

植物生态学报 ›› 2021, Vol. 45 ›› Issue (2): 105-118.DOI: 10.17521/cjpe.2020.0323
所属专题: 生态学研究的方法和技术
• 综述 • 下一篇
丁键浠1, 周蕾1,2, 王永琳1, 庄杰1, 陈集景1, 周稳1, 赵宁1, 宋珺1, 迟永刚1,*( )
)
收稿日期:2020-09-25
接受日期:2020-12-10
出版日期:2021-02-20
发布日期:2021-03-09
通讯作者:
迟永刚
作者简介:*(chiyonggang@zjnu.cn)基金资助:
DING Jian-Xi1, ZHOU Lei1,2, WANG Yong-Lin1, ZHUANG Jie1, CHEN Ji-Jing1, ZHOU Wen1, ZHAO Ning1, SONG Jun1, CHI Yong-Gang1,*( )
)
Received:2020-09-25
Accepted:2020-12-10
Online:2021-02-20
Published:2021-03-09
Contact:
CHI Yong-Gang
Supported by:摘要:
叶绿素荧光是研究植物光合生理机制、量化植被光合作用时空格局以及准确理解气候变化背景下陆地生态系统生产力的关键。然而, 目前对于叶绿素荧光主动与被动联合观测的研究还较少。该文对比了叶绿素荧光主动观测与被动观测的优缺点, 展示了叶片尺度和冠层尺度主动与被动联合观测的仪器设备组成, 探讨了主动与被动联合观测在探索叶绿体尺度-叶片尺度-冠层尺度能量在光合、荧光以及热耗散中的分配, 阐明叶绿素荧光与总初级生产力的关联机制, 验证星基日光诱导叶绿素荧光, 解译叶绿素荧光光谱形状4个方面的应用前景。综上, 叶绿素荧光的主动与被动联合观测对于揭示各尺度上荧光与光合作用之间的关联机制, 改善全球尺度植被生产力模型至关重要。
丁键浠, 周蕾, 王永琳, 庄杰, 陈集景, 周稳, 赵宁, 宋珺, 迟永刚. 叶绿素荧光主动与被动联合观测应用前景. 植物生态学报, 2021, 45(2): 105-118. DOI: 10.17521/cjpe.2020.0323
DING Jian-Xi, ZHOU Lei, WANG Yong-Lin, ZHUANG Jie, CHEN Ji-Jing, ZHOU Wen, ZHAO Ning, SONG Jun, CHI Yong-Gang. Application prospects for combining active and passive observations of chlorophyll fluorescence. Chinese Journal of Plant Ecology, 2021, 45(2): 105-118. DOI: 10.17521/cjpe.2020.0323
| 传感器 Sensor | 卫星 Satellite | 发射时间 Launch time | 波段 Band (nm) | 状态 State | 相关文献 Related literature |
|---|---|---|---|---|---|
| SCIAMACHY | ENVISAT | 2002-03 | 650-790 | 失联 Lost contact | Joiner et al., |
| GOME-2 | METOP | 2006-07 | 650-790 | 在轨 On orbit | Joiner et al., |
| TANSO-FTS-1 | GOSAT | 2009-01 | 757-775 | 在轨 On orbit | Frankenberg et al., |
| OCO-2 | OCO-2 | 2014-07 | 757-775 | 在轨 On orbit | Frankenberg et al., |
| ACGS | TANSAT | 2016-12 | 758-778 | 在轨 On orbit | Du et al., |
| TROPOMI | Sentinel-5P | 2017-10 | 675-775 | 在轨 On orbit | Guanter et al., |
| TANSO-FTS-2 | GOSAT-2 | 2018-10 | 757-775 | 在轨 On orbit | Nakajima et al., |
| OCO-3 | OCO-3 | 2019-05 | 757-775 | 在轨 On orbit | Eldering et al., |
| - | TEMPO | 预计2020 Estimate 2020 | 650-740 | 计划发射 Planned launch | Zoogman et al., |
| - | GeoCARB | 预计2021 Estimate 2021 | 757-772 | 计划发射 Planned launch | O’Brienet al., |
| FLORIS | FLEX | 预计2023 Estimate 2023 | 650-780 | 计划发射 Planned launch | Drusch et al., |
表1 可用于星基日光诱导叶绿素荧光监测的卫星传感器
Table 1 Satellite-based sensors used for the retrieval of satellite-based sun-induced chlorophyll fluorescence
| 传感器 Sensor | 卫星 Satellite | 发射时间 Launch time | 波段 Band (nm) | 状态 State | 相关文献 Related literature |
|---|---|---|---|---|---|
| SCIAMACHY | ENVISAT | 2002-03 | 650-790 | 失联 Lost contact | Joiner et al., |
| GOME-2 | METOP | 2006-07 | 650-790 | 在轨 On orbit | Joiner et al., |
| TANSO-FTS-1 | GOSAT | 2009-01 | 757-775 | 在轨 On orbit | Frankenberg et al., |
| OCO-2 | OCO-2 | 2014-07 | 757-775 | 在轨 On orbit | Frankenberg et al., |
| ACGS | TANSAT | 2016-12 | 758-778 | 在轨 On orbit | Du et al., |
| TROPOMI | Sentinel-5P | 2017-10 | 675-775 | 在轨 On orbit | Guanter et al., |
| TANSO-FTS-2 | GOSAT-2 | 2018-10 | 757-775 | 在轨 On orbit | Nakajima et al., |
| OCO-3 | OCO-3 | 2019-05 | 757-775 | 在轨 On orbit | Eldering et al., |
| - | TEMPO | 预计2020 Estimate 2020 | 650-740 | 计划发射 Planned launch | Zoogman et al., |
| - | GeoCARB | 预计2021 Estimate 2021 | 757-772 | 计划发射 Planned launch | O’Brienet al., |
| FLORIS | FLEX | 预计2023 Estimate 2023 | 650-780 | 计划发射 Planned launch | Drusch et al., |
| 仪器设备 Equipment | 光谱仪 Spectrometer | 波段 Band (nm) | 光学分辨率 Optical resolution (nm) | 相关文献 Related literature |
|---|---|---|---|---|
| TriFLEX | HR2000+ | 630-815 | 0.50 | Daumard et al., |
| HR2000+ | 630-815 | 0.50 | ||
| HR2000+ | 300-900 | 2.00 | ||
| SpectroFLEX | HR2000+ | 630-820 | 0.20 | Fournier et al., |
| AutoSIF | QE65pro | 645-805 | 0.30 | Hu et al., |
| S-FluorBox | HR4000 | 700-800 | 0.10 | Cogliati et al., |
| HR4000 | 400-1 000 | 1.00 | ||
| SIF-SYS | STS-VIS | 337-823 | 3.00 | Burkart et al., |
| FluoSpec | HR2000+ | 680-775 | 0.13 | Yang et al., |
| FluoSpec2 | QEpro | 730-780 | 0.15 | Miao et al., |
| HR2000+ | 350-1 100 | 1.10 | ||
| PhotoSpec | QEpro1 | 670-732 | 0.30 | Grossmann et al., |
| QEpro2 | 729-784 | 0.30 | ||
| Flame | 177-874 | 1.20 | ||
| FLOX | QEpro | 650-800 | 0.30 | Wohlfahrt et al., |
| VIS-NIR | 400-950 | 1.50 | ||
| SIFSpec | QE65pro | 649-805 | 0.34 | Du et al., |
| SIFPrism | QEpro | 650-800 | 0.30 | Zhang et al., |
| FAME | QEpro | 730-786 | 0.15 | Gu et al., |
表2 近地面日光诱导叶绿素荧光连续观测仪器设备
Table 2 Instruments used for the ground-based continuous observation of sun-induced chlorophyll fluorescence
| 仪器设备 Equipment | 光谱仪 Spectrometer | 波段 Band (nm) | 光学分辨率 Optical resolution (nm) | 相关文献 Related literature |
|---|---|---|---|---|
| TriFLEX | HR2000+ | 630-815 | 0.50 | Daumard et al., |
| HR2000+ | 630-815 | 0.50 | ||
| HR2000+ | 300-900 | 2.00 | ||
| SpectroFLEX | HR2000+ | 630-820 | 0.20 | Fournier et al., |
| AutoSIF | QE65pro | 645-805 | 0.30 | Hu et al., |
| S-FluorBox | HR4000 | 700-800 | 0.10 | Cogliati et al., |
| HR4000 | 400-1 000 | 1.00 | ||
| SIF-SYS | STS-VIS | 337-823 | 3.00 | Burkart et al., |
| FluoSpec | HR2000+ | 680-775 | 0.13 | Yang et al., |
| FluoSpec2 | QEpro | 730-780 | 0.15 | Miao et al., |
| HR2000+ | 350-1 100 | 1.10 | ||
| PhotoSpec | QEpro1 | 670-732 | 0.30 | Grossmann et al., |
| QEpro2 | 729-784 | 0.30 | ||
| Flame | 177-874 | 1.20 | ||
| FLOX | QEpro | 650-800 | 0.30 | Wohlfahrt et al., |
| VIS-NIR | 400-950 | 1.50 | ||
| SIFSpec | QE65pro | 649-805 | 0.34 | Du et al., |
| SIFPrism | QEpro | 650-800 | 0.30 | Zhang et al., |
| FAME | QEpro | 730-786 | 0.15 | Gu et al., |
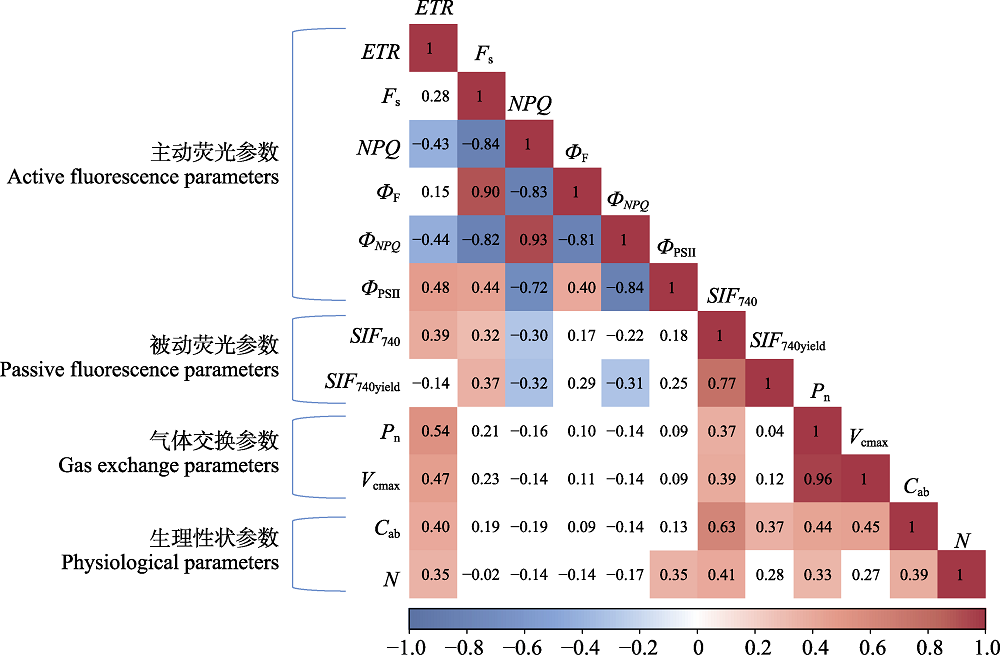
图1 水稻叶绿素荧光主动与被动联合观测耦合气体交换测定参数线性相关性矩阵。该图为野外实际观测数据。色块表示p < 0.05, 空白表示p > 0.05。Cab, 叶片叶绿素浓度; ETR, 表观光合电子传递速率; Fs, 稳态荧光值; N, 叶片氮含量; NPQ, 非光化学淬灭; Pn, 净光合速率; SIF740, 740 nm的日光诱导叶绿素荧光值; SIF740yield, 740 nm的日光诱导叶绿素荧光量子产率; Vcmax, 最大羧化速率; ФF, 荧光效率; ФNPQ, NPQ效率; ФPSII, 光化学效率。
Fig. 1 Linear correlation matrix for combined observation of actively and passively induced chlorophyll fluorescence coupled gas exchange measurement parameter in rice. The figure showed actual observation data in the field. Color black means p < 0.05, blank means p > 0.05. Cab, leaf chlorophyll concentration; ETR, apparent combined electron transfer rate; Fs, steady-state fluorescence value; N, leaf nitrogen content; NPQ, non-photochemical quenching; Pn, net photosynthetic rate; SIF740, value of sun-induced chlorophyll fluorescence at 740 nm; SIF740yield, quantum yield of sun-induced chlorophyll fluorescence at 740 nm; Vcmax, maximum carboxylation rate; ФF, fluorescence efficiency; ФNPQ, NPQ efficiency; ФPSII, photochemical efficiency.
