108, 115-124. |
| 28 | Sundberg S (2010). Size matters for violent discharge height and settling speed of Sphagnum spores: Important attributes for dispersal potential.Annals of Botany, 105, 291-300. |
| 29 | Sundberg S (2013). Spore rain in relation to regional sources and beyond.Ecography, 36, 364-373. |
| 30 | Sundberg S, Rydin H (1998). Spore number in Sphagnum and its dependence on spore and capsule size.Journal of Bryology, 20, 1-16. |
| 31 | Sundberg S, Rydin H (2000). Experimental evidence for a persistent spore bank in Sphagnum.New Phytologist, 148, 105-116. |
| 32 | Whitaker DL, Edwards J (2010). Sphagnum moss disperses spores with vortex rings.Science, 329, 406. |
| [1] | 索南吉, 李博文, 吕汪汪, 王文颖, 拉本, 陆徐伟, 宋扎磋, 陈程浩, 苗琪, 孙芳慧, 汪诗平. 增温增水情景下钉柱委陵菜物候序列的变化及其抗冻性[J]. 植物生态学报, 2024, 48(2): 158-170. |
| [2] | 胡昭佚, 陈天松, 赵丽, 许培轩, 吴正江, 董李勤, 张昆. 水位下降对若尔盖高寒草本沼泽木里薹草氮磷重吸收的影响[J]. 植物生态学报, 2023, 47(6): 847-855. |
| [3] | 李小玲, 朱道明, 余玉蓉, 吴浩, 牟利, 洪柳, 刘雪飞, 卜贵军, 薛丹, 吴林. 模拟氮沉降对鄂西南贫营养泥炭地两种藓类植物生长与分解的影响[J]. 植物生态学报, 2023, 47(5): 644-659. |
| [4] | 李兆光, 文高, 和桂青, 徐天才, 和琼姬, 侯志江, 李燕, 薛润光. 滇西北藜麦氮磷钾生态化学计量特征的物候期动态[J]. 植物生态学报, 2023, 47(5): 724-732. |
| [5] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [6] | 夏璟钰, 张扬建, 郑周涛, 赵广, 赵然, 朱艺旋, 高洁, 沈若楠, 李文宇, 郑家禾, 张雨雪, 朱军涛, 孙建新. 青藏高原那曲高山嵩草草甸植物物候对增温的异步响应[J]. 植物生态学报, 2023, 47(2): 183-194. |
| [7] | 陈心怡, 吴晨, 黄锦学, 熊德成. 增温对林木细根物候影响的研究进展[J]. 植物生态学报, 2023, 47(11): 1471-1482. |
| [8] | 余玉蓉, 吴浩, 高娅菲, 赵媛博, 李小玲, 卜贵军, 薛丹, 刘正祥, 武海雯, 吴林. 模拟氮沉降对鄂西南湿地泥炭藓生理及形态特征的影响[J]. 植物生态学报, 2023, 47(11): 1493-1506. |
| [9] | 魏瑶, 马志远, 周佳颖, 张振华. 模拟增温改变青藏高原植物繁殖物候及植株高度[J]. 植物生态学报, 2022, 46(9): 995-1004. |
| [10] | 陈奕竹, 郎伟光, 陈效逑. 中国北方树木秋季物候的过程模拟及其区域分异归因[J]. 植物生态学报, 2022, 46(7): 753-765. |
| [11] | 张迪, 都业勤, 王磊, 陈鑫, 闫兴富, 唐占辉. 两种生境间大花百合不同性别表型开花及传粉特征的差异[J]. 植物生态学报, 2022, 46(5): 580-592. |
| [12] | 田磊, 朱毅, 李欣, 韩国栋, 任海燕. 不同降水条件下内蒙古荒漠草原主要植物物候对长期增温和氮添加的响应[J]. 植物生态学报, 2022, 46(3): 290-299. |
| [13] | 丛楠, 张扬建, 朱军涛. 北半球中高纬度地区近30年植被春季物候温度敏感性[J]. 植物生态学报, 2022, 46(2): 125-135. |
| [14] | 原媛, 母艳梅, 邓钰洁, 李鑫豪, 姜晓燕, 高圣杰, 查天山, 贾昕. 植被覆盖度和物候变化对典型黑沙蒿灌丛生态系统总初级生产力的影响[J]. 植物生态学报, 2022, 46(2): 162-175. |
| [15] | 于海英, 杨莉琳, 付素静, 张志敏, 姚琦馥. 暖温带森林木本植物展叶始期对低温和热量累积变化的响应[J]. 植物生态学报, 2022, 46(12): 1573-1584. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19