

植物生态学报 ›› 2023, Vol. 47 ›› Issue (3): 331-347.DOI: 10.17521/cjpe.2021.0452
所属专题: 光合作用
收稿日期:2021-12-06
接受日期:2022-05-20
出版日期:2023-03-20
发布日期:2023-02-28
通讯作者:
陈军文
作者简介:* (cjw31412@163.com)基金资助:
ZHANG Jin-Yan, CUN Zhu, SHUANG Sheng-Pu, HONG Jie, MENG Zhen-Gui, CHEN Jun-Wen( )
)
Received:2021-12-06
Accepted:2022-05-20
Online:2023-03-20
Published:2023-02-28
Contact:
CHEN Jun-Wen
Supported by:摘要:
氮(N)对植物光合作用至关重要。阴生植物在自然生长条件下, 接受的是高度动态的光照。然而, 探讨N水平对阴生植物动态光照下的光合调控作用的研究相对较少。为了阐明N对阴生植物动态光合作用的调控机制, 该研究以典型阴生植物三七(Panax notoginseng)为材料, 设置了低氮(LN, 112.5 kg·hm-2)和高氮(HN, 450.0 kg·hm-2) 2个N水平, 研究动态光和稳态光条件下植株叶片的光合气体交换参数及卡尔文循环酶蛋白的活性和数量。结果表明单位叶面积氮含量(Narea)与光照60 s的诱导状态(IS60)负相关, 与达到光合作用稳态90%所需的时间(tP90)和达到光合作用稳态100%所需的时间(tP-steady)正相关, 表明Narea并不是通过影响核酮糖-1,5-二磷酸羧化酶/加氧酶(Rubisco)的总活性来调控光诱导反应。短时间的低光间隔对Rubisco活性影响不显著, 但明显降低了果糖-1,6-二磷酸酶(FBPase)和景天庚酮糖-1,7-二磷酸酶(SBPase)的活性; 当高光照光斑突然出现时, Rubisco活性不受影响, 但是SBPase和FBPase需要被重新激活以匹配Rubisco的活性。因此, 低光间隔后的光合诱导主要受限于SBPase和FBPase的再激活。此外, HN处理叶片中Rubisco蛋白的含量高于FBPase和SBPase的含量; 在动态光的高光照阶段, HN处理叶片需要激活较高比例的FBPase和SBPase及较长的时间来恢复光合速率。该研究结果揭示: 动态光条件下, LN处理能缓解光诱导速率的下降, HN处理反而加剧光诱导速率的下降。核酮糖-1,5-二磷酸(RuBP)再生相关酶的限制可能是动态光条件下, HN处理加剧光诱导效率下降的原因之一。
张金燕, 寸竹, 双升普, 洪杰, 孟珍贵, 陈军文. 阴生植物三七稳态和动态光合特性对氮水平的响应. 植物生态学报, 2023, 47(3): 331-347. DOI: 10.17521/cjpe.2021.0452
ZHANG Jin-Yan, CUN Zhu, SHUANG Sheng-Pu, HONG Jie, MENG Zhen-Gui, CHEN Jun-Wen. Steady-state and dynamic photosynthetic characteristics of shade-tolerant species Panax notoginseng in response to nitrogen levels. Chinese Journal of Plant Ecology, 2023, 47(3): 331-347. DOI: 10.17521/cjpe.2021.0452
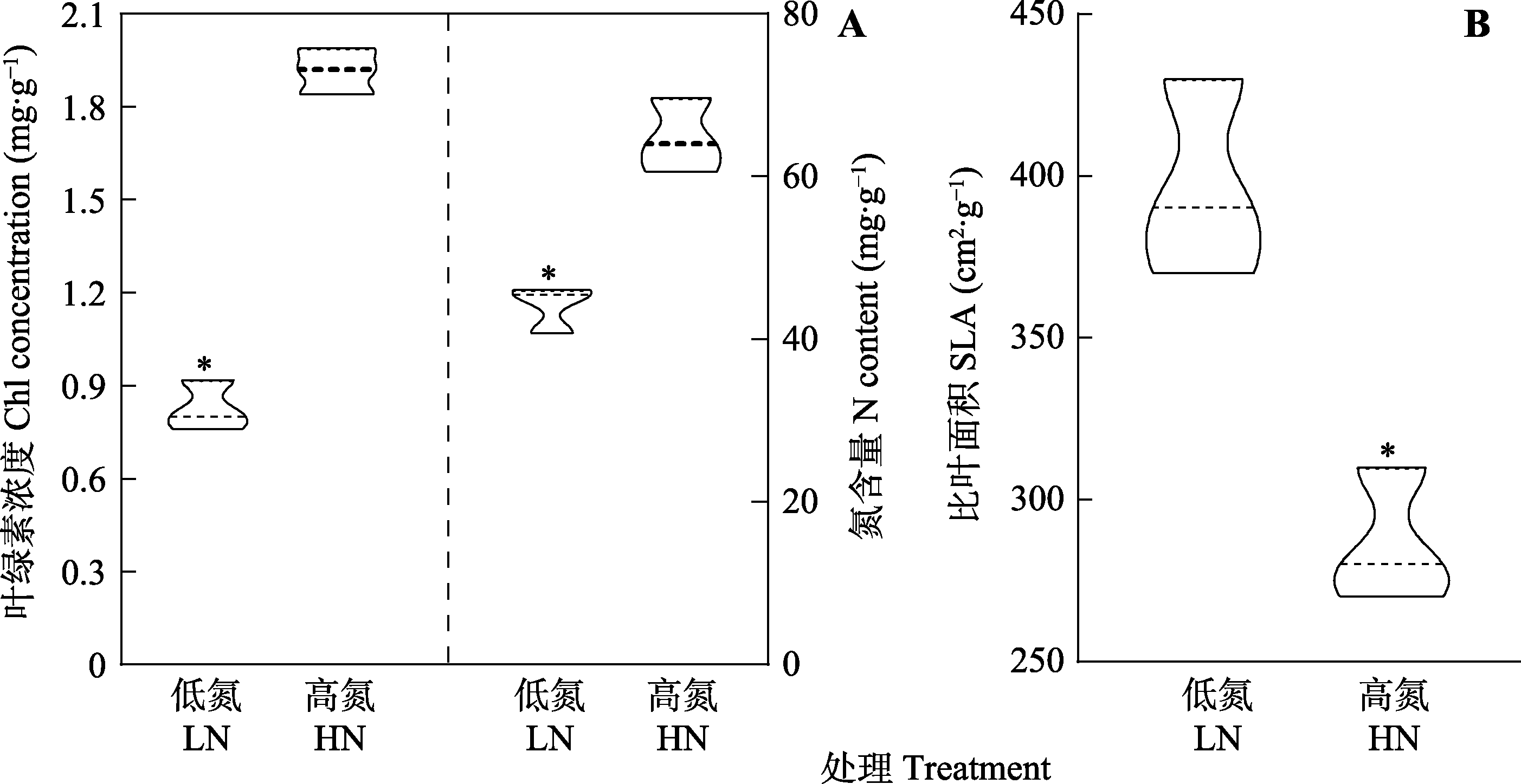
图1 不同氮处理下三七叶片氮含量、叶绿素浓度和比叶面积的变化特征。*, 两处理间相关指标差异显著(p < 0.05)。
Fig. 1 Leaf nitrogen (N) content, chlorophyll (Chl) concentration and specific leaf area (SLA) of Panax notoginseng at different N levels. HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two N treatments (p < 0.05).
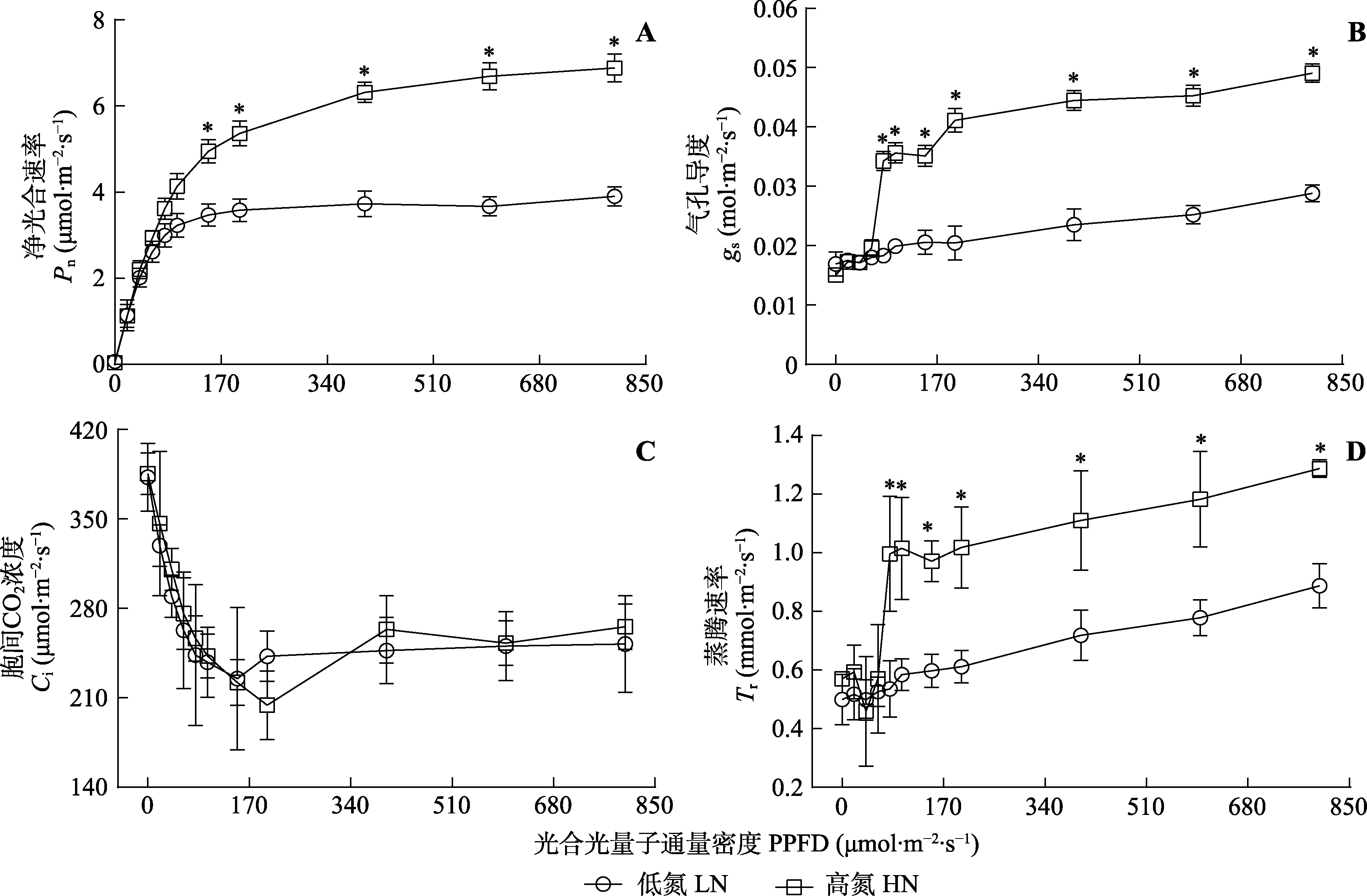
图2 不同氮处理下三七净光合速率、气孔导度、胞间CO2浓度和蒸腾速率对光合光量子通量密度的影响(平均值±标准差)。*, 两处理间气体交换参数差异显著(p < 0.05)。
Fig. 2 Net photosynthetic rate (Pn), stomatal conductance (gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) of Panax notoginseng in response to photosynthetic photon flux density (PPFD) at two different nitrogen levels (mean ± SD). HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two nitrogen treatments (p < 0.05).
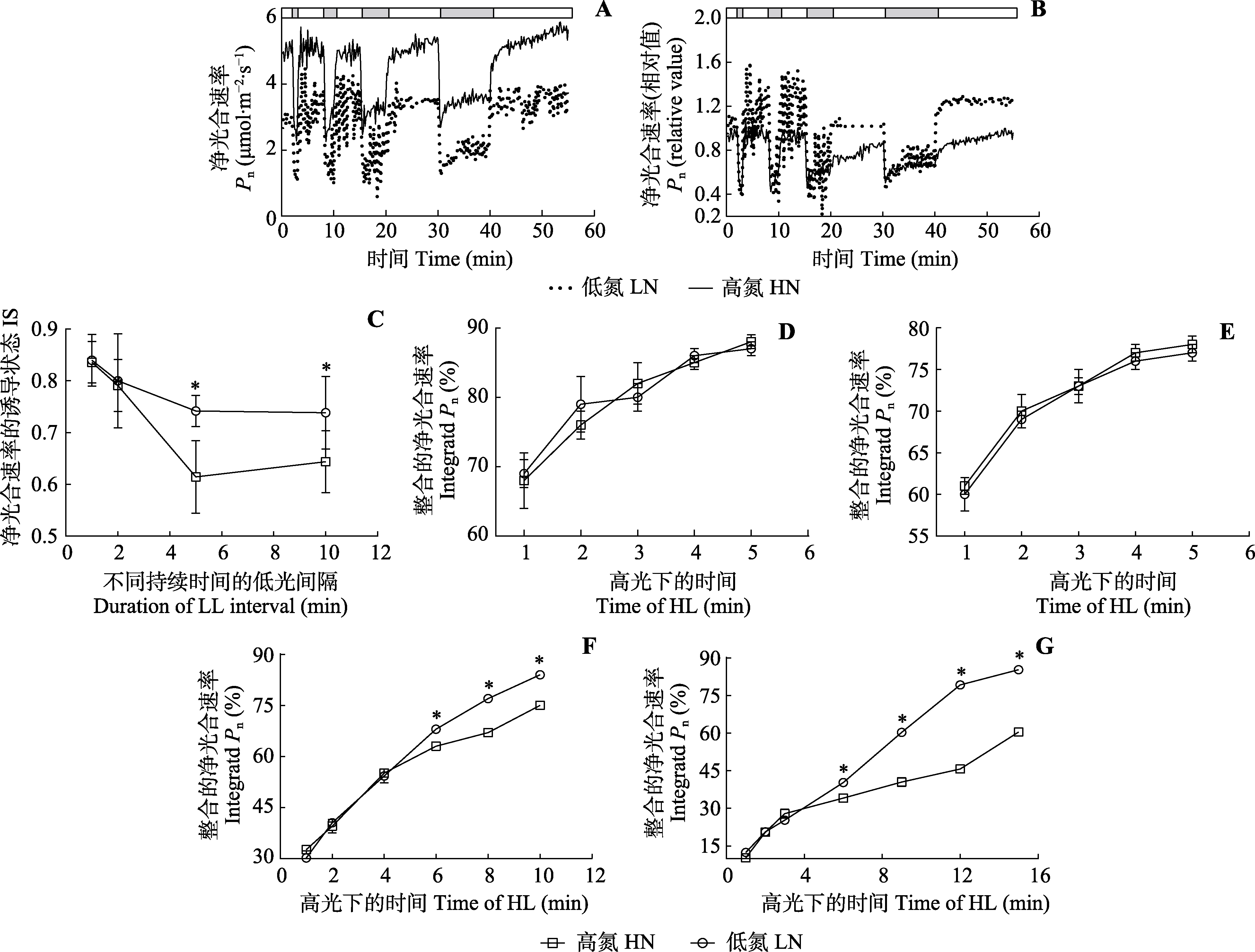
图3 非规律性动态光条件下三七的光合作用气体交换参数(平均值±标准差)。A、B, 低氮和高氮供应条件下的净光合速率。图上方的条形图显示了高光(800 μmol·m-2·s-1, 白色)和低光(50 μmol·m-2·s-1, 灰色)时期。叶片在高光下适应20-40 min, 直到净光合速率稳定下来。之后, 叶片暴露在变化的光照条件下。灰色条从左到右代表60、120、300和600 s的低光。白色条从左到右代表300、300、600和900 s的高光。C, 不同持续时间的低光间隔后净光合速率的诱导状态。D-G, 60、120、300、600 s低光间隔后高光期整合的净光合速率。*, 两处理间气体交换参数差异显著(p < 0.05)。
Fig. 3 Gas exchange parameters of leaves of Panax notoginseng under irregular dynamic light condition (mean ± SD). A, B, Net photosynthetic rates (Pn) under low nitrogen (LN) and high nitrogen (HN) conditions. The bars at the top of the figures show the net photosynthetic rates under high light (HL, 800 μmol·m-2·s-1, white) and low light (LL, 50 μmol·m-2·s-1, grey) conditions. The leaves were exposed to high light for 20-40 min until the net photosynthetic rates stabilized. After that, the leaves were exposed to changing light conditions. The grey bars from left to right represent low light at 60, 120, 300 and 600 s. The white bars from left to right represent high light at 300, 300, 600 and 900 s. C, Induction state of net photosynthetic rates (IS) after low light intervals of different durations. D-G, Integrated net photosynthetic rates during high light period after 60, 120, 300 and 600 s low light intervals. * indicates significant differences between the two nitrogen treatments (p < 0.05).
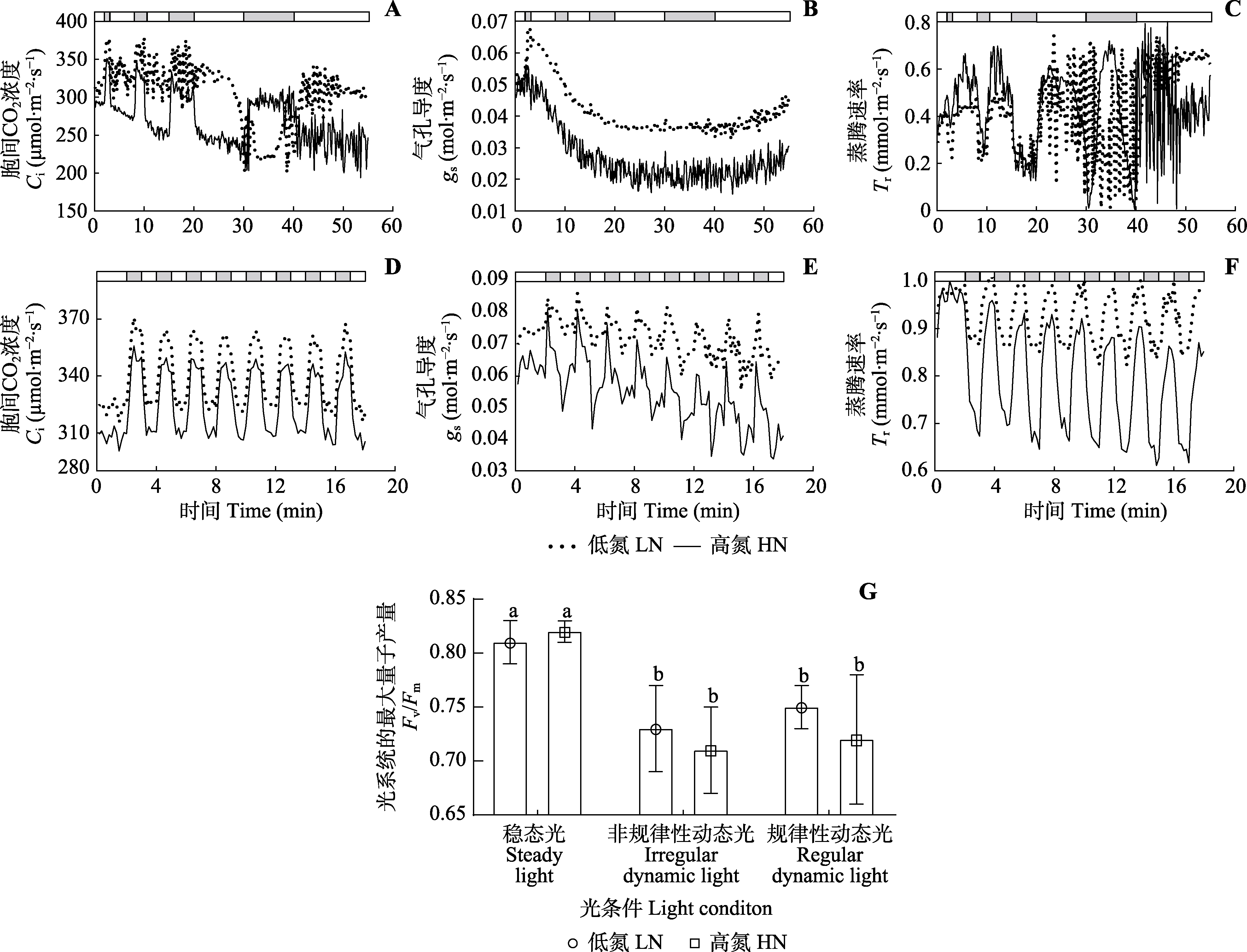
图4 两种动态光条件下三七的细胞间CO2浓度、气孔导度和蒸腾速率的变化规律及动态光合气体交换测定引起的光系统II光抑制(平均值±标准差)。A?F上方的条形图显示高光(800 μmol·m?2·s?1, 白色)和低光(50 μmol·m?2·s?1, 灰色)时期。G中不同小写字母表示差异显著(p < 0.05)。
Fig. 4 Intercellular CO2 concentration (Ci), stomatal conductance (gs) and transpiration rate (Tr) under two dynamic light conditions, and photosystem II photoinhibition induced by dynamic light of Panax notoginseng (mean ± SD). The bars at the top of A?F show the high light (800 μmol·m?2·s?1, white) and low light (50 μmol·m?2·s?1, grey) periods. Fv/Fm, maximum photochemistry efficiency of photosystem II; HN, high nitrogen; LN, low nitrogen. Different lowercase letters in G indicate significant differences (p < 0.05).
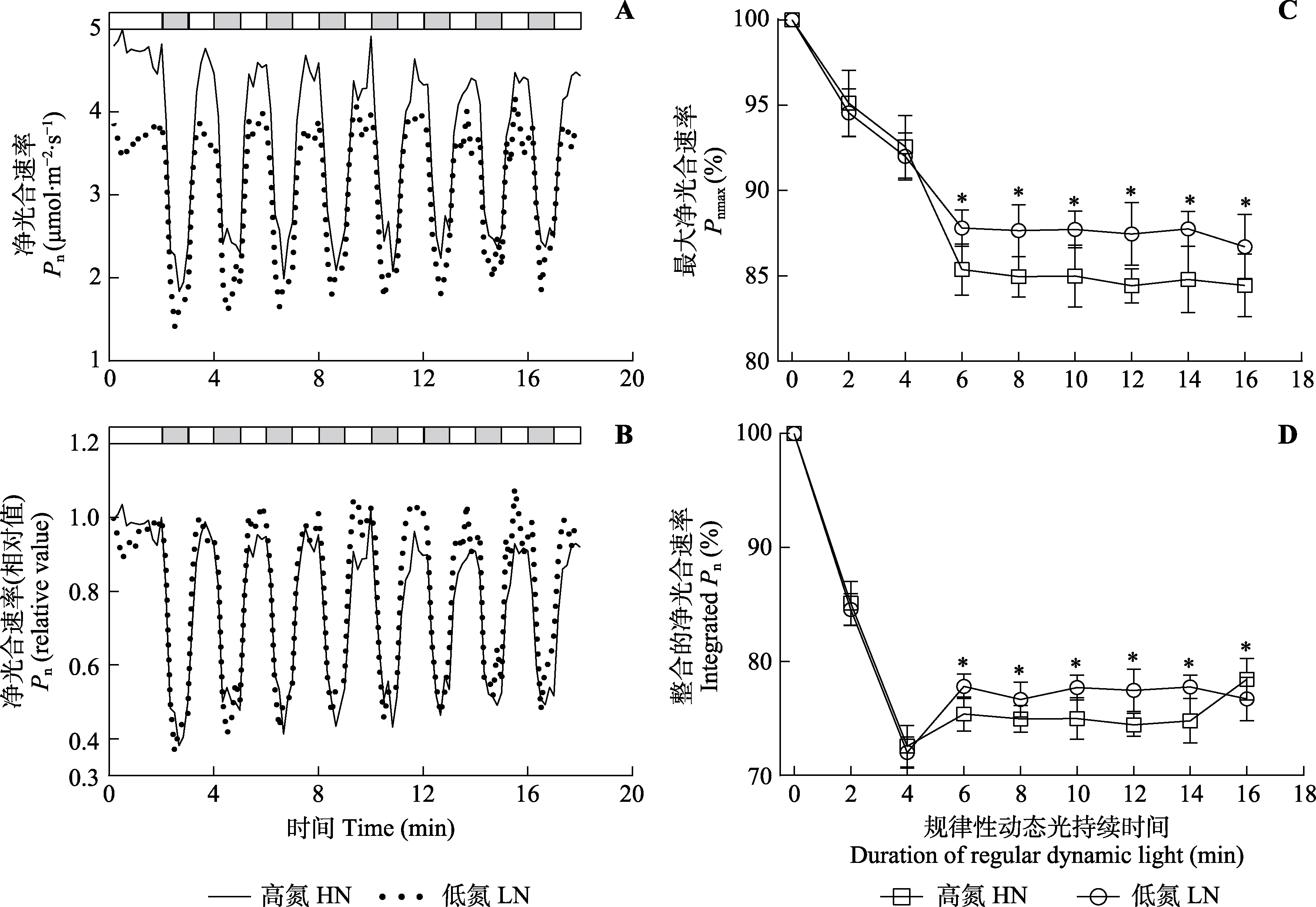
图5 规律性动态光条件下三七的光合气体交换参数(平均值±标准差)。A、B中, 叶片净光合速率稳定后, 在动态光下每60 s经历一次高光(800 μmol·m-2·s-1, 白色)和低光(50 μmol·m-2·s-1, 灰色)的光强交替。C中, 取稳态高光下净光合速率为100%, 动态光照条件下净光合速率为稳态高光下净光合速率的百分比。*, 两处理间气体交换参数差异显著(p < 0.05)。
Fig. 5 Gas exchange parameters of Panax notoginseng under regular dynamic light condition (mean ± SD). In A and B, after photosynthetic rate was stabilized, the light intensity alternated between high light (HL, 800 μmol·m-2·s-1, white) and low light (LL, 50 μmol·m-2·s-1, grey) every 60 s under fluctuating light. In C, net photosynthetic rate (Pn) under steady-state high light was 100%, net photosynthetic rate under fluctuating light was the percentage of net photosynthetic rate under steady-state high light. Pnmax, maximum net photosynthetic rate. HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two nitrogen treatments (p < 0.05).
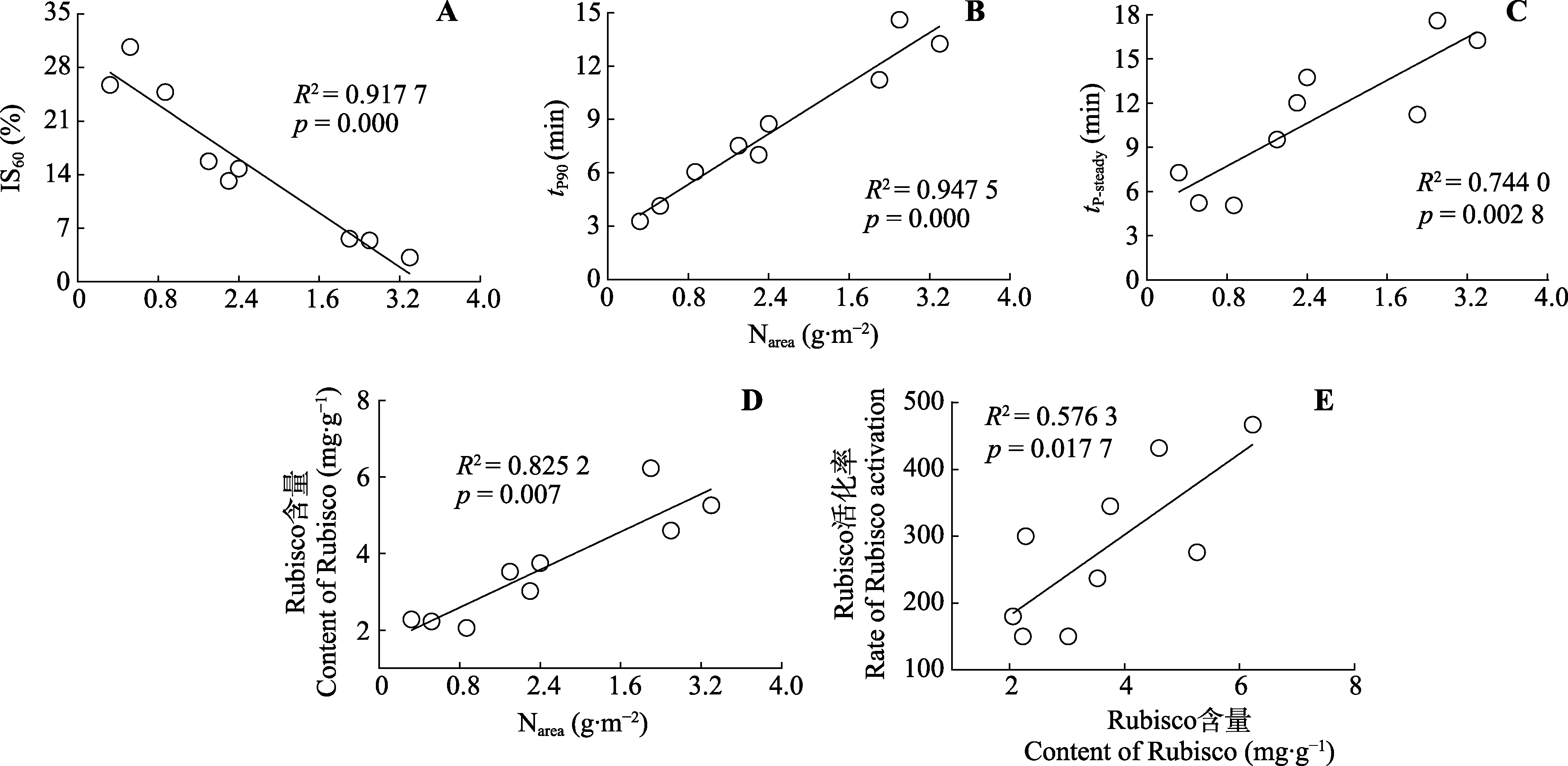
图6 三七气体交换参数相关性分析。IS60, 光照60s的诱导状态; Narea, 单位叶面积氮含量; Rubisco, 核酮糖-1,5-二磷酸羧化酶/加氧酶; tP90, 达到光合作用稳态90%所需的时间; tP-steady, 达到光合作用稳态100%所需的时间。
Fig. 6 Correlation between gas exchange parameters of Panax notoginseng. IS60, induction state at 60 s of light; Narea, nitrogen content per unit leaf area; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; tP90, time required to reach 90% of photosynthetic steady state; tP-steady, time required to reach 100% of photosynthetic steady state.
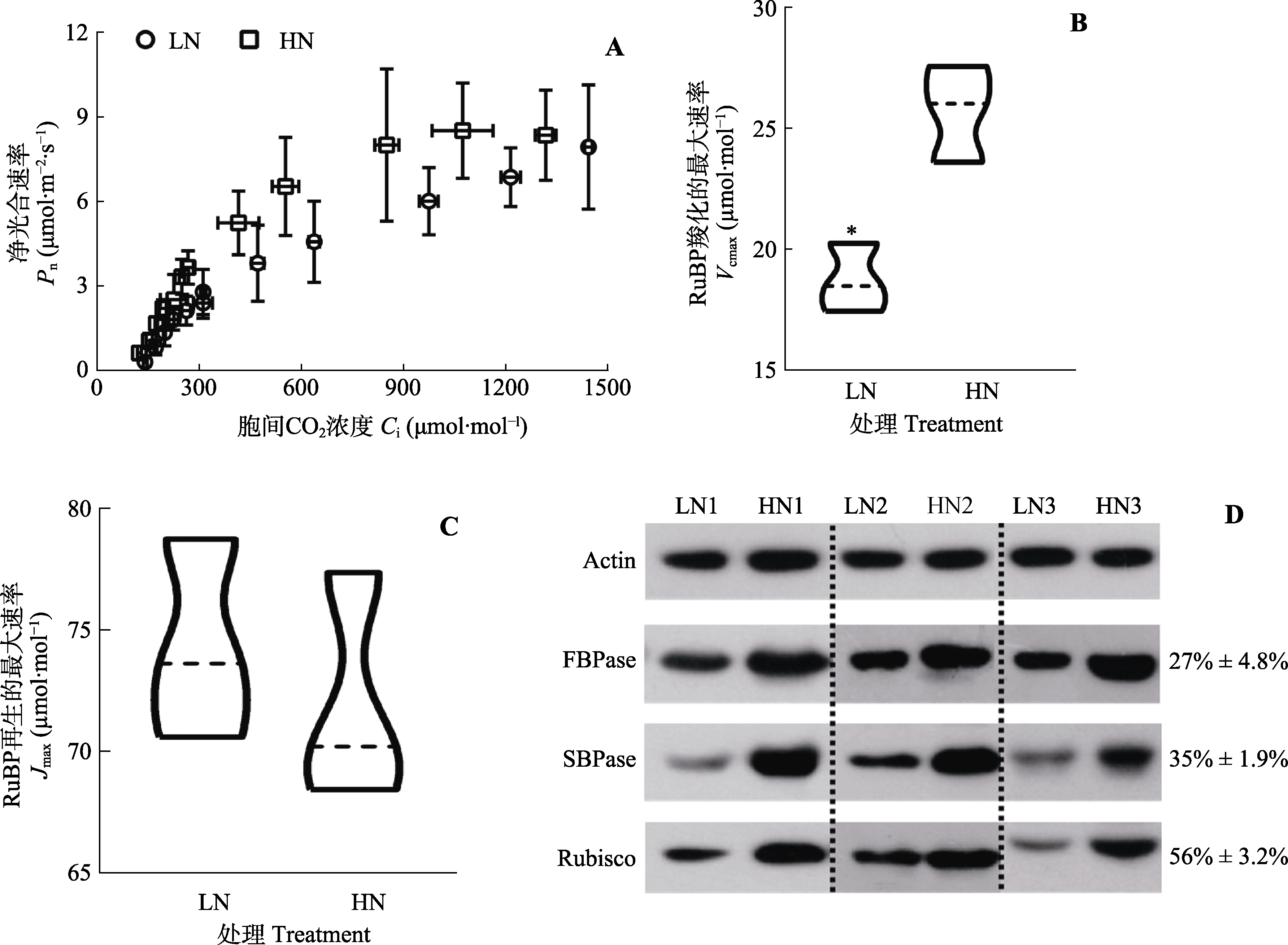
图7 低氮(LN)和高氮(HN)处理下三七叶片的核酮糖-1,5-二磷酸(RuBP)羧化和再生能力。D, 核酮糖-1,5-二磷酸羧化酶/加氧酶(Rubisco), 景天庚酮糖-1,7-二磷酸酶(SBPase)和果糖-1,6-二磷酸酶(FBPase)的免疫印迹分析, 右边的数字表示低氮叶片蛋白质含量为高氮叶片蛋白质含量的百分比。Actin, 肌动蛋白。*, 两处理间气体交换参数差异显著(p < 0.05)。
Fig. 7 Ribulose 1,5-bisphosphate carboxylation (RuBP) and regeneration capacity of Panax notoginseng leaves exposed to low-nitrogen (LN) and high-nitrogen (HN). D, Immunoblot analysis of 1,5-bisphosphate carboxylase (Rubisco), scenedesmus heptulose-1,7-bisphosphatase (SBPase) and fructose-1,6-bisphosphatase (FBPase), with the numbers on the right indicates the percentage of protein content of low-nitrogen leaves as a percentage of protein content of high-nitrogen leaves. Ci, intercellular CO2 concentration; Jmax, maximum rate of RuBP-regeneration; Pn, net photosynthetic rate; Vcmax, maximum carboxylation efficiency. * indicates significant differences between the two nitrogen treatments (p < 0.05).
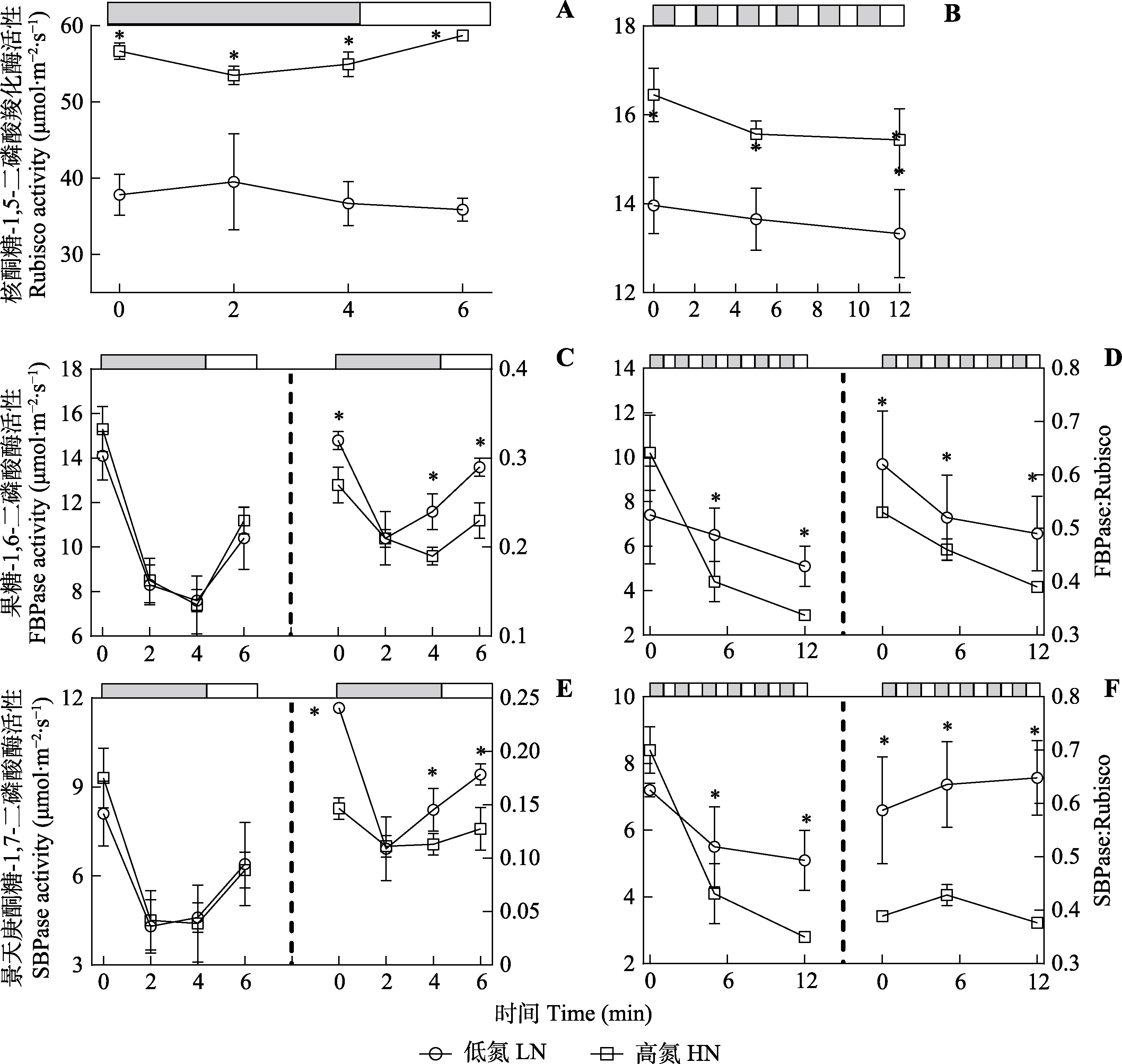
图8 在稳态和动态光条件下三七酶的活性(平均值±标准差)。图上方的条形图显示了高光(800 μmol·m-2·s-1, 白色)和低光(50 μmol·m-2·s-1, 灰色)时期。非规律动态光条件下的叶片(A, C, E)在高光(800 μmol·m-2·s-1)下适应20-40 min, 然后将叶片暴露于低光(50 μmol·m-2·s-1)下240 s, 然后将光转换为高光下120 s。在规律性动态光条件下(B, D, F), 高光处理20-40 min; 规律性动态光条件下, 叶片每120 s发生高光(800 μmol·m-2·s-1)和低光(50 μmol·m-2·s-1)光强交替, 持续12 min。*, 两处理间气体交换参数差异显著(p < 0.05)。
Fig. 8 Enzyme activities of Panax notoginseng under steady-state and dynamic light conditions (mean ± SD). The bars at the top of each figure show the high light (800 μmol·m-2·s-1, white) and low light (50 μmol·m-2·s-1, grey) periods. Under irregular dynamic light condition (A, C, E), leaves were exposed under high light (800 μmol·m-2·s-1) condition for 20-40 min and then the leaves were exposed to low light (50 μmol·m-2·s-1) for 240 s and then the light was converted to high light for 120 s. Under regular dynamic light condition (B, D, F), leaves were treated with high light for 20-40 min, followed by alternating high light (800 μmol·m-2·s-1) and low light (50 μmol·m-2·s-1) every 120 s for 12 min. FBPase, fructose-1,6-bisphosphatase; Rubisco, 1,5-bisphosphate carboxylase; SBPase, scenedesmusheptulose-1,7-bisphosphatase. HN, high nitrogen; LN, low nitrogen. * indicates significant differences between the two nitrogen treatments (p < 0.05).
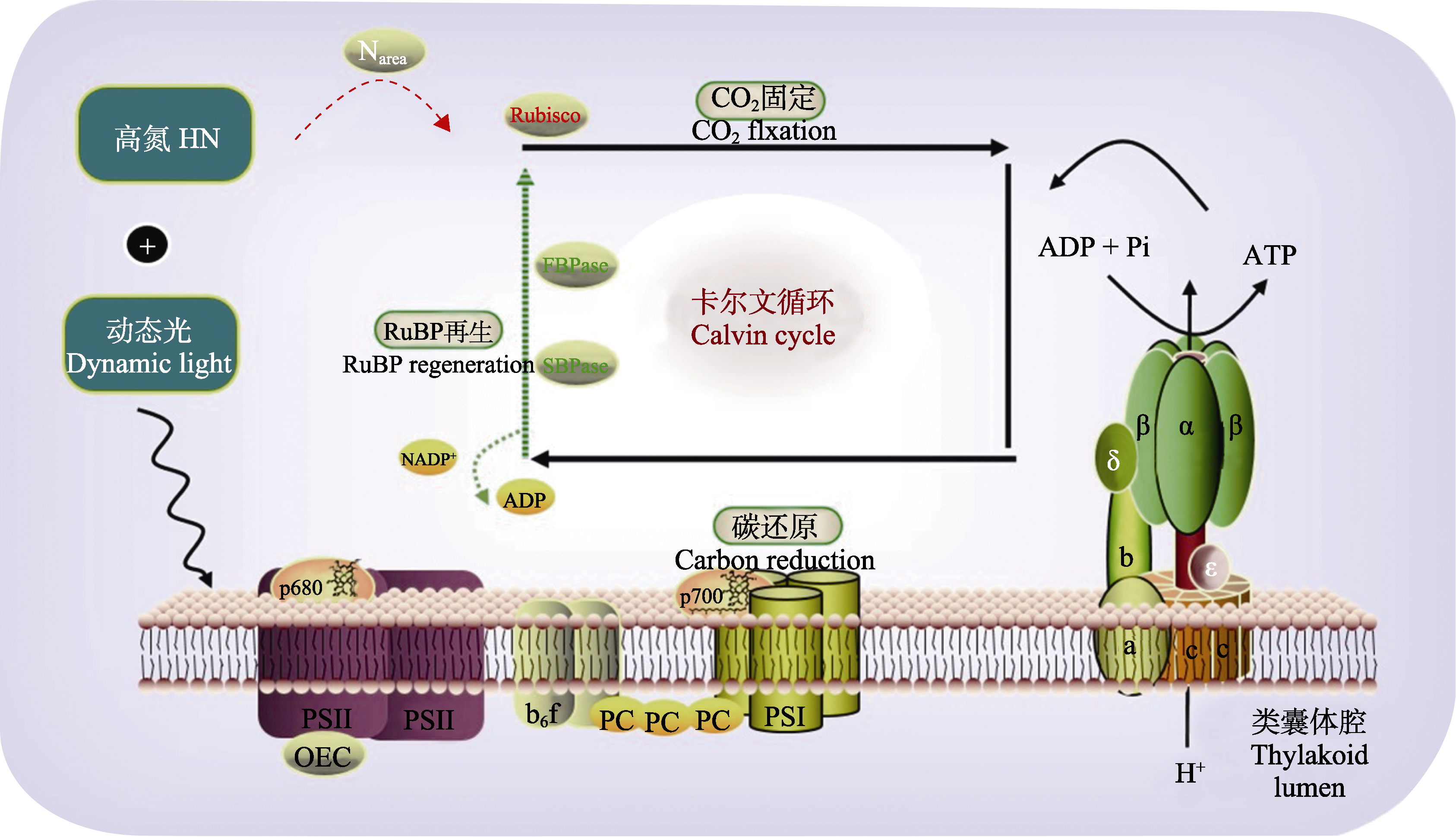
图9 高氮加剧典型阴生植物三七光诱导速率的下降。高氮叶片中核酮糖-1,5-二磷酸羧化酶/加氧酶(Rubisco)蛋白的含量高于果糖-1,6-二磷酸酶(FBPase)和景天庚酮糖-1,7-二磷酸酶(SBPase)的含量; 在动态光的高光照阶段, 高氮叶片需要激活较高比例的FBPase和SBPase及较长的时间来恢复光合速率。三七在高氮动态光环境中, 低光间隔后的光合诱导主要受到SBPase和FBPase再激活的限制, 而不是受到Rubisco活性限制。该模型展示的是三七光合电子传递的过程, 红色虚线表示该过程被充分激活, 绿色虚线表示该过程被抑制, 红色字体表示该物质的含量(浓度)增加, 绿色字体表示该物质的含量(浓度)减少。ADP, 二磷酸腺苷; ATP, 三磷酸腺苷; OEC, 放氧复合体; PC, 质体蓝素; Pi, 磷酸基团; PSI, 光系统I; PSII, 光系统II。a、b、c、β、δ、α、ε, ATP合酶的不同亚基; ADP, 二磷酸腺苷; ATP, 二磷酸腺苷; b6f, 细胞色素b6f复合物; FBPase, 果糖-1,6-二磷酸酶; Narea, 单位叶面积氮含量; NADP+, 烟酰胺腺嘌呤二核苷磷酸; OEC, 放氧复合体; p680, 叶绿素II; p700, 叶绿素I; PC, 质体蓝素; Pi, 磷酸基团; PSI, 光系统I; PSII, 光系统II; RuBP, 核酮糖-1,5-二磷酸; Rubisco, 核酮糖-1,5-二磷酸羧化酶/加氧酶; SBPase, 景天庚酮糖-1,7-二磷酸酶。
Fig. 9 High nitrogen (HN) exacerbates the decline of photosynthetic induction rate in a typically shade-tolerant species Panax notoginseng. The protein content of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) in high-nitrogen leaves was higher than that of fructose-1,6-bisphosphatase (FBPase) and scenedesmus heptulose-1,7-bisphosphatase (SBPase); during the high light phase of dynamic light, HN leaves required activation of a higher proportion of FBPase and SBPase and a longer time to restore photosynthetic rate. The photosynthetic induction in the dynamic light of HN condition after low light interval was mainly limited by SBPase and FBPase reactivation rather than by Rubisco activity. The model shows the process of photosynthetic electron transfer, the red dashed line indicates that the process is fully activated and the green dashed line indicates that the process is inhibited, the red font indicates an increase in the amount (concentration) of the substance and the green font indicates a decrease in the amount (concentration) of the substance. ADP, adenosine diphosphate; ATP, adenosine triphosphate; OEC, oxygen-evolving complex; PC, plastocyanin; Pi, phosphate group; PSI, photosystem I; PSII, photosystem II. a, b, c, β, δ, α and ε, different subunits of ATP synthase; ADP, adenosine diphosphate; ATP, adenosine triphosphate; b6f, cytochrome b6-f complex; FBPase, fructose-1,6-bisphosphatase; Narea, N content per unit of leaf area; NADP+, nicotinamide adenine dinucleotide phosphate; OEC, oxygen-evolving complex; p680, chlorophyll II; p700, chlorophyl; PC, plastocyanin; Pi, phosphate group; PSI, photosystem I; PSII, photosystem II; RuBP, ribulose-1,5-disphosphate; Rubisco, ribulose-1,5-bisphosphate carboxylase; SBPase, sedoheptulose-1,7-bisphosphatase.
| [1] | Cai ZQ, Qi X, Cao KF (2004). Response of stomatal characteristics and its plasticity to different light intensities in leaves of seven tropical woody seedlings. Chinese Journal of Applied Ecology, 15, 201-204. |
| [蔡志全, 齐欣, 曹坤芳 (2004). 七种热带雨林树苗叶片气孔特征及其可塑性对不同光照强度的响应. 应用生态学报, 15, 201-204.] | |
| [2] |
Carmo-Silva AE, Salvucci ME (2013). The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiology, 161, 1645-1655.
DOI PMID |
| [3] |
Chen JW, Kuang SB, Long GQ, Yang SC, Meng ZG, Li LG, Chen ZJ, Zhang GH (2016). Photosynthesis, light energy partitioning, and photoprotection in the shade-demanding species Panax notoginseng under high and low level of growth irradiance. Functional Plant Biology, 43, 479-491.
DOI URL |
| [4] |
Chen JW, Zhang Q, Li XS, Cao KF (2011). Steady and dynamic photosynthetic responses of seedlings from contrasting successional groups under low-light growth conditions. Physiologia Plantarum, 141, 84-95.
DOI URL |
| [5] | Cun Z, Zhang JY, Chen JW (2020). Effects of nitrogen addition on growth, photosynthetic characteristics and saponin content in two-year-old Panax notoginseng. Chinese Journal of Ecology, 39, 1101-1111. |
| [寸竹, 张金燕, 陈军文 (2020). 氮添加对二年生三七生长、光合特性及皂苷含量的影响. 生态学杂志, 39, 1101-1111.] | |
| [6] |
Cun Z, Zhang JY, Wu HM, Zhang L, Chen JW (2021). High nitrogen inhibits photosynthetic performance in a shade-tolerant and N-sensitive species Panax notoginseng. Photosynthesis Research, 147, 283-300.
DOI |
| [7] |
de Souza AP, Wang Y, Orr DJ, Carmo-Silva E, Long SP (2020). Photosynthesis across African cassava germplasm is limited by Rubisco and mesophyll conductance at steady state, but by stomatal conductance in fluctuating light. New Phytologist, 225, 2498-2512.
DOI PMID |
| [8] |
Ernstsen J, Woodrow IE, Mott KA (1997). Responses of Rubisco activation and deactivation rates to variations in growth-light conditions. Photosynthesis Research, 52, 117-125.
DOI URL |
| [9] |
Evans JR, Clarke VC (2019). The nitrogen cost of photosynthesis. Journal of Experimental Botany, 70, 7-15.
DOI PMID |
| [10] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
DOI PMID |
| [11] | Fu Z, Xie SQ, Xu WG, Yan S, Chen JW (2016). Characteristics of photosynthesis and light energy partitioning in Amorphophallus xiei grown along a light-intensity gradient. Chinese Journal of Applied Ecology, 27, 1177-1188. |
|
[付忠, 谢世清, 徐文果, 岩所, 陈军文 (2016). 不同光照强度下谢君魔芋的光合作用及能量分配特征. 应用生态学报, 27, 1177-1188.]
DOI |
|
| [12] | Gao F, Wang RS, Xu HS, Wang DM, Yang ZR (2016). Effects of water and fertilizer coupling on photosynthetic characteristics of maize leaves in ear position at filling stage in an apple-maize intercropping system in Losses Plateau of west Shanxi Province, China. Chinese Journal of Applied Ecology, 27, 2477-2490. |
|
[高飞, 王若水, 许华森, 王冬梅, 杨宗儒 (2016). 晋西黄土区水肥调控对苹果-玉米间作系统玉米灌浆期穗位叶光合生理特性的影响. 应用生态学报, 27, 2477-2490.]
DOI |
|
| [13] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [14] | Grieco M, Roustan V, Dermendjiev G, Rantala S, Jain A, Leonardelli M, Neumann K, Berger V, Engelmeier D, Bachmann G, Ebersberger I, Aro EM, Weckwerth W, Teige M (2020). Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant, Cell & Environment, 43, 1484-1500. |
| [15] |
Han JM, Zhang WF, Xiong DL, Flexas J, Zhang YL (2017). Mesophyll conductance and its limiting factors in plant leaves. Chinese Journal of Plant Ecology, 41, 914-924.
DOI URL |
|
[韩吉梅, 张旺锋, 熊栋梁, Flexas J, 张亚黎 (2017). 植物光合作用叶肉导度及主要限制因素研究进展. 植物生态学报, 41, 914-924.]
DOI |
|
| [16] |
Hauser T, Popilka L, Hartl FU, Hayer-Hartl M (2015). Role of auxiliary proteins in Rubisco biogenesis and function. Nature Plants, 1, 15065. DOI: 10.1038/nplants.2015.65.
DOI |
| [17] | He P, Cheng F, Yang M, He FZ (2020). Light environment analysis of artificial and natural canopy of Parashorea chinensis in Guangxi. Journal of Northeast Forestry University, 48(7), 29-33. |
| [何鹏, 程飞, 杨梅, 何方中 (2020). 广西望天树人工林和天然林冠层光环境特征与其生长的关系. 东北林业大学学报, 48(7), 29-33.] | |
| [18] |
Howard TP, Metodiev M, Lloyd JC, Raines CA (2008). Thioredoxin-mediated reversible dissociation of a stromal multiprotein complex in response to changes in light availability. Proceedings of the National Academy of Sciences of the United States of America, 105, 4056-4061.
DOI PMID |
| [19] |
Ikeuchi M, Uebayashi N, Sato F, Endo T (2014). Physiological functions of PsbS-dependent and PsbS-independent NPQ under naturally fluctuating light conditions. Plant and Cell Physiology, 55, 1286-1295.
DOI PMID |
| [20] | Jiang QQ, Liu C, Hu ZH, Yu LF, Yang ZQ, Chen ST (2021). Effects of different levels of elevated CO2 concentration and nitrogen fertilization on chlorophyll fluorescence characteristics of rice. Acta Ecologica Sinica, 41, 4953-4962. |
| [姜倩倩, 刘超, 胡正华, 于凌飞, 杨再强, 陈书涛 (2021). 不同CO2浓度升高和氮肥水平对水稻叶绿素荧光特性的影响. 生态学报, 41, 4953-4962.] | |
| [21] |
Jin C, Li XH, Jiang Y, Xu MZ, Tian Y, Liu P, Jia X, Zha TS (2021). Relative changes and regulation of photosynthetic energy partitioning components in Artemisia ordosica during growing season. Chinese Journal of Plant Ecology, 45, 870-879.
DOI URL |
|
[靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山 (2021). 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制. 植物生态学报, 45, 870-879.]
DOI |
|
| [22] |
Kaiser E, Kromdijk J, Harbinson J, Heuvelink E, Marcelis LFM (2016a). Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance. Annals of Botany, 119, 191-205.
DOI URL |
| [23] |
Kaiser E, Matsubara S, Harbinson J, Heuvelink E, Marcelis LFM (2018). Acclimation of photosynthesis to lightflecks in tomato leaves: interaction with progressive shading in a growing canopy. Physiologia Plantarum, 162, 506-517.
DOI URL |
| [24] |
Kaiser E, Morales A, Harbinson J (2017a). Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiology, 176, 977-989.
DOI URL |
| [25] |
Kaiser E, Morales A, Harbinson J, Heuvelink E, Prinzenberg AE, Marcelis LFM (2016b). Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Scientific Reports, 6, 31252. DOI: 10.1038/srep31252.
DOI |
| [26] |
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM (2014). Dynamic photosynthesis in different environmental conditions. Journal of Experimental Botany, 66, 2415-2426.
DOI URL |
| [27] |
Kaiser E, Zhou D, Heuvelink E, Harbinson J, Morales A, Marcelis LFM (2017b). Elevated CO2 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. Journal of Experimental Botany, 68, 5629-5640.
DOI URL |
| [28] |
Kang H, Zhu X, Yamori W, Tang Y (2020). Concurrent increases in leaf temperature with light accelerate photosynthetic induction in tropical tree seedlings. Frontiers in Plant Science, 11, 1216. DOI: 10.3389/fpls.2020.01216.
DOI |
| [29] |
Kono M, Kawaguchi H, Mizusawa N, Yamori W, Suzuki Y, Terashima I (2019). Far-red light accelerates photosynthesis in the low-light phases of fluctuating light. Plant and Cell Physiology, 61, 192-202.
DOI URL |
| [30] | Kuang SB, Xu XZ, Meng ZG, Zhang GH, Yang SC, Chen ZJ, Wei FG, Chen JW (2015). Effects of light transmittance on plant growth and root ginsenoside content of Panax notoginseng. Chinese Journal of Applied and Environmental Biology, 21, 279-286. |
| [匡双便, 徐祥增, 孟珍贵, 张广辉, 杨生超, 陈中坚, 魏富刚, 陈军文 (2015). 不同透光率对三七生长特征及根皂苷含量的影响. 应用与环境生物学报, 21, 279-286.] | |
| [31] |
Lang Y, Wang M, Zhang GC, Zhao QK (2013). Experimental and simulated light responses of photosynthesis in leaves of three tree species under different soil water conditions. Photosynthetica, 51, 370-378.
DOI URL |
| [32] |
Lawson T, Kramer DM, Raines CA (2012). Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Current Opinion in Biotechnology, 23, 215-220.
DOI PMID |
| [33] | Leakey ADB, Press MC, Scholes JD (2003). Patterns of dynamic irradiance affect the photosynthetic capacity and growth of dipterocarp tree seedlings. Oecologia, 135, 184-193. |
| [34] |
LeBauer DS, Treseder KK (2008). Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology, 89, 371-379.
PMID |
| [35] | Li M, Li YC, Niu XG, Ma F, Wei N, Hao XY, Dong LB, Guo LP (2021). Effects of elevated atmospheric CO2 concentration and nitrogen fertilizer on the yield of summer maize and carbon and nitrogen metabolism after flowering. Scientia Agricultura Sinica, 54, 3647-3665. |
|
[李明, 李迎春, 牛晓光, 马芬, 魏娜, 郝兴宇, 董李冰, 郭李萍 (2021). 大气CO2浓度升高与氮肥互作对玉米花后碳氮代谢及产量的影响. 中国农业科学, 54, 3647-3665.]
DOI |
|
| [36] | Li Q, Luo YH, Yu DH, Kong FL, Yang SM, Yuan JC (2015). Effects of low nitrogen stress on photosynthetic characteristics and chlorophyll fluorescence parameters of maize cultivars tolerant to low nitrogen stress at the seedling stage. Journal of Plant Nutrition and Fertilizer, 21, 1132-1141. |
| [李强, 罗延宏, 余东海, 孔凡磊, 杨世民, 袁继超 (2015). 低氮胁迫对耐低氮玉米品种苗期光合及叶绿素荧光特性的影响. 植物营养与肥料学报, 21, 1132-1141.] | |
| [37] | Li Y (2011). Studies on Mechanisms of the Effects of Different Nitrogen Supplies on Photosynthesis and Photosynthetic Nitrogen Use Efficiency of Rice Plants. PhD dissertation, Nanjing Agricultural University, Nanjing. |
| [李勇 (2011). 氮素营养对水稻光合作用与光合氮素利用率的影响机制研究. 博士学位论文, 南京农业大学, 南京.] | |
| [38] |
Li YT, Li Y, Li YN, Liang Y, Sun Q, Li G, Liu P, Zhang ZS, Gao HY (2020). Dynamic light caused less photosynthetic suppression, rather than more, under nitrogen deficit conditions than under sufficient nitrogen supply conditions in soybean. BMC Plant Biology, 20, 339. DOI: 10.1186/s12870-020-02516-y.
DOI |
| [39] |
Li ZZ, Liu DH, Zhao SW, Jiang CD, Shi L (2014). Mechanisms of photoinhibition induced by high light in Hosta grown outdoors. Chinese Journal of Plant Ecology, 38, 720-728.
DOI |
|
[李志真, 刘东焕, 赵世伟, 姜闯道, 石雷 (2014). 环境强光诱导玉簪叶片光抑制的机制. 植物生态学报, 38, 720-728.]
DOI |
|
| [40] | Liu J, Last RL (2017). A chloroplast thylakoid lumen protein is required for proper photosynthetic acclimation of plants under fluctuating light environments. Proceedings of the National Academy of Sciences of the United States of America, 114, E8110-E8117. |
| [41] |
Liu J, Zhang J, Estavillo GM, Luo T, Hu L (2021). Leaf N content regulates the speed of photosynthetic induction under fluctuating light among canola genotypes (Brassica napus L.). Physiologia Plantarum, 172, 1844-1852.
DOI URL |
| [42] |
Long SP, Bernacchi CJ (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany, 54, 2393-2401.
DOI PMID |
| [43] |
Marri L, Zaffagnini M, Collin V, Issakidis-Bourguet E, Lemaire SD, Pupillo P, Sparla F, Miginiac-Maslow M, Trost P (2009). Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Molecular Plant, 2, 259-269.
DOI URL |
| [44] |
Matthews JSA, Vialet-Chabrand S, Lawson T (2018). Acclimation to fluctuating light impacts the rapidity of response and diurnal rhythm of stomatal conductance. Plant Physiology, 176, 1939-1951.
DOI PMID |
| [45] |
Mu XH, Chen YL (2021). The physiological response of photosynthesis to nitrogen deficiency. Plant Physiology and Biochemistry, 158, 76-82.
DOI PMID |
| [46] |
Naranjo B, Diaz-Espejo A, Lindahl M, Cejudo FJ (2016). Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. Journal of Experimental Botany, 67, 1951-1964.
DOI PMID |
| [47] |
Niedermaier S, Schneider T, Bahl MO, Matsubara S, Huesgen PF (2020). Photoprotective acclimation of the Arabidopsis thaliana leaf proteome to fluctuating light. Frontiers in Genetics, 11, 154. DOI: 10.3389/fgene.2020.00154.
DOI |
| [48] |
Niyogi KK, Truong TB (2013). Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Current Opinion in Plant Biology, 16, 307-314.
DOI PMID |
| [49] |
Ohkubo S, Tanaka Y, Yamori W, Adachi S (2020). Rice cultivar takanari has higher photosynthetic performance under fluctuating light than koshihikari, especially under limited nitrogen supply and elevated CO2. Frontiers in Plant Science, 11, 1308. DOI: 10.3389/fpls.2020.01308.
DOI |
| [50] |
Pilon C, Snider JL, Sobolev V, Chastain DR, Sorensen RB, Meeks CD, Massa AN, Walk T, Singh B, Earl HJ (2018). Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). Journal of Plant Physiology, 231, 124-134.
DOI URL |
| [51] |
Raines CA (2003). The Calvin cycle revisited. Photosynthesis Research, 75, 1-10.
DOI PMID |
| [52] |
Rascher U, Nedbal L (2006). Dynamics of photosynthesis in fluctuating light. Current Opinion in Plant Biology, 9, 671-678.
DOI PMID |
| [53] |
Scheibe R, Fickenscher K, Ashton AR (1986). Studies on the mechanism of the reductive activation of NADP-malate dehydrogenase by thioredoxin m and low-molecular-weight thiols. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 870, 191-197.
DOI URL |
| [54] |
Seuter A, Busch M, Hain R (2002). Overexpression of the potential herbicide target sedoheptulose-1,7-bisphosphatase from Spinacia oleracea in transgenic tobacco. Molecular Breeding, 9, 53-61.
DOI URL |
| [55] |
Slattery RA, Walker BJ, Weber APM, Ort DR (2017). The impacts of fluctuating light on crop performance. Plant Physiology, 176, 990-1003.
DOI URL |
| [56] | Soleh MA, Tanaka Y, Nomoto Y, Iwahashi Y, Nakashima K, Fukuda Y, Long SP, Shiraiwa T (2016). Factors underlying genotypic differences in the induction of photosynthesis in soybean [Glycine max (L.) Merr]. Plant, Cell & Environment, 39, 685-693. |
| [57] |
Sun H, Shi Q, Zhang SB, Huang W (2022). The response of photosystem I to fluctuating light is influenced by leaf nitrogen content in tomato. Environmental and Experimental Botany, 193, 104665. DOI: 10.1016/j.envexpbot.2021.104665.
DOI |
| [58] |
Sun J, Ye M, Peng S, Li Y (2016). Nitrogen can improve the rapid response of photosynthesis to changing irradiance in rice (Oryza sativa L.) plants. Scientific Reports, 6, 31305. DOI: 10.1038/srep31305.
DOI |
| [59] |
Terashima I, Matsuo M, Suzuki Y, Yamori W, Kono M (2021). Photosystem I in low light-grown leaves of Alocasia odora, a shade-tolerant plant, is resistant to fluctuating light-induced photoinhibition. Photosynthesis Research, 149, 69-82.
DOI PMID |
| [60] |
Warren CR, Dreyer E, Adams MA (2003). Photosynthesis-Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees, 17, 359-366.
DOI |
| [61] | Wei Z (2021). Physiological and Molecular Mechanisms of Rice in Response to Fluctuating Light. PhD dissertation, Shandong Agricultural University, Taian, Shangdong. |
| [卫泽 (2021). 水稻响应波动光的生理及分子机制. 博士学位论文, 山东农业大学, 山东泰安.] | |
| [62] |
Woodrow IE, Mott KA (1989). Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in spinach. Functional Plant Biology, 16, 487. DOI: 10.1071/pp9890487.
DOI |
| [63] |
Wu HM, Shuang SP, Zhang JY, Cun Z, Meng ZG, Li LG, Sha BC, Chen JW (2021). Photodamage to photosystem in a typically shade-tolerant species Panax notoginseng exposed to a sudden increase in light intensity. Chinese Journal of Plant Ecology, 45, 404-419.
DOI URL |
|
[武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文 (2021). 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制. 植物生态学报, 45, 404-419.]
DOI |
|
| [64] | Xiong DL (2016). Coordination of Leaf Morpho-Anatomical Traits, Photosynthesis and Leaf Hydraulic Conductance in Oryza. PhD dissertation, Huazhong Agricultural University, Wuhan. |
| [熊栋梁 (2016). 水稻叶片结构对水力导度与光合作用的影响及其机理. 博士学位论文, 华中农业大学, 武汉.] | |
| [65] |
Xiong DL, Flexas J, Yu TT, Peng SB, Huang JL (2017). Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 in Oryza. New Phytologist, 213, 572-583.
DOI URL |
| [66] | Xu XZ, Zhang JY, Zhang GH, Long GQ, Yang SC, Chen ZJ, Wei FG, Chen JW (2018). Effects of light intensity on photosynthetic capacity and light energy allocation in Panax notoginseng. Chinese Journal of Applied Ecology, 29, 193-204. |
|
[徐祥增, 张金燕, 张广辉, 龙光强, 杨生超, 陈中坚, 魏富刚, 陈军文 (2018). 光强对三七光合能力及能量分配的影响. 应用生态学报, 29, 193-204.]
DOI |
|
| [67] | Yamori W, Kusumi K, Iba K, Terashima I (2020). Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant, Cell & Environment, 43, 1230-1240. |
| [68] | Ye SH (2004). Experimental Course of Plant Physiology and Biochemistry. Yunnan Science and Technology Press, Kunming. |
| [叶尚红 (2004). 植物生理生化实验教程. 云南科技出版社, 昆明.] | |
| [69] | Ye ZP, Yu Q (2008). Comparison of new and several classical models of photo-synthesis in response to irradiance. Journal of Plant Ecology (Chinese Version), 32, 1356-1361. |
|
[叶子飘, 于强 (2008). 光合作用光响应模型的比较. 植物生态学报, 32, 1356-1361.]
DOI |
|
| [70] |
Yin XY, Schapendonk AHCM, Struik PC (2018). Exploring the optimum nitrogen partitioning to predict the acclimation of C3 leaf photosynthesis to varying growth conditions. Journal of Experimental Botany, 70, 2435-2447.
DOI URL |
| [71] | Yuan JM, He L, Yang XQ, Xu ZP, Kong WX, Zhao QL, Qu WL, Lei X (2020). Light response curve of photosynthesis of three Phyllanthus emblica provenances in dry-hot valley of Jinsha River. Acta Agriculturae Jiangxi, 32, 55-60. |
| [袁建民, 何璐, 杨晓琼, 许智萍, 孔维喜, 赵琼玲, 瞿文林, 雷虓 (2020). 干热河谷区3个种源余甘子的光响应曲线特性研究. 江西农业学报, 32, 55-60.] | |
| [72] |
Zhang JY, Cun Z, Chen JW (2020a). Photosynthetic performance and photosynthesis-related gene expression coordinated in a shade-tolerant species Panax notoginseng under nitrogen regimes. BMC Plant Biology, 20, 273. DOI: 10.1186/s12870-020-02434-z.
DOI |
| [73] |
Zhang JY, Cun Z, Wu HM, Chen JW (2020b). Integrated analysis on biochemical profiling and transcriptome revealed nitrogen-driven difference in accumulation of saponins in a medicinal plant Panax notoginseng. Plant Physiology and Biochemistry, 154, 564-580.
DOI URL |
| [74] |
Zhang JY, Shuang SP, Zhang L, Xie SQ, Chen JW (2021a). Photosynthetic and photoprotective responses to steady-state and fluctuating light in the shade-demanding crop Amorphophallus xiei grown in intercropping and monoculture systems. Frontiers in Plant Science, 12, 663473. DOI: 10.3389/fpls.2021.663473.
DOI |
| [75] |
Zhang JY, Xie SQ, Yan S, Xu WG, Chen JW (2021b). Light energy partitioning and photoprotection from excess light energy in shade-tolerant plant Amorphophallus xiei under steady-state and fluctuating high light. Acta Physiologiae Plantarum, 43, 1-17.
DOI |
| [76] |
Zhang JY, Xu XZ, Kuang SB, Cun Z, Wu HM, Shuang SP, Chen JW (2021c). Constitutive activation of genes involved in triterpene saponins enhances the accumulation of saponins in three-year-old Panax notoginseng growing under moderate light intensity. Industrial Crops and Products, 171, 113938. DOI: 10.1016/j.indcrop.2021.113938.
DOI |
| [77] |
Zhang JY, Zhang QH, Shuang SP, Cun Z, Wu HM, Chen JW (2021d). The responses of light reaction of photosynthesis to dynamic sunflecks in a typically shade-tolerant species Panax notoginseng. Frontiers in Plant Science, 12, 718981. DOI: 10.3389/fpls.2021.718981.
DOI |
| [78] |
Zhang QQ, Peng SB, Li Y (2019). Increase rate of light-induced stomatal conductance is related to stomatal size in the genus Oryza. Journal of Experimental Botany, 70, 5259-5269.
DOI URL |
| [79] | Zhao SJ, Cang J (2016). Experimental Guide for Plant Physiology. Chinese Agriculture Press, Beijing. |
| [赵世杰, 苍晶 (2016). 植物生理学实验指导. 中国农业出版社, 北京.] |
| [1] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [2] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| [3] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [4] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [5] | 周稳, 迟永刚, 周蕾. 基于日光诱导叶绿素荧光的北半球森林物候研究[J]. 植物生态学报, 2021, 45(4): 345-354. |
| [6] | 丁键浠, 周蕾, 王永琳, 庄杰, 陈集景, 周稳, 赵宁, 宋珺, 迟永刚. 叶绿素荧光主动与被动联合观测应用前景[J]. 植物生态学报, 2021, 45(2): 105-118. |
| [7] | 郭庆华, 胡天宇, 马勤, 徐可心, 杨秋丽, 孙千惠, 李玉美, 苏艳军. 新一代遥感技术助力生态系统生态学研究[J]. 植物生态学报, 2020, 44(4): 418-435. |
| [8] | 刘双娥, 李义勇, 方熊, 黄文娟, 龙凤玲, 刘菊秀. 不同氮添加量和添加方式对南亚热带4个主要树种幼苗生长的影响[J]. 植物生态学报, 2015, 39(10): 950-961. |
| [9] | 陈志刚, 樊大勇, 张旺锋, 谢宗强. 林隙与林下环境对锐齿槲栎和米心水青冈种群更新的影响[J]. 植物生态学报, 2005, 29(3): 354-360. |
| [10] | 王俊峰, 冯玉龙. 光强对两种入侵植物生物量分配、叶片形态和相对生长速率的影响[J]. 植物生态学报, 2004, 28(6): 781-786. |
| [11] | 樊大勇, 谢宗强, 王强, 张其德. 亚热带常绿阔叶林下富贵草 (Pachysandra Terminalis)对模拟光斑的光合响应(英文)[J]. 植物生态学报, 2002, 26(4): 447-453. |
| [12] | 张其德, 蒋高明, 朱新广, 王强, 卢从明, 白克智, 匡廷云, 魏其克, 李振声. 12个不同基因型冬小麦的光合能力[J]. 植物生态学报, 2001, 25(5): 532-536. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19