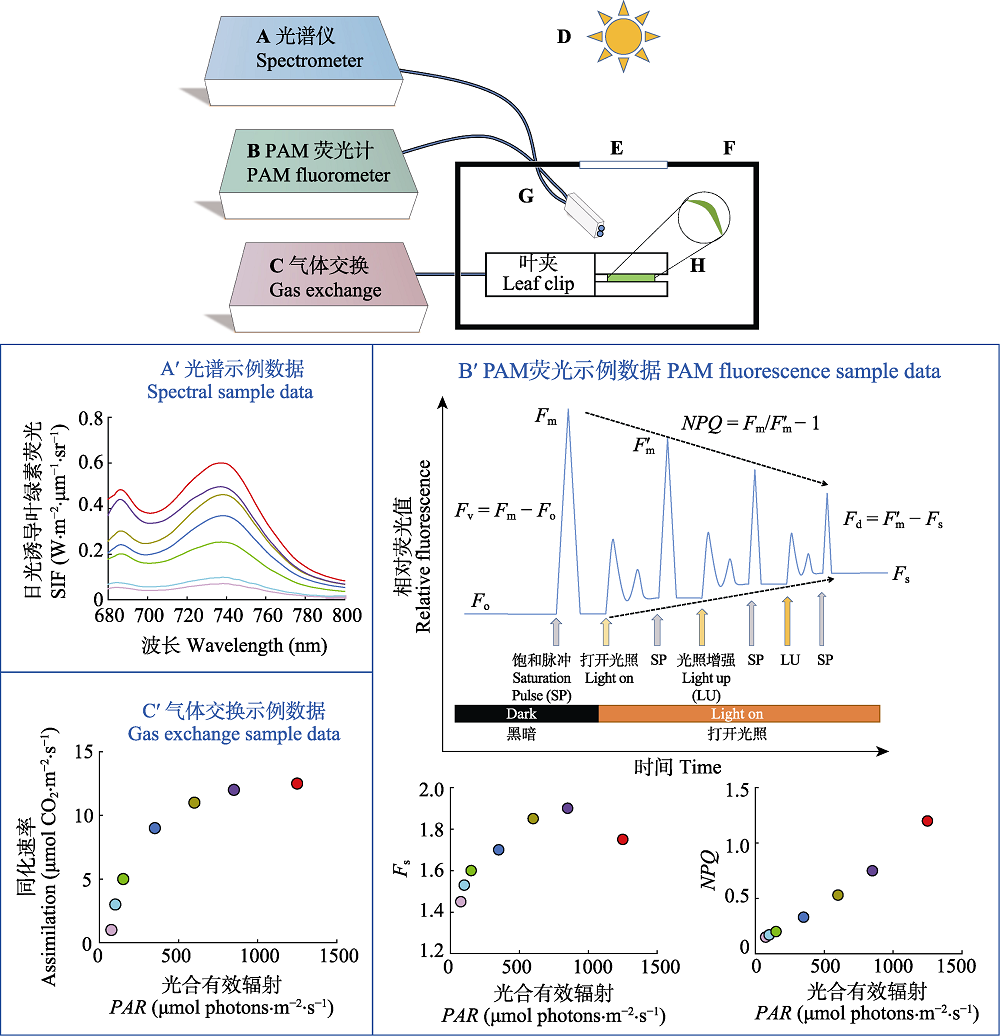
图2 叶绿素荧光主动与被动联合观测耦合气体交换测定系统及其示例数据。测定系统主要包括: 光谱仪(A); PAM荧光计(B); 气体交换(C); 入射光线(D); 进光口(E); 封闭的叶室(F); 光纤(G); 被测叶片(H)。Fd, 光适应下可变荧光值; Fm, 最大荧光值; Fm', 光适应下最大荧光值; Fo, 最小荧光值; Fs, 稳态荧光值; Fv, 最大可变荧光值; NPQ, 非光化学淬灭。
Fig. 2 Combined observation of actively and passively induced chlorophyll fluorescence coupled gas exchange measurement system and its sample data. The measurement system mainly includes: spectrometer (A); PAM fluorometer (B); gas exchange (C); incident light (D); light inlet (E); enclosed leaf chamber (F); optical fiber (G); leaves (H). Fd, variable fluorescence value under light adaptation; Fm, maximum fluorescence value; Fm', maximum fluorescence value under light adaptation; Fo, minimum fluorescence value; Fs, steady-state fluorescence value; Fv, maximum variable fluorescence value; NPQ, non-photochemical quenching; PAR, photosynthetically active radiation; SIF, sun-induced chlorophgll flhorescence.
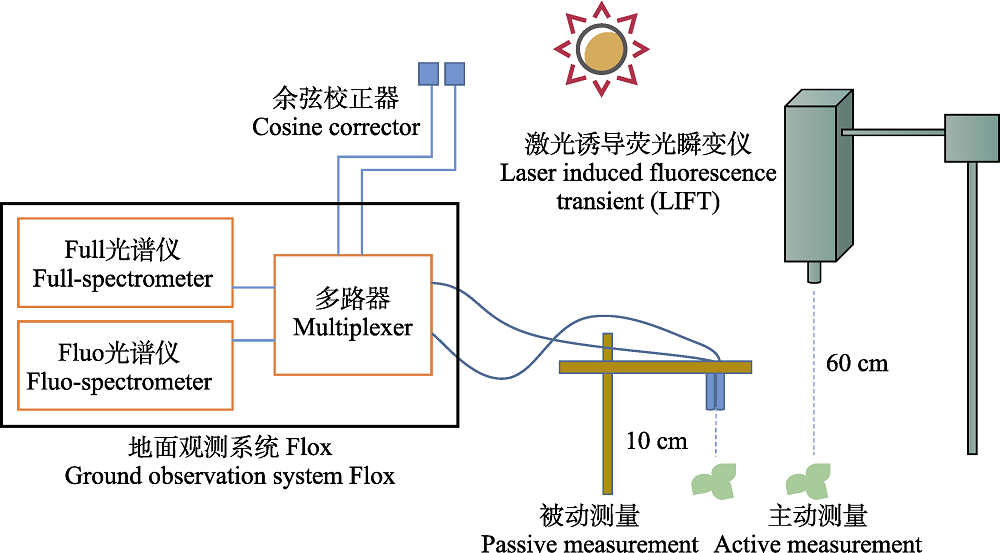
图3 利用LIFT技术及地面观测系统Flox搭建的主被动荧光联合观测系统(修改自Acebron (2020)的Fig. S2)。
Fig. 3 Combined observation system of actively and passively induced chlorophyll fluorescence built by the LIFT technology and ground observation system Flox (modified from Fig. S2 of Acebron (2020)).
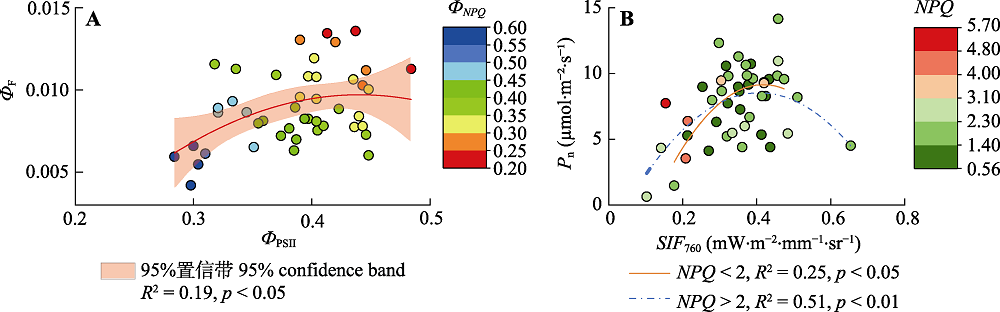
图4 水稻ФF和ФPSII在ФNPQ影响下的关系变化(A), Pn与SIF760在NPQ影响下的关系变化(B)。该图为野外实际观测数据。NPQ, 非光化学淬灭; Pn, 净光合速率; SIF760, 760 nm的日光诱导叶绿素荧光值; ФF, 荧光效率; ФNPQ, NPQ效率; ФPSII, 光化学效率。
Fig. 4 Relationship between ФF and ФPSII under the influence of ФNPQ(A), relationship change between Pn and SIF760 under the influence of NPQ (B) in rice. The figure showed actual observation data in the field. NPQ, non-photochemical quenching; Pn, net photosynthetic rate; SIF760, value of sun-induced chlorophyll fluorescence at 760 nm; ФF, fluorescence efficiency; ФNPQ, NPQ efficiency; ФPSII, photochemical efficiency.
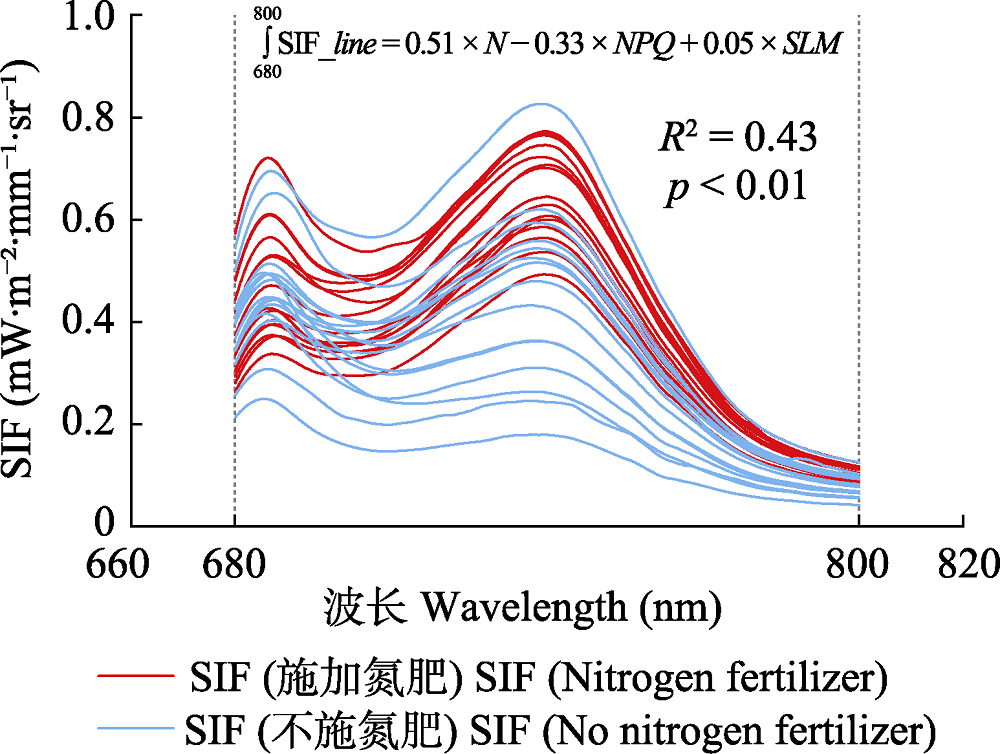
图5 水稻不同处理下叶片日光诱导叶绿素荧光光谱曲线。该图为野外实际观测数据。N, 叶片氮含量; NPQ, 非光化学淬灭; SIF_line, SIF光谱曲线; SLM, 比叶质量。
Fig. 5 Leaves spectral curve of sun-induced chlorophyll fluorescence (SIF) under different treatments of rice. The figure showed actual observation data in the field. N, leaf nitrogen content; NPQ, non-photochemical quenching; SIF_line, SIF spectral curve; SLM, specific leaf mass.
| [1] |
Aasen H, van Wittenberghe S, Medina NS, Damm A, Goulas Y, Wieneke S, Hueni A, Malenovský Z, Alonso L, Pacheco-Labrador J, Cendrero-Mateo MP, Tomelleri E, Burkart A, Cogliati S, Rascher U, Arthur AM (2019). Sun-induced chlorophyll fluorescence II: review of passive measurement setups, protocols, and their application at the leaf to canopy level. Remote Sensing, 11,927. DOI: 10.3390/rs11080927.
DOI URL |
| [2] |
Acebron K, Matsubara S, Jedmowski C, Emin D, Muller O, Rascher U (2020). Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar- induced fluorescence and reflectance measurements in the field. New Phytologist, 229, 4,2104-2119.
DOI URL |
| [3] |
Agati G, Foschi L, Grossi N, Guglielminetti L, Cerovic ZG, Volterrani M (2013). Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. European Journal of Agronomy, 45,39-51.
DOI URL |
| [4] |
Amoros-Lopez J, Gomez-Chova L, Vila-Frances J, Alonso L, Calpe J, Moreno J, del Valle-Tascon S (2008). Evaluation of remote sensing of vegetation fluorescence by the analysis of diurnal cycles. International Journal of Remote Sensing, 29,5423-5436.
DOI URL |
| [5] |
Araus JL, Sánchez C, Cabrera-Bosquet L (2010). Is heterosis in maize mediated through better water use? New Phytologist, 187,392-406.
DOI URL |
| [6] |
Atherton J, Liu W, Porcar-Castell A (2019). Nocturnal Light Emitting Diode Induced Fluorescence (LEDIF): a new technique to measure the chlorophyll a fluorescence emission spectral distribution of plant canopies in situ. Remote Sensing of Environment, 231,111137. DOI: 10.1016/j.rse.2019.03.030.
DOI URL |
| [7] |
Atherton J, Nichol CJ, Porcar-Castell A (2016). Using spectral chlorophyll fluorescence and the photochemical reflectance index to predict physiological dynamics. Remote Sensing of Environment, 176,17-30.
DOI URL |
| [8] |
Baker NR (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59,89-113.
DOI PMID |
| [9] |
Barranguet C, Kromkamp J (2000). Estimating primary production rates from photosynthetic electron transport in estuarine microphytobenthos. Marine Ecology Progress Series, 204,39-52.
DOI URL |
| [10] |
Burkart A, Schickling A, Mateo MPC, Wrobel TJ, Rossini M, Cogliati S, Julitta T, Rascher U (2015). A method for uncertainty assessment of passive sun-induced chlorophyll fluorescence retrieval using an infrared reference light. IEEE Sensors Journal, 15,4603-4611.
DOI URL |
| [11] |
Campbell P, Huemmrich K, Middleton E, Ward L, Julitta T, Daughtry C, Burkart A, Russ A, Kustas W (2019). Diurnal and seasonal variations in chlorophyll fluorescence associated with photosynthesis at leaf and canopy scales. Remote Sensing, 11,488. DOI: 10.3390/rs11050488.
DOI URL |
| [12] |
Cendrero-Mateo MP, Moran MS, Papuga SA, Thorp KR, Alonso L, Moreno J, Ponce-Campos G, Rascher U, Wang G (2016). Plant chlorophyll fluorescence: active and passive measurements at canopy and leaf scales with different nitrogen treatments. Journal of Experimental Botany, 67,275-286.
DOI PMID |
| [13] | Chen JM, Yu XP, Cheng JA (2006). The application of chlorophyll fluorescence kinetics in the study of physiological responses of plants to environmental stresses. Acta Agriculturae Zhejiangensis, 18,51-55. |
| [ 陈建明, 俞晓平, 程家安 (2006). 叶绿素荧光动力学及其在植物抗逆生理研究中的应用. 浙江农业学报, 18,51-55.] | |
| [14] |
Chen SG, Yang J, Zhang MS, Strasser RJ, Qiang S (2016). Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environmental and Experimental Botany, 122,126-140.
DOI URL |
| [15] |
Chen XJ, Mo XG, Hu S, Liu SX (2018). Relationship between fluorescence yield and photochemical yield under water stress and intermediate light conditions. Journal of Experimental Botany, 70,301-313.
DOI URL |
| [16] |
Cheng YB, Middleton E, Zhang QY, Huemmrich K, Campbell P, Corp L, Cook B, Kustas W, Daughtry C (2013). Integrating solar induced fluorescence and the photochemical reflectance index for estimating gross primary production in a cornfield. Remote Sensing, 5,6857-6879.
DOI URL |
| [17] |
Cogliati S, Verhoef W, Kraft S, Sabater N, Alonso L, Vicent J, Moreno J, Drusch M, Colombo R (2015). Retrieval of sun-induced fluorescence using advanced spectral fitting methods. Remote Sensing of Environment, 169,344-357.
DOI URL |
| [18] |
Consalvey M, Perkins RG, Paterson DM, Underwood GJC (2005). Pam fluorescence: a beginners guide for benthic diatomists. Diatom Research, 20,1-22.
DOI URL |
| [19] |
Cui TX, Sun R, Qiao C (2016). Assessing the factors determining the relationship between solar-induced chlorophyll fluorescence and GPP//IEEE. IEEE International Geoscience and Remote Sensing Symposium. IEEE, Beijing. DOI: 10.1109/IGARSS.2016.7729910.
DOI |
| [20] |
Damm A, Guanter L, Paul-Limoges E, van der Tol C, Hueni A, Buchmann N, Eugster W, Ammann C, Schaepman ME (2015). Far-red sun-induced chlorophyll fluorescence shows ecosystem-specific relationships to gross primary production: an assessment based on observational and modeling approaches. Remote Sensing of Environment, 166,91-105.
DOI URL |
| [21] |
Daumard F, Champagne S, Fournier A, Goulas Y, Ounis A, Hanocq JF, Moya I (2010). A field platform for continuous measurement of canopy fluorescence. IEEE Transactions on Geoscience and Remote Sensing, 48,3358-3368.
DOI URL |
| [22] |
Drusch M, Moreno J, del Bello U, Franco R, Goulas Y, Huth A, Kraft S, Middleton EM, Miglietta F, Mohammed G, Nedbal L, Rascher U, Schuttemeyer D, Verhoef W (2016). The FLuorescence EXplorer mission concept—ESA’s earth explorer 8. IEEE Transactions on Geoscience and Remote Sensing, 55,1273-1284.
DOI URL |
| [23] |
Du SS, Liu LY, Liu XJ, Guo J, Hu JC, Wang SQ, Zhang YG (2019). SIFSpec: measuring solar-induced chlorophyll fluorescence observations for remote sensing of photosynthesis. Sensors, 19,3009. DOI: 10.3390/s19133009.
DOI URL |
| [24] |
Du SS, Liu LY, Liu XJ, Zhang X, Zhang XY, Bi YM, Zhang LC (2018). Retrieval of global terrestrial solar-induced chlorophyll fluorescence from TanSat satellite. Science Bulletin, 63,1502-1512.
DOI URL |
| [25] |
Eldering A, Taylor TE, O’dell CW, Pavlick R (2019). The OCO-3 mission: measurement objectives and expected performance based on 1 year of simulated data. Atmospheric Measurement Techniques, 12,2341-2370.
DOI URL |
| [26] |
Faraloni C, Cutino I, Petruccelli R, Leva AR, Lazzeri S, Torzillo G (2011). Chlorophyll fluorescence technique as a rapid tool for in vitro screening of olive cultivars ( Olea europaea L.) tolerant to drought stress. Environmental and Experimental Botany, 73,49-56.
DOI URL |
| [27] |
Farooq S, Chmeliov J, Wientjes E, Koehorst R, Bader A, Valkunas L, Trinkunas G, van Amerongen H (2018). Dynamic feedback of the photosystem II reaction centre on photoprotection in plants. Nature Plants, 4,225-231.
DOI URL |
| [28] |
Flexas J, Medrano H (2002). Energy dissipation in C3 plants under drought. Functional Plant Biology, 29,1209-1215.
DOI URL |
| [29] |
Fournier A, Daumard F, Champagne S, Ounis A, Goulas Y, Moya I (2012). Effect of canopy structure on sun-induced chlorophyll fluorescence. ISPRS Journal of Photogrammetry and Remote Sensing, 68,112-120.
DOI URL |
| [30] |
Franck F, Dewez D, Popovic R (2005). Changes in the room-temperature emission spectrum of chlorophyll during fast and slow phases of the Kautsky effect in intact leaves. Photochemistry and Photobiology, 81,431-436.
DOI URL |
| [31] | Franck F, Juneau P, Popovic R (2002). Resolution of the photosystem I and photosystem II contributions to chlorophyll fluorescence of intact leaves at room temperature. Biochimica et Biophysica Acta (BBA): Bioenergetics, 1556,239-246. |
| [32] |
Frankenbach S, Ezequiel J, Plecha S, Goessling JW, Vaz L, Kühl M, Dias JM, Vaz N, Serôdio J (2020). Synoptic spatio- temporal variability of the photosynthetic productivity of microphytobenthos and phytoplankton in a tidal estuary. Frontiers in Marine Science, 7,170. DOI: 10.3389/fmars.2020.00170.
DOI URL |
| [33] |
Frankenberg C, Fisher JB, Worden J, Badgley G, Saatchi SS, Lee JE, Toon GC, Butz A, Jung M, Kuze A, Yokota T (2011). New global observations of the terrestrial carbon cycle from GOSAT: patterns of plant fluorescence with gross primary productivity. Geophysical Research Letters, 38,L17706. DOI: 10.1029/2011GL048738.
DOI |
| [34] |
Frankenberg C, O’dell C, Berry J, Guanter L, Joiner J, Köhler P, Pollock R, Taylor TE (2014). Prospects for chlorophyll fluorescence remote sensing from the Orbiting Carbon Observatory-2. Remote Sensing of Environment, 147,1-12.
DOI URL |
| [35] |
Goulas Y, Fournier A, Daumard F, Champagne S, Ounis A, Marloie O, Moya I (2017). Gross primary production of a wheat canopy relates stronger tofar red than to red solar- induced chlorophyll fluorescence. Remote Sensing, 9,97. DOI: 10.3390/rs9010097.
DOI URL |
| [36] |
Grossmann K, Frankenberg C, Magney TS, Hurlock SC, Seibt U, Stutz J (2018). PhotoSpec: a new instrument to measure spatially distributed red and far-red solar-induced chlorophyll fluorescence. Remote Sensing of Environment, 216,311-327.
DOI URL |
| [37] |
Gu L, Wood JD, Chang CYY, Sun Y, Riggs JS (2018). Advancing terrestrial ecosystem science with a novel automated measurement system for sun-induced chlorophyll fluorescence for integration with eddy covariance flux networks. Journal of Geophysical Research: Biogeosciences, 124,127-146.
DOI URL |
| [38] |
Guanter L, Aben I, Tol P, Krijger JM, Hollstein A, Köhler P, Damm A, Joiner J, Frankenberg C, Landgraf J (2015). Potential of the TROPOspheric Monitoring Instrument (TROPOMI) onboard the Sentinel-5 Precursor for the monitoring of terrestrial chlorophyll fluorescence. Atmospheric Measurement Techniques, 8,1337-1352.
DOI URL |
| [39] |
Guanter L, Alonso L, Gómez-Chova L, Meroni M, Preusker R, Fischer J, Moreno J (2010). Developments for vegetation fluorescence retrieval from spaceborne high-resolution spectrometry in the O2-A and O2-B absorption bands. Journal of Geophysical Research: Atmospheres, 115,D19303. DOI: 10.1029/2009JD013716.
DOI URL |
| [40] |
Guanter L, Frankenberg C, Dudhia A, Lewