

植物生态学报 ›› 2023, Vol. 47 ›› Issue (11): 1493-1506.DOI: 10.17521/cjpe.2022.0426
所属专题: 生态化学计量
余玉蓉1,2, 吴浩1,2, 高娅菲1,2, 赵媛博1,2, 李小玲2,3, 卜贵军1,2, 薛丹4, 刘正祥5, 武海雯5, 吴林1,2,*( )
)
收稿日期:2022-10-28
接受日期:2023-02-15
出版日期:2023-11-20
发布日期:2023-12-22
通讯作者:
吴林 (基金资助:
YU Yu-Rong1,2, WU Hao1,2, GAO Ya-Fei1,2, ZHAO Yuan-Bo1,2, LI Xiao-Ling2,3, BU Gui-Jun1,2, XUE Dan4, LIU Zheng-Xiang5, WU Hai-Wen5, WU Lin1,2,*( )
)
Received:2022-10-28
Accepted:2023-02-15
Online:2023-11-20
Published:2023-12-22
Contact:
WU Lin (Supported by:摘要:
泥炭藓(Sphagnum)作为泥炭藓湿地中的优势种, 是泥炭藓湿地最主要的固碳(C)植物, 其生理与形态特征关系着泥炭藓湿地的碳汇潜力。氮(N)沉降对泥炭藓生理和形态特征具有显著影响, 但有关N沉降对湿地泥炭藓生理及形态特征的影响还存在很大的争议, 并且N沉降对亚热带湿地泥炭藓生理及形态特征影响的研究鲜有报道。该研究以鄂西南泥炭藓湿地为研究对象, 通过原位喷洒不同浓度的NH4Cl溶液, 探讨模拟N沉降对泥炭藓生理及形态特征的影响。模拟N浓度设置4个水平, 分别为0 (N0)、3 (N3)、6 (N6)、12 g·m-2·a-1 (N12), 其中N0为对照(CK)。研究结果表明: (1) N沉降对泥炭藓体内总C、总N含量有显著影响。其中, N3处理下的总C、总N含量最高, 相比于CK分别增加了3.78%和88.52%。(2) N沉降对泥炭藓叶绿素含量和荧光活性影响不显著, 但对泥炭藓抗氧化酶活性和渗透调节物质的含量有显著促进作用, 尤其是可溶性糖含量与过氧化物酶活性。(3)随N沉降量增加, 泥炭藓株高、分枝数、单株质量及叶细胞面积均表现为先增加后降低趋势, 且最大值出现在N3处理。(4)泥炭藓对N沉降很敏感, 2年的N沉降对泥炭藓的生理及形态特征的影响存在一定的N沉降负荷值, 约为3 g·m-2·a-1, 大于该值后, 泥炭藓各类形态指标受到抑制, 受到胁迫显著增加。该研究表明, 当前自然大气N沉降量对鄂西南湿地泥炭藓生长有一定的促进作用, 但持续或加倍的N沉降可能会对泥炭藓生长造成伤害。
余玉蓉, 吴浩, 高娅菲, 赵媛博, 李小玲, 卜贵军, 薛丹, 刘正祥, 武海雯, 吴林. 模拟氮沉降对鄂西南湿地泥炭藓生理及形态特征的影响. 植物生态学报, 2023, 47(11): 1493-1506. DOI: 10.17521/cjpe.2022.0426
YU Yu-Rong, WU Hao, GAO Ya-Fei, ZHAO Yuan-Bo, LI Xiao-Ling, BU Gui-Jun, XUE Dan, LIU Zheng-Xiang, WU Hai-Wen, WU Lin. Effects of simulated nitrogen deposition on physiological and morphological characteristics of Sphagnum in wetland, southwestern Hubei Province, China. Chinese Journal of Plant Ecology, 2023, 47(11): 1493-1506. DOI: 10.17521/cjpe.2022.0426
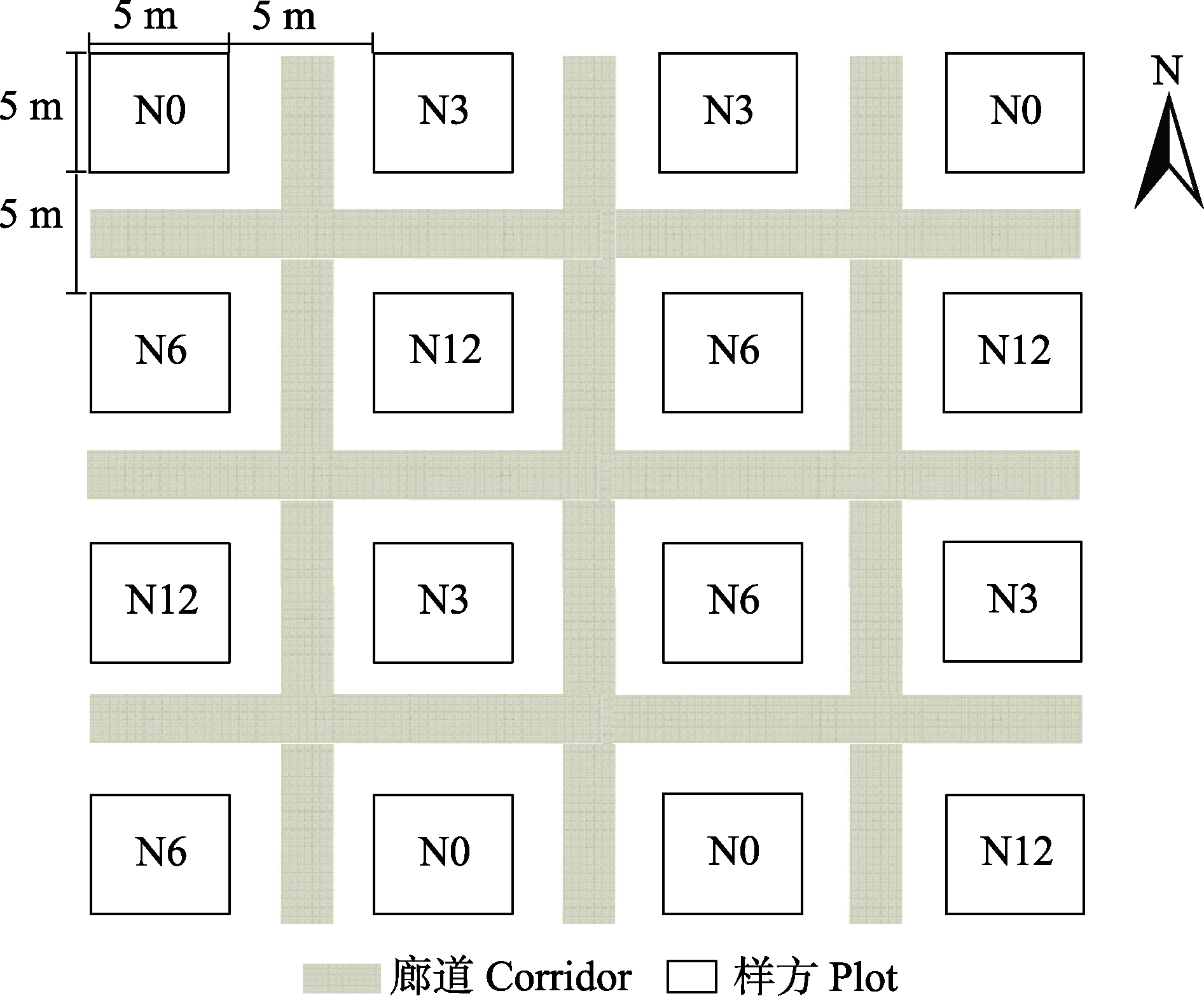
图1 鄂西南泥炭藓湿地模拟氮沉降实验样方示意图。N0、N3、N6及N12分别表示氮添加浓度为0、3、6和12 g·m-2·a-1。
Fig. 1 Schematic diagram of the simulated nitrogen deposition plot in Sphagnum wetland in southwestern Hubei Province. N0, N3, N6, and N12 indicate the nitrogen concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively.
| N浓度 N concentration (g·m-2·a-1) | pH | 总酚含量 Total polyphenol content (%) | 总碳(C)含量 Total carbon (C) content (%) | 总N含量 Total N content (%) | C:N |
|---|---|---|---|---|---|
| 0 (N0) | 4.1 ± 0.1a | 4.80 ± 0.94a | 29.62 ± 3.26a | 1.29 ± 0.07a | 22.86 ± 1.31a |
| 3 (N3) | 4.0 ± 0.1a | 5.00 ± 0.94a | 33.21 ± 2.69ab | 1.44 ± 0.09ab | 23.07 ± 1.29a |
| 6 (N6) | 4.1 ± 0.1a | 5.95 ± 1.24a | 40.21 ± 0.24c | 1.63 ± 0.04bc | 24.75 ± 0.60a |
| 12 (N12) | 3.9 ± 0a | 6.66 ± 0.69a | 38.16 ± 0.51bc | 1.74 ± 0.06c | 21.94 ± 0.73a |
表1 氮(N)沉降对鄂西南泥炭藓湿地土壤理化性质的影响(平均值±标准误, n = 5)
Table 1 Effects of simulated nitrogen (N) deposition on soil physicochemical characteristics in Sphagnum wetland in southwestern Hubei Province (mean ± SE, n = 5)
| N浓度 N concentration (g·m-2·a-1) | pH | 总酚含量 Total polyphenol content (%) | 总碳(C)含量 Total carbon (C) content (%) | 总N含量 Total N content (%) | C:N |
|---|---|---|---|---|---|
| 0 (N0) | 4.1 ± 0.1a | 4.80 ± 0.94a | 29.62 ± 3.26a | 1.29 ± 0.07a | 22.86 ± 1.31a |
| 3 (N3) | 4.0 ± 0.1a | 5.00 ± 0.94a | 33.21 ± 2.69ab | 1.44 ± 0.09ab | 23.07 ± 1.29a |
| 6 (N6) | 4.1 ± 0.1a | 5.95 ± 1.24a | 40.21 ± 0.24c | 1.63 ± 0.04bc | 24.75 ± 0.60a |
| 12 (N12) | 3.9 ± 0a | 6.66 ± 0.69a | 38.16 ± 0.51bc | 1.74 ± 0.06c | 21.94 ± 0.73a |
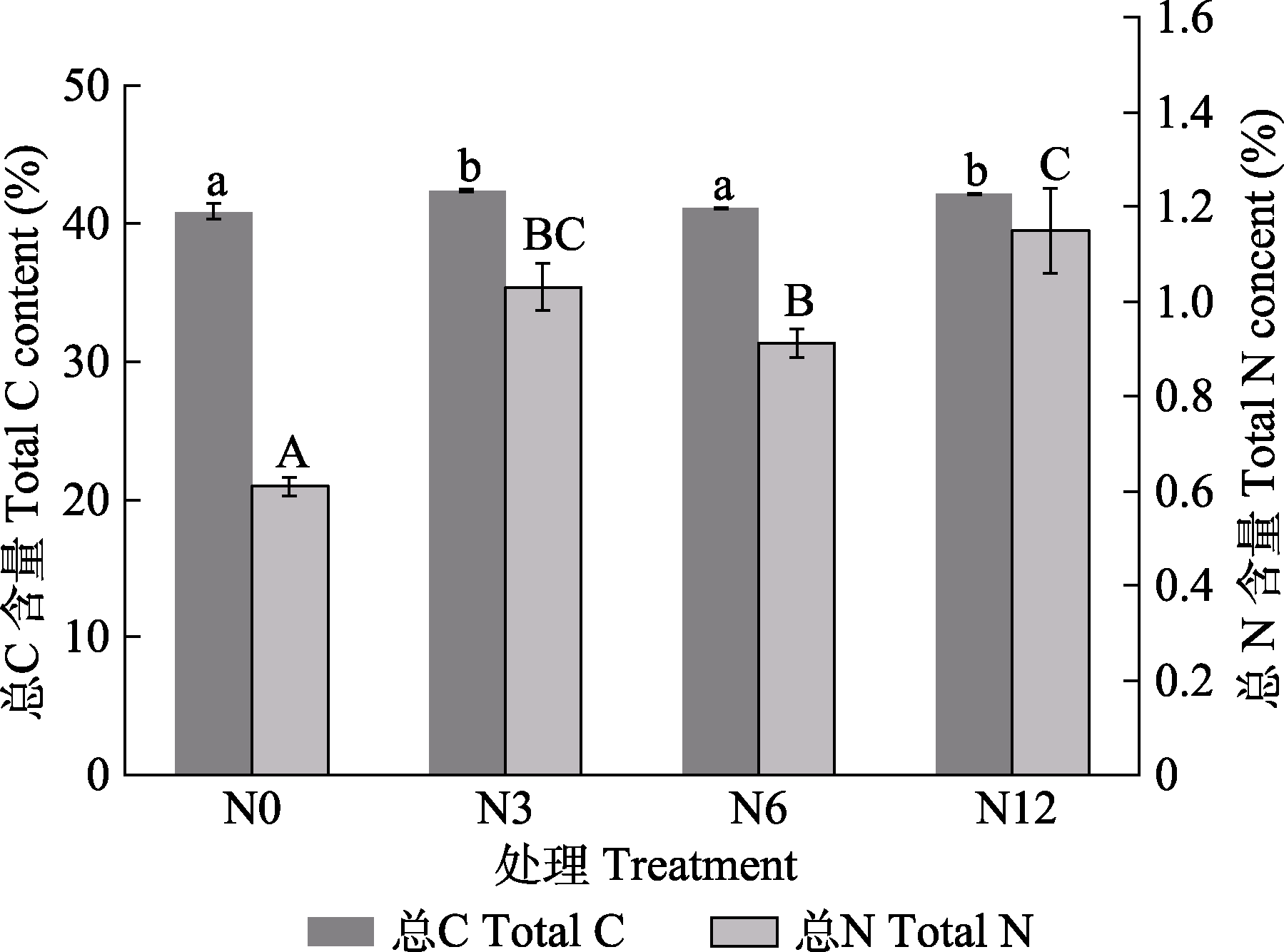
图2 模拟氮沉降对泥炭藓总碳(C)、总氮(N)含量的影响(平均值±标准误, n = 5)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示总C含量在不同氮处理间差异显著(p < 0.05), 不同大写字母表示总N含量在不同氮处理间差异显著(p < 0.05)。
Fig. 2 Effects of simulated nitrogen (N) deposition on the total carbon (C) and total N contents of Sphagnum palustre (mean ± SE, n = 5). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference between treatments in total C content; different uppercase letters indicate significant difference between treatments in total N content (p < 0.05).
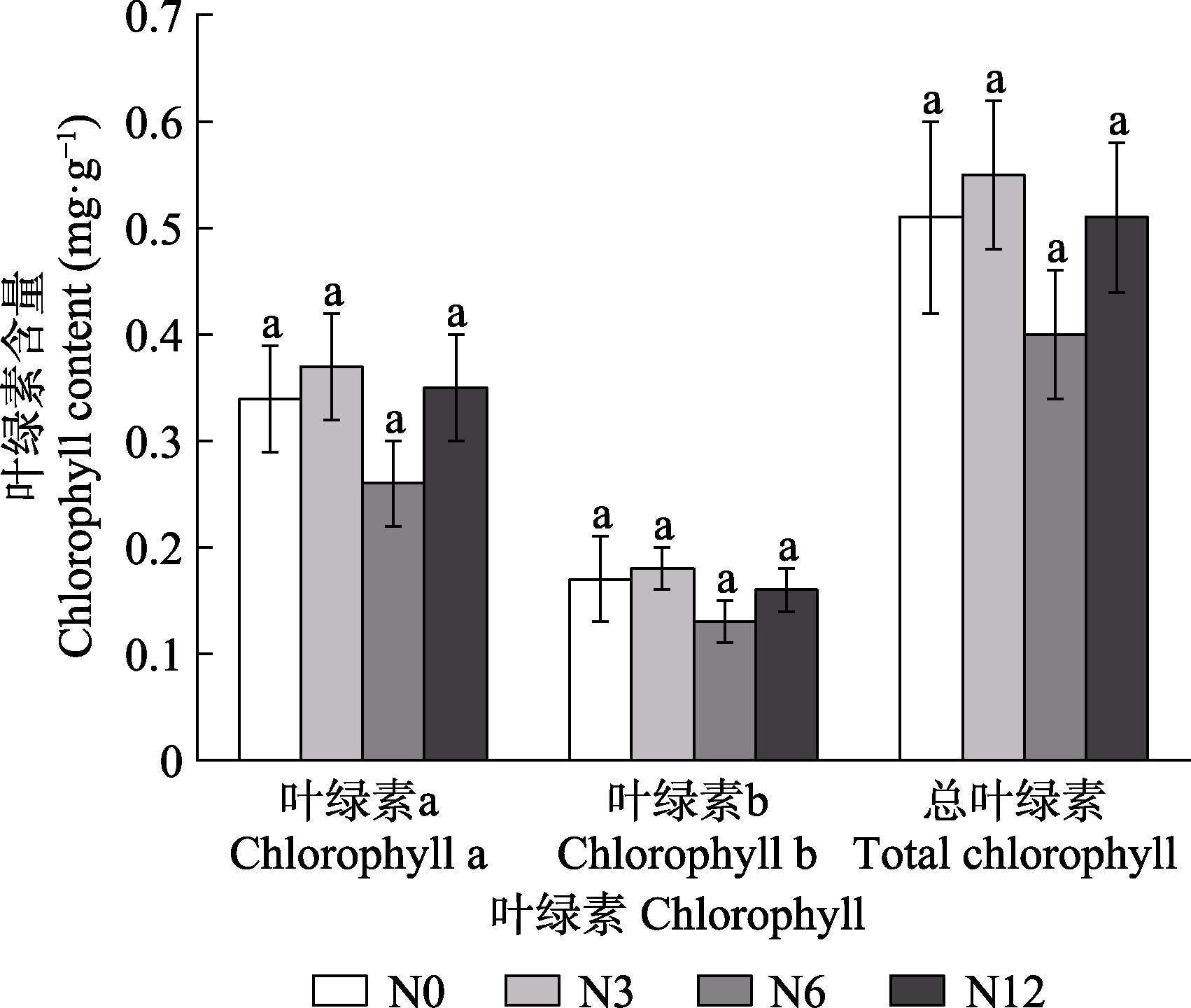
图3 模拟氮沉降对泥炭藓叶绿素含量的影响(平均值±标准误, n = 5)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 3 Effects of simulated nitrogen deposition on chlorophyll content of Sphagnum palustre (mean ± SE, n = 5). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
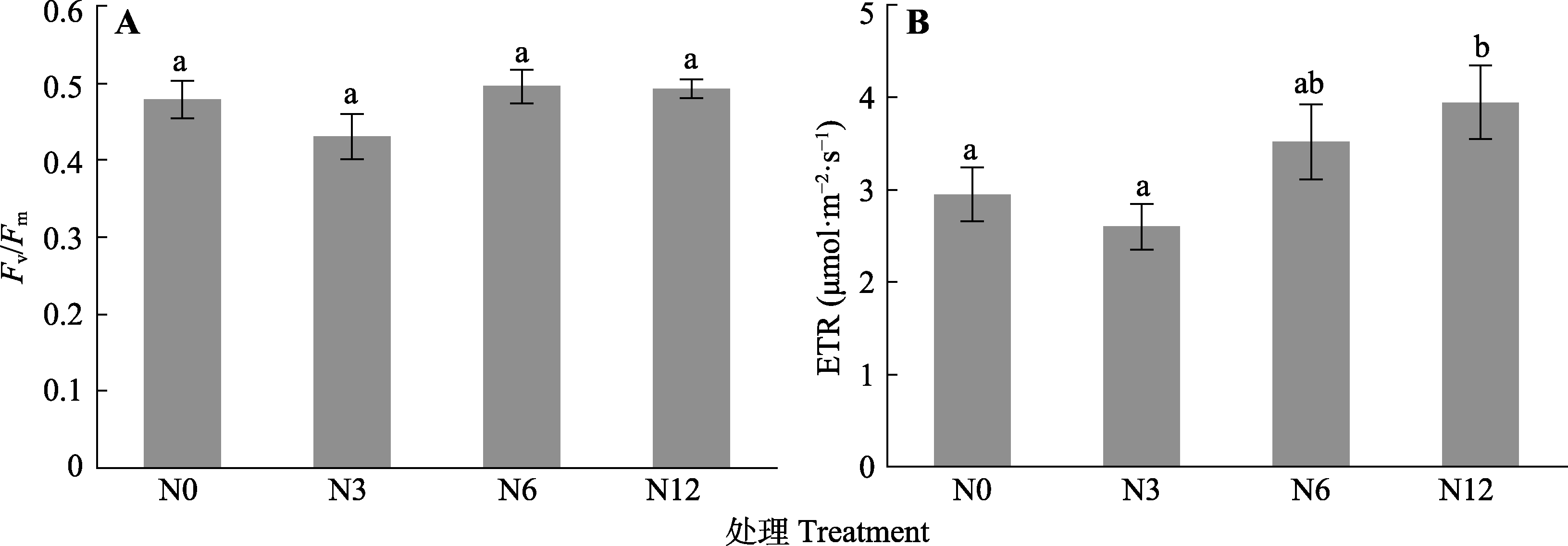
图4 模拟氮沉降对泥炭藓最大荧光量子产量(Fv/Fm) (A)和电子传递速率(ETR) (B)的影响(平均值±标准误, n = 5)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 4 Effects of simulated nitrogen deposition on the measurement of maximum quantum yield (Fv/Fm) (A) and electron transport rate (ETR) (B) of Sphagnum palustre (mean ± SE, n = 5). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g· m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
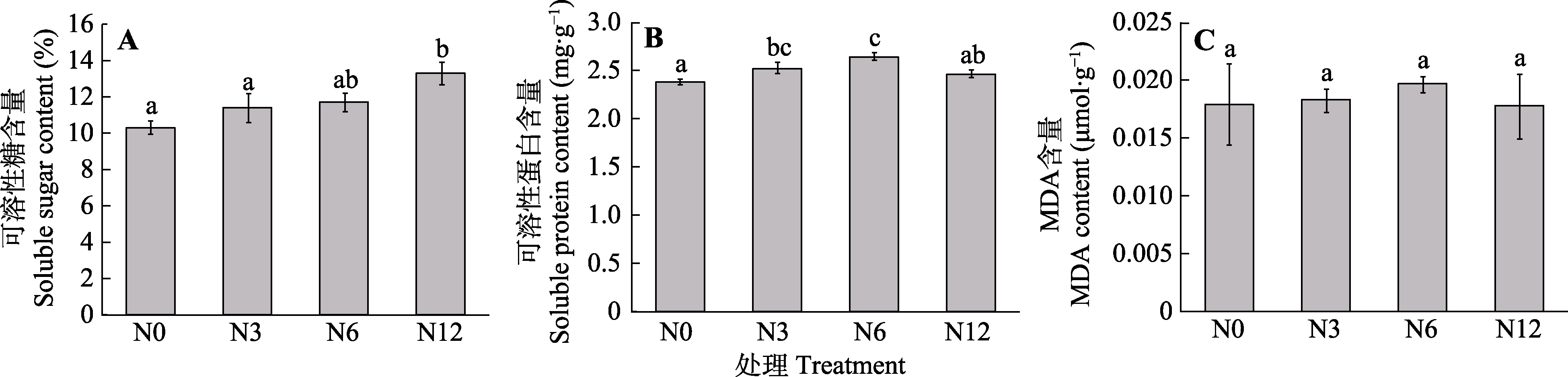
图5 模拟氮沉降对泥炭藓可溶性糖(A)、可溶性蛋白(B)及丙二醛(MDA)含量(C)的影响(平均值±标准误, n = 5)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 5 Effects of simulated nitrogen deposition on contents of soluble sugar (A), soluble protein (B), and malondialdehyde (MDA) (C) of Sphagnum palustre (mean ± SE, n = 5). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
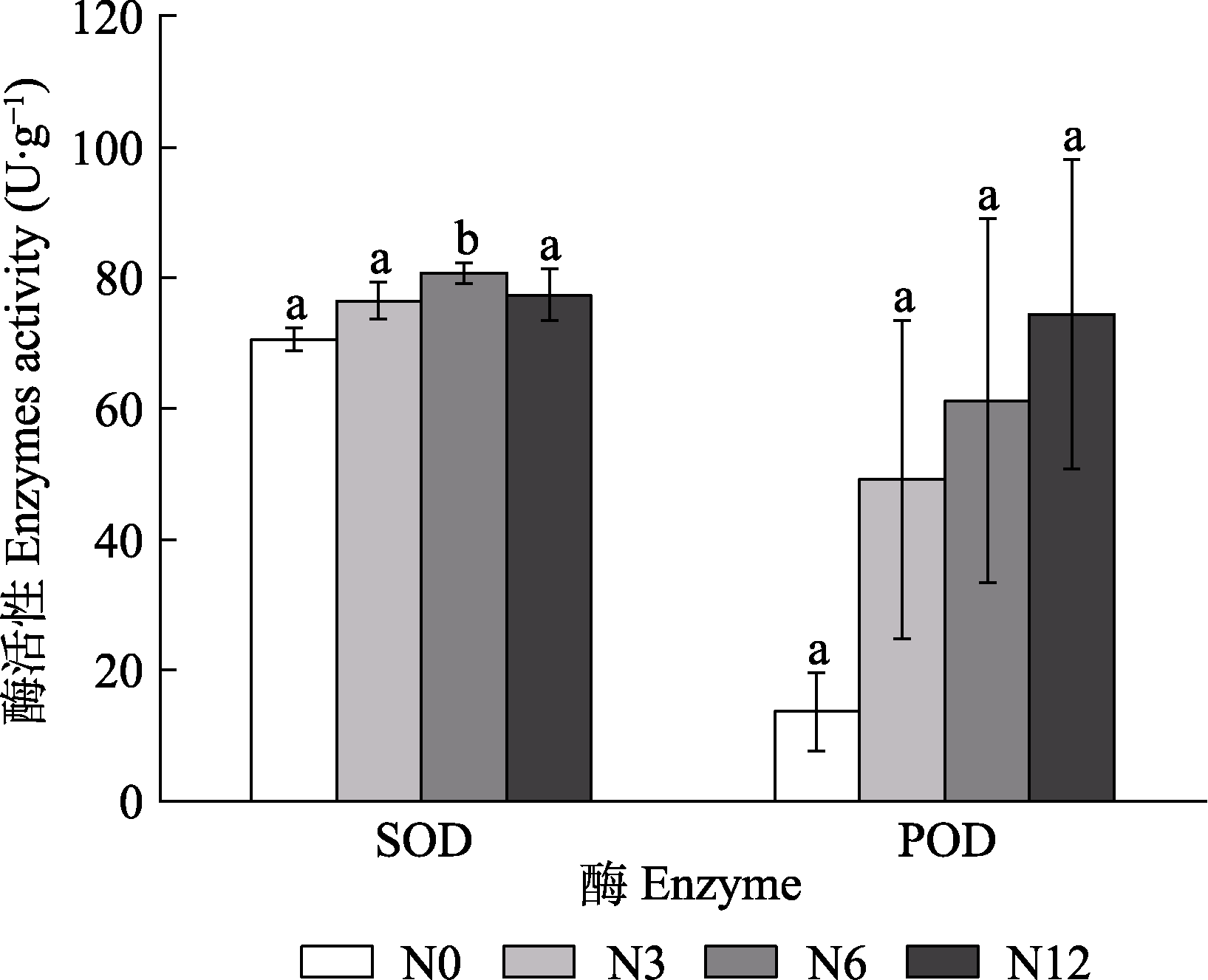
图6 模拟氮沉降对泥炭藓超氧化物歧化酶(SOD)和过氧化物酶(POD)活性的影响(平均值±标准误, n = 5)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 6 Effects of simulated nitrogen deposition on activities of superoxide dismutase (SOD) and peroxidase (POD) of Sphagnum palustre (mean ± SE, n = 5). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
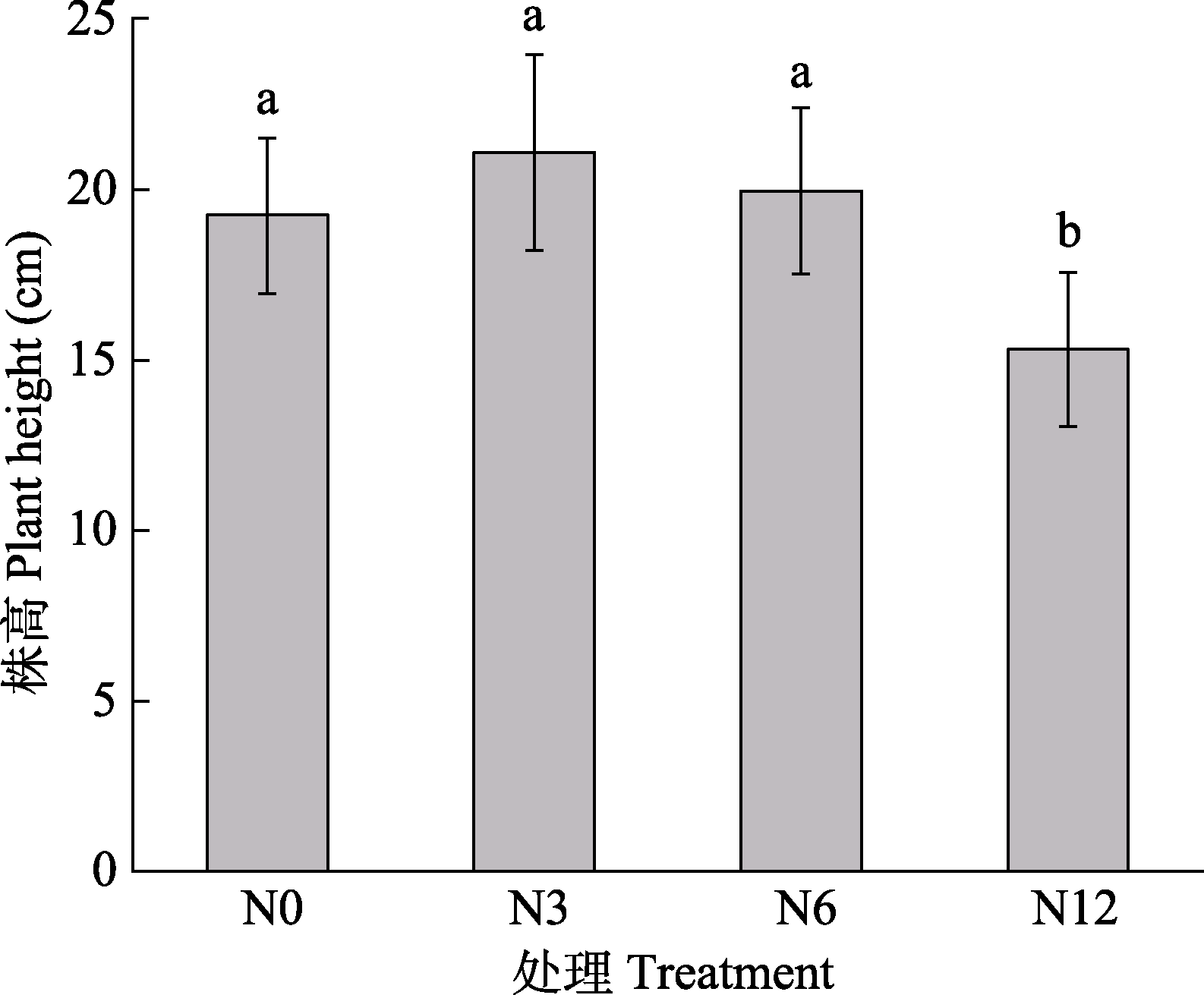
图7 模拟氮沉降对泥炭藓株高的影响(平均值±标准误, n = 10)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 7 Effects of simulated nitrogen deposition on the plant height of Sphagnum palustre (mean ± SE, n = 10). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
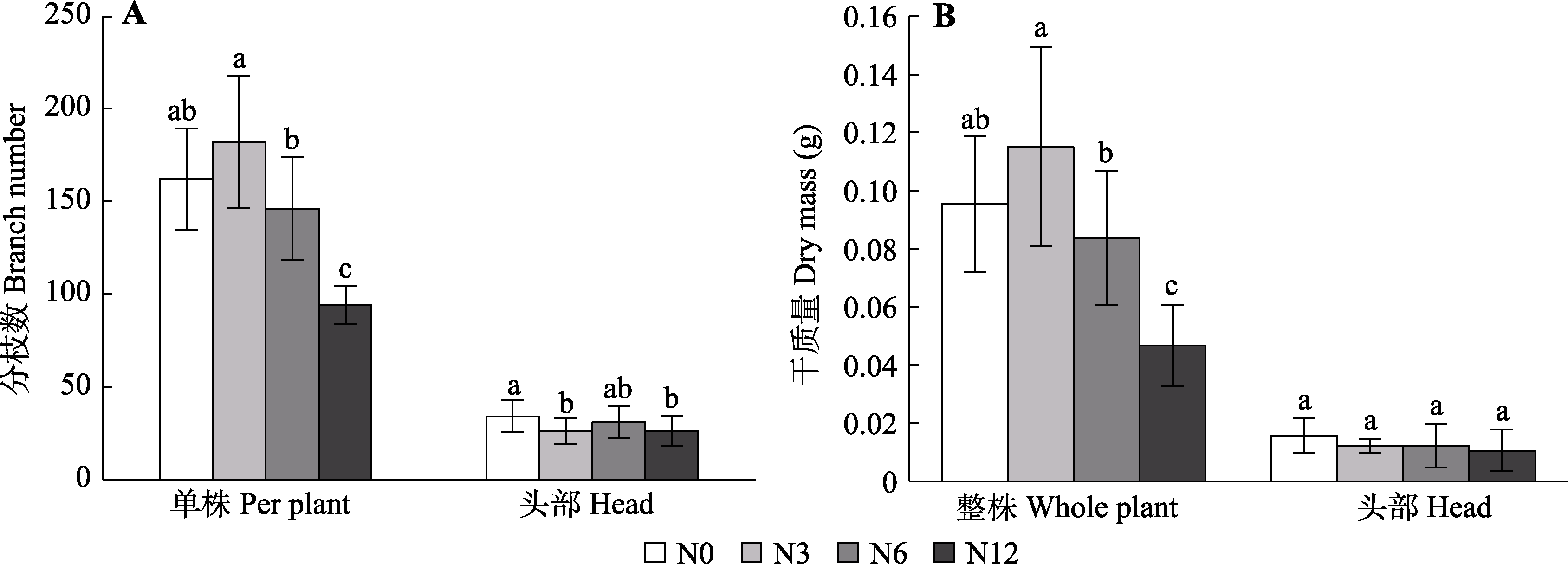
图8 模拟氮沉降对泥炭藓分枝数(A)及泥炭藓干质量(B)的影响(平均值±标准误, n = 10)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 8 Effects of simulated nitrogen deposition on the branch number (A) and dry mass (B) of Sphagnum palustre (mean ± SE, n = 10). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
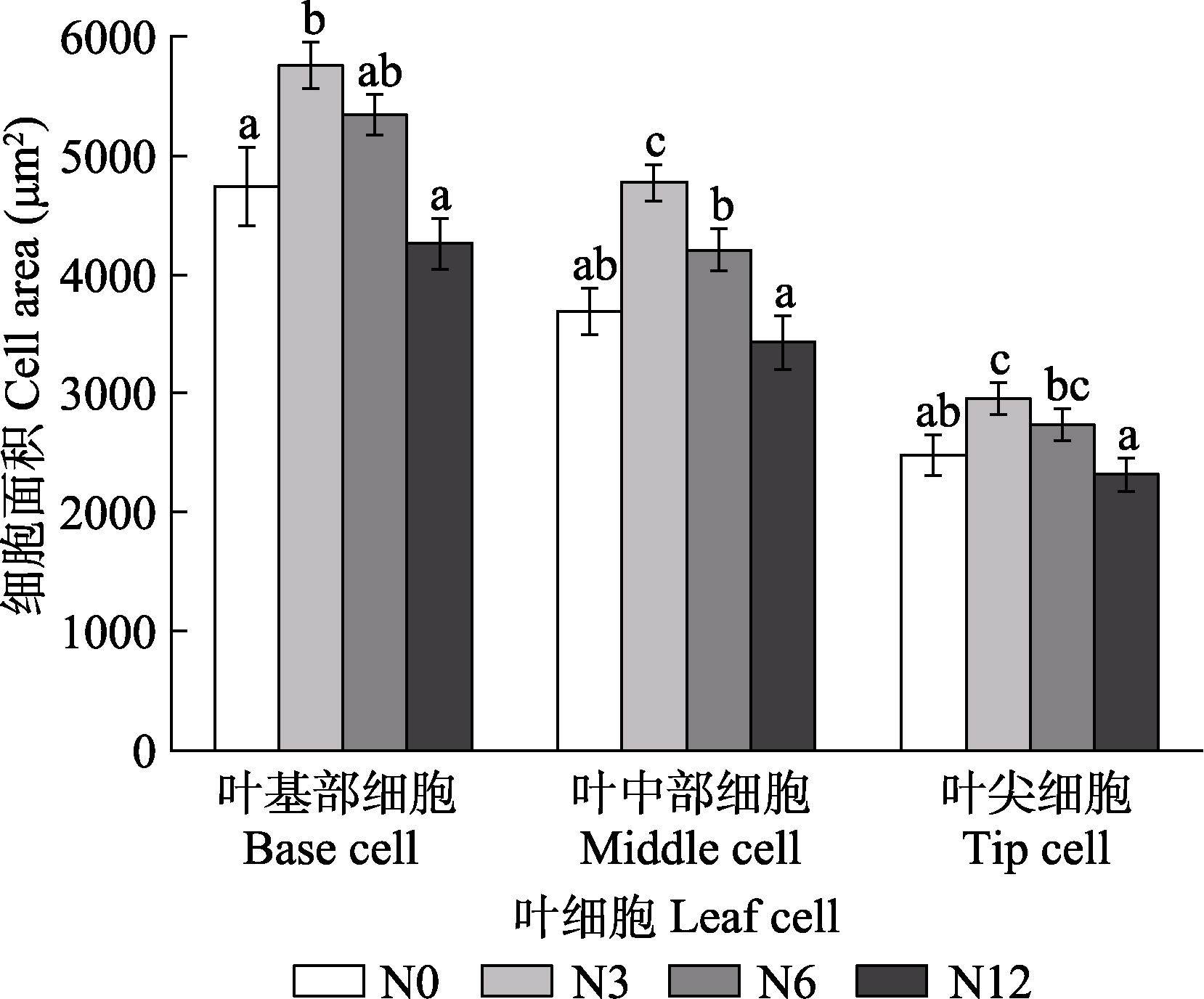
图9 模拟氮沉降对泥炭藓叶片细胞面积的影响(平均值±标准误, n = 5)。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。不同小写字母表示不同氮处理间差异显著(p < 0.05)。
Fig. 9 Effects of simulated nitrogen deposition on the cell area of Sphagnum palustre leaves (mean ± SE, n = 5). N0, N3, N6, and N12 represent nitrogen addition concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively. Different lowercase letters indicate significant difference among the treatments (p < 0.05).
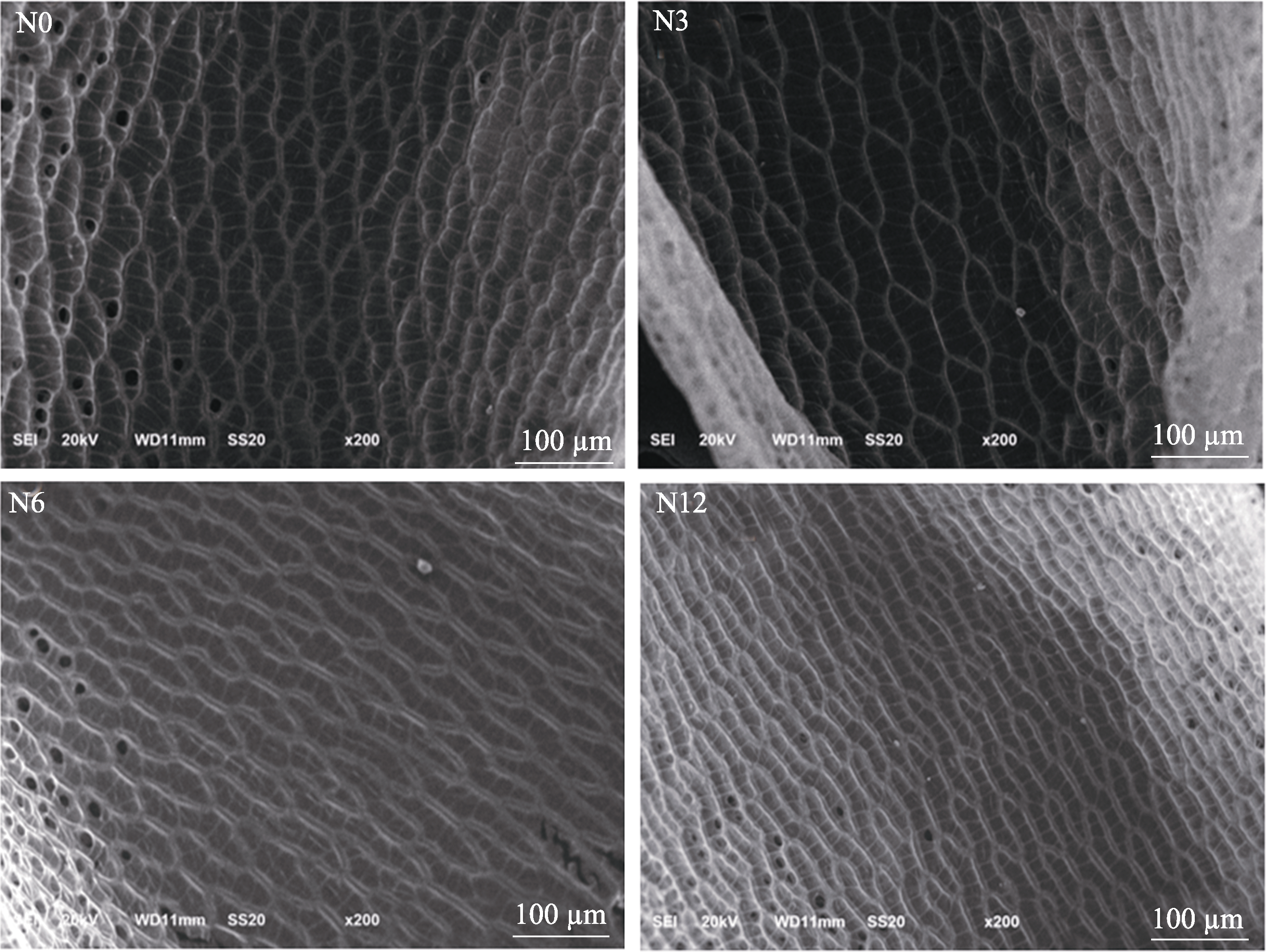
图10 不同氮沉降处理下的泥炭藓叶片细胞电镜图。N0、N3、N6及N12分别表示氮添加浓度为0、3、6及12 g·m-2·a-1。
Fig. 10 Electron micrographs of cells of Sphagnum palustre leaves under different nitrogen deposition treatments. N0, N3, N6, and N12 indicate the nitrogen concentrations of 0, 3, 6, and 12 g·m-2·a-1, respectively.
| [1] | Ahanger MA, Gul F, Ahmad P, Akram NA (2018). Environmental stresses and metabolomics—Deciphering the role of stress responsive metabolites//Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN. Plant Metabolites and Regulation Under Environmental Stress. Elsevier, Amsterdam, the Netherlands. 53-67. |
| [2] |
Ainsworth EA, Gillespie KM (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nature Protocols, 2, 875-877.
DOI PMID |
| [3] |
Baxter R, Emes MJ, Lee JA (1992). Effects of an experimentally applied increase in ammonium on growth and amino-acid metabolism of Sphagnum cuspidatum Ehrh. ex. Hoffm. from differently polluted areas. New Phytologist, 120, 265-274.
DOI URL |
| [4] |
Berendse F, van Breemen N, Rydin H, Buttler A, Heijmans M, Hoosbeek MR, Lee JA, Mitchell E, Saarinen T, Vasander H (2001). Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Global Change Biology, 7, 591-598.
DOI URL |
| [5] |
Berg A, Danielsson Å, Svensson BH (2013). Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant and Soil, 362, 271-278.
DOI URL |
| [6] | Bobbink R, Ashmore M, Braun S, Flückiger W, van den Wyngaert IJJ(2003). Empirical Nitrogen Critical Loads for Natural and Semi-natural Ecosystems: 2002 Update. [2022-10-28].https://www.iap.ch/publikationen/nworkshop-background.pdf. |
| [7] |
Bragazza L, Tahvanainen T, Kutnar L, Rydin H, Limpens J, Hájek M, Grosvernier P, Hájek T, Hajkova P, Hansen I, Iacumin P, Gerdol R (2004). Nutritional constraints in ombrotrophic Sphagnum plants under increasing atmospheric nitrogen deposition in Europe. New Phytologist, 163, 609-616.
DOI PMID |
| [8] |
Breeuwer A, Heijmans MMPD, Gleichman M, Robroek BJM, Berendse F (2009). Response of Sphagnum species mixtures to increased temperature and nitrogen availability. Plant Ecology, 204, 97-111.
DOI URL |
| [9] |
Brouns K, Keuskamp JA, Potkamp G, Verhoeven JTA, Hefting MM (2016). Peat origin and land use effects on microbial activity, respiration dynamics and exo-enzyme activities in drained peat soils in the Netherlands. Soil Biology & Biochemistry, 95, 144-155.
DOI URL |
| [10] | Bussotti F, Gerosa G, Digrado A, Pollastrini M (2020). Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecological Indicators, 108, 1-10. |
| [11] |
Cakmak I, Marschner H (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiology, 98, 1222-1227.
DOI PMID |
| [12] |
Carfrae JA, Sheppard LJ, Raven JA, Leith ID, Crossley A (2007). Potassium and phosphorus additions modify the response of Sphagnum capillifolium growing on a Scottish ombrotrophic bog to enhanced nitrogen deposition. Applied Geochemistry, 22, 1111-1121.
DOI URL |
| [13] | Chen ML, Chang L, Zhang JM, Guo FC, Vymazal J, He Q, Chen Y (2020). Global nitrogen input on wetland ecosystem: the driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environmental Science and Ecotechnology, 4, 26-38. |
| [14] |
Cho UH, Park JO (2000). Mercury-induced oxidative stress in tomato seedlings. Plant Science, 156, 1-9.
DOI PMID |
| [15] | Clymo RS, Hayward PM (1982). The ecology of Sphagnum//Smith AJE. Bryophyte Ecology. Springer, Dordrecht, the Netherlands. 229-289. |
| [16] |
Delucia EH, Sasek TW, Strain BR (1985). Photosynthetic inhibition after long-term exposure to elevated levels of atmospheric carbon dioxide. Photosynthesis Research, 7, 175-184.
DOI PMID |
| [17] |
Fenner N, Ostle N, Freeman C, Sleep D, Reynolds B (2004). Peatland carbon efflux partitioning reveals that Sphagnum photosynthate contributes to the DOC pool. Plant and Soil, 259, 345-354.
DOI URL |
| [18] | Fritz C, Lamers LPM, Riaz M, van den Berg LJL, Elzenga TJTM (2014). Sphagnum mosses—Masters of efficient N-uptake while avoiding intoxication. PLoS ONE, 9, e79991. DOI: 10.1371/journal.pone.0079991. |
| [19] | Fudyma JD, Lyon J, Amini Tabrizi R, Gieschen H, Chu RK, Hoyt DW, Kyle JE, Toyoda J, Tolic N, Heyman HM, Hess NJ, Metz TO, Tfaily MM (2019). Untargeted metabolomic profiling of Sphagnum fallax reveals novel antimicrobial metabolites. Plant Direct, 3, 1-17. |
| [20] |
Gaudig G, Krebs M, Joosten H (2020). Sphagnum growth under N saturation: interactive effects of water level and P or K fertilization. Plant Biology, 22, 394-403.
DOI PMID |
| [21] |
Gerdol R, Petraglia A, Bragazza L, Iacumin P, Brancaleoni L (2007). Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Global Change Biology, 13, 1810-1821.
DOI URL |
| [22] |
Gonzalez CM, Pignata ML (1994). The influence of air pollution on soluble proteins, chlorophyll degradation, MDA, sulphur and heavy metals in a transplanted lichen. Chemistry and Ecology, 9, 105-113.
DOI URL |
| [23] |
Gorham E (1991). Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications, 1, 182-195.
DOI PMID |
| [24] |
Granath G, Strengbom J, Breeuwer A, Heijmans MMPD, Berendse F, Rydin H (2009a). Photosynthetic performance in Sphagnum transplanted along a latitudinal nitrogen deposition gradient. Oecologia, 159, 705-715.
DOI URL |
| [25] |
Granath G, Wiedermann MM, Strengbom J (2009b). Physiological responses to nitrogen and sulphur addition and raised temperature in Sphagnum balticum. Oecologia, 161, 481-490.
DOI URL |
| [26] |
Gunnarsson U (2005). Global patterns of Sphagnum productivity. Journal of Bryology, 27, 269-279.
DOI URL |
| [27] |
Gunnarsson U, Granberg G, Nilsson M (2004). Growth, production and interspecific competition in Sphagnum: effects of temperature, nitrogen and sulphur treatments on a boreal mire. New Phytologist, 163, 349-359.
DOI PMID |
| [28] |
Gunnarsson U, Rydin H (2000). Nitrogen fertilization reduces Sphagnum production in bog communities. New Phytologist, 147, 527-537.
DOI PMID |
| [29] |
Hájek T, Beckett RP (2008). Effect of water content components on desiccation and recovery in Sphagnum mosses. Annals of Botany, 101, 165-173.
DOI URL |
| [30] | Hamard S, Robroek BJM, Allard PM, Signarbieux C, Zhou SZ, Saesong T, Baaker F, Buttler A, Chiapusio G, Wolfender JL, Bragazza L, Jassey VEJ (2019). Effects of Sphagnum leachate on competitive Sphagnum microbiome depend on species and time. Frontiers in Microbiology, 10, 2042. DOI: 10.3389/fmicb.2019.02042. |
| [31] |
Hashempour A, Ghasemnezhad M, Ghazvini RF, Sohani MM (2014). Olive (Olea europaea L.) freezing tolerance related to antioxidant enzymes activity during cold acclimation and non acclimation. Acta Physiologiae Plantarum, 36, 3231-3241.
DOI URL |
| [32] | He G (2014). Physiological Responses of Bryophyte in Alpine Ecosystem to Increasing Temperature and N Depostion. Master degree dissertation, Sichuan Normal University, Chengdu. |
| [何刚 (2014). 高山生态系统苔藓植物对升温和氮沉降的生理响应. 硕士学位论文, 四川师范大学, 成都.] | |
| [33] | Jia YL (2016). Spatio-temporal Patterns of Atmospheric Nitrogen Depositon in China and Global Land. PhD dissertation, University of Chinese Academy of Science, Beijing. |
| 贾彦龙 (2016). 中国及全球大气氮沉降的时空格局研究. 博士学位论文, 中国科学院大学, 北京.] | |
| [34] |
Juutinen S, Moore TR, Laine AM, Bubier JL, Tuittila ES, de Young A, Chong M (2016). Responses of the mosses Sphagnum capillifolium and Polytrichum strictum to nitrogen deposition in a bog: growth, ground cover, and CO2 exchange. Botany, 94, 127-138.
DOI URL |
| [35] | Kashi NN (2014). Sphagnum Moss Enzymatic Activity Responses To Elevated Nitrogen Inputs in Ombrotrophic Peatlands. PhD dissertation, Villanova University, Pennsylvania. |
| [36] | Kivimäki S (2011). Changes in Carbon and Nitrogen Dynamics in Sphagnum capillifolium Under Enhanced Nitrogen Deposition. PhD dissertation, University of Edinburgh, Scotland. |
| [37] | Kong LG, Xie Y, Hu L, Si JS, Wang ZS (2017). Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Scientific Reports, 7, 43363. DOI: 10.1038/srep43363. |
| [38] | Lambers H (1985). Respiration in intact plants and tissues: its regulation and dependence on environmental factors, metabolism and invaded organisms//Douce R, Day DA. Higher Plant Cell Respiration. Springer, Berlin. 418-473. |
| [39] |
Lamers LPM, Bobbink R, Roelofs JGM (2000). Natural nitrogen filter fails in polluted raised bogs. Global Change Biology, 6, 583-586.
DOI URL |
| [40] | Lassouane N, Aïd F, Lutts S (2013). Water stress impact on young seedling growth of Acacia Arabica. Acta Physiologiae Plantarum, 35, 2157-2169. |
| [41] | Le TB, Wu J, Gong Y (2022). Vascular plants regulate responses of boreal peatland Sphagnum to climate warming and nitrogen addition. Science of the Total Environment, 819, 152077. DOI: 10.1016/j.scitotenv.2021.152077. |
| [42] |
León CA, Neila-Pivet M, Benítez-Mora A, Lara L (2019). Effect of phosphorus and nitrogen on Sphagnum regeneration and growth: an experience from Patagonia. Wetlands Ecology and Management, 27, 257-266.
DOI |
| [43] | Li XL, Zhou JS, Mou L, Wang H, Bu GJ, Wu L (2021). Effects of simulated nitrogen deposition on decomposition of Sphagnum in a subalpine wetland, south-western Hubei Province. Chinese Journal of Applied and Environmental Biology, 27, 916-922. |
| [李小玲, 周建山, 牟利, 王涵, 卜贵军, 吴林 (2021). 模拟氮沉降对鄂西南亚高山湿地泥炭藓(Sphagnum)凋落物分解的影响. 应用与环境生物学报, 27, 916-922.] | |
| [44] |
Li XL, Zhu DM, Yu YR, Wu H, Mou L, Hong L, Liu XF, Bu GJ, Xue D, Wu L (2023). Effects of simulated nitrogen deposition on growth and decomposition of two bryophytes in ombrotrophic peatland, southwestern Hubei, China. Chinese Journal of Plant Ecology, 47, 644-659.
DOI URL |
|
[李小玲, 朱道明, 余玉蓉, 吴浩, 牟利, 洪柳, 刘雪飞, 卜贵军, 薛丹, 吴林 (2023). 模拟氮沉降对鄂西南贫营养泥炭地两种藓类植物生长与分解的影响. 植物生态学报, 47, 644-659.]
DOI |
|
| [45] |
Lichtenthaler HK, Wellburn AR (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions, 11, 591-592.
DOI URL |
| [46] | Limpens J (2003). Prospects for Sphagnum Bogs Subject to High Nitrogen Deposition. PhD dissertation, Wageningen University, Wageningen. |
| [47] |
Limpens J, Berendse F (2003). Growth reduction of Sphagnum magellanicum subjected to high nitrogen deposition: the role of amino acid nitrogen concentration. Oecologia, 135, 339-345.
PMID |
| [48] | Limpens J, Heijmans MMPD, Berendse F (2006). The nitrogen cycle in boreal peatlands//Wider RK, Vitt DH. Boreal Peatland Ecosystems. Springer, Berlin. 195-230. |
| [49] | Liu HT (1995). Definition and classification of wetlands. Chinese Journal of Ecology, 14, 73-77. |
| [刘厚田 (1995). 湿地的定义和类型划分. 生态学杂志, 14, 73-77.] | |
| [50] |
Manninen S, Woods C, Leith ID, Sheppard LJ (2011). Physiological and morphological effects of long-term ammonium or nitrate deposition on the green and red (shade and open grown) Sphagnum capillifolium. Environmental and Experimental Botany, 72, 140-148.
DOI URL |
| [51] |
Neves NR, Oliva MA, da Cruz Centeno D, Costa AC, Ribas RF, Pereira EG (2009). Photosynthesis and oxidative stress in the restinga plant species Eugenia uniflora L. exposed to simulated acid rain and iron ore dust deposition: potential use in environmental risk assessment. Science of the Total Environment, 407, 3740-3745.
DOI URL |
| [52] | Press MC, Woodin SJ, Lee JA (1986). The potential importance of an increased atmospheric nitrogen supply to the growth of ombrotrophic Sphagnum species. New Phytologist, 103, 45-55. |
| [53] | Ptushenko VV, Ptushenko OS, Tikhonov AN (2014). Chlorophyll fluorescence induction, chlorophyll content, and chromaticity characteristics of leaves as indicators of photosynthetic apparatus senescence in arboreous plants. Biochemistry, 79, 260-272. |
| [54] |
Rudolph H, Voigt JU (1986). Effects of NH4+-N and NO3--N on growth and metabolism of Sphagnum magellanicum. Physiologia Plantarum, 66, 339-343.
DOI URL |
| [55] | Rydin H, Jeglum JK, Bennett KD (2013). The Biology of Peatlands. 2nd ed. Oxford University Press, Oxford. |
| [56] | Sharma P, Jha AB, Dubey RS, Pessarakli M (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany, 2012, 1-26. |
| [57] | Soares A, Pearson J (1997). Short-term physiological responses of mosses to atmospheric ammonium and nitrate. Water, Air, and Soil Pollution, 93, 225-242. |
| [58] | Steinfeld JI (1998). Atmospheric chemistry and physics: from air pollution to climate change. Environment: Science and Policy for Sustainable Development, 40(7), 26. |
| [59] |
van Breemen N (1995). How Sphagnum bogs down other plants. Trends in Ecology & Evolution, 10, 270-275.
DOI URL |
| [60] |
van der Heijden E, Verbeek SK, Kuiper PJC (2000). Elevated atmospheric CO2 and increased nitrogen deposition: effects on C and N metabolism and growth of the peat moss Sphagnum recurvum P. Beauv. var. mucronatum (Russ.) Warnst. Global Change Biology, 6, 201-212.
DOI URL |
| [61] |
Verhoeven JTA, Liefveld WM (1997). The ecological significance of organochemical compounds in Sphagnum. Acta Botanica Neerlandica, 46, 117-130.
DOI URL |
| [62] |
Vingiani S, Adamo P, Giordano S (2004). Sulphur, nitrogen and carbon content of Sphagnum capillifolium and Pseudevernia furfuracea exposed in bags in the Naples urban area. Environmental Pollution, 129, 145-158.
PMID |
| [63] | Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997). Technical report: human alteration of the global nitrogen cycle: sources and consequences Ecological Applications, 7, 737-750. |
| [64] |
Vitt DH, Halsey LA, Bauer IE, Campbell C (2000). Spatial and temporal trends in carbon storage of peatlands of continental western Canada through the Holocene. Canadian Journal of Earth Sciences, 37, 683-693.
DOI URL |
| [65] | Wang H, Wu L, Xue D, Liu XF, Hong L, Mou L, Li XL (2020). Distribution and environmental characteristics of Sphagnum peat bogs in Taishanmiao in Enshi City, Hubei Province. Wetland Science, 18, 266-274. |
| [王涵, 吴林, 薛丹, 刘雪飞, 洪柳, 牟利, 李小玲 (2020). 湖北省恩施市太山庙泥炭藓泥炭沼泽分布及其环境特征研究. 湿地科学, 18, 266-274.] | |
| [66] | Wieder RK, Vitt DH, Vile MA, Graham JA, Hartsock JA, Popma JMA, Fillingim H, House M, Quinn JC, Scott KD, Petix M, McMillen KJ (2020). Experimental nitrogen addition alters structure and function of a boreal poor fen: implications for critical loads. Science of the Total Environment, 733, 138619. DOI: 10.1016/j.scitotenv.2020.138619. |
| [67] |
Wiedermann MM, Gunnarsson U, Ericson L, Nordin A (2009). Ecophysiological adjustment of two Sphagnum species in response to anthropogenic nitrogen deposition. New Phytologist, 181, 208-217.
DOI PMID |
| [68] | Yee DA, Tissue DT (2005). Relationships between non-structural carbohydrate concentration and flowering in a subtropical herb, Heliconia caribaea (Heliconiaceae). Caribbean Journal of Science, 41, 243-249. |
| [69] | Zhang R, Wu JS, Li Q, Hänninen H, Peng CJ, Yao H, Song XZ, Ying YQ (2017). Nitrogen deposition enhances photosynthesis in moso bamboo but increases susceptibility to other stress factors. Frontiers in Plant Science, 8, 1975. DOI: 10.3389/fpls.2017.01975. |
| [70] |
Zhang YM, Zhou XB, Yin BF, Downing A (2016). Sensitivity of the xerophytic moss Syntrichia caninervis to prolonged simulated nitrogen deposition. Annals of Botany, 117, 1153-1161.
DOI URL |
| [71] | Zhao SJ, Xu CC, Zou Q, Meng QW (1994). Improvement of determination method of malondialdehyde in plant tissues. Plant Physiology Communications, 30, 207-210. |
| [赵世杰, 许长成, 邹琦, 孟庆伟 (1994). 植物组织中丙二醛测定方法的改进. 植物生理学通讯, 30, 207-210.] | |
| [72] |
Zhou XB, Zhang YM, Ji XH, Downing A, Serpe M (2011). Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China. Environmental and Experimental Botany, 74, 1-8.
DOI URL |
| [73] |
Zhou XB, Zhang YM, Yin BF (2016). Divergence in physiological responses between cyanobacterial and lichen crusts to a gradient of simulated nitrogen deposition. Plant and Soil, 399, 121-134.
DOI URL |
| [74] | Zhou Y, Huang Y, Peng X, Xu J, Hu Y (2021). Sphagnum response to nitrogen deposition and nitrogen critical load: a meta-analysis. Global Ecology and Conservation, 30, e01791. DOI: 10.21203/rs.3.rs-193829/v1. |
| [1] | 曲泽坤, 朱丽琴, 姜琦, 王小红, 姚晓东, 蔡世锋, 罗素珍, 陈光水. 亚热带常绿阔叶林丛枝菌根树种养分觅食策略及其与细根形态间的关系[J]. 植物生态学报, 2024, 48(4): 416-427. |
| [2] | 黄玲, 王榛, 马泽, 杨发林, 李岚, SEREKPAYEV Nurlan, NOGAYEV Adilbek, 侯扶江. 长期放牧和氮添加对黄土高原典型草原长芒草种群生长的影响[J]. 植物生态学报, 2024, 48(3): 317-330. |
| [3] | 舒韦维, 杨坤, 马俊旭, 闵惠琳, 陈琳, 刘士玲, 黄日逸, 明安刚, 明财道, 田祖为. 氮添加对红锥不同序级细根形态和化学性状的影响[J]. 植物生态学报, 2024, 48(1): 103-112. |
| [4] | 吴帆, 吴晨, 张宇辉, 余恒, 魏智华, 郑蔚, 刘小飞, 陈仕东, 杨智杰, 熊德成. 增温对成熟杉木人工林不同季节细根生长、形态及生理代谢特征的影响[J]. 植物生态学报, 2023, 47(6): 856-866. |
| [5] | 余继梅, 吴福忠, 袁吉, 金遐, 魏舒沅, 袁朝祥, 彭艳, 倪祥银, 岳楷. 全球尺度上凋落物初始酚类含量特征及影响因素[J]. 植物生态学报, 2023, 47(5): 608-617. |
| [6] | 李小玲, 朱道明, 余玉蓉, 吴浩, 牟利, 洪柳, 刘雪飞, 卜贵军, 薛丹, 吴林. 模拟氮沉降对鄂西南贫营养泥炭地两种藓类植物生长与分解的影响[J]. 植物生态学报, 2023, 47(5): 644-659. |
| [7] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [8] | 杜英东, 袁相洋, 冯兆忠. 不同形态氮对杨树光合特性及生长的影响[J]. 植物生态学报, 2023, 47(3): 348-360. |
| [9] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [10] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [11] | 叶洁泓, 于成龙, 卓少菲, 陈新兰, 杨科明, 文印, 刘慧. 木兰科植物叶片光合系统耐热性与叶片形态及温度生态位的关系[J]. 植物生态学报, 2023, 47(10): 1432-1440. |
| [12] | 万春燕, 余俊瑞, 朱师丹. 喀斯特与非喀斯特森林乔木叶性状及其相关性网络的差异[J]. 植物生态学报, 2023, 47(10): 1386-1397. |
| [13] | 郑宁, 李素英, 王鑫厅, 吕世海, 赵鹏程, 臧琛, 许玉珑, 何静, 秦文昊, 高恒睿. 基于环境因子对叶绿素影响的典型草原植物生活型优势研究[J]. 植物生态学报, 2022, 46(8): 951-960. |
| [14] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [15] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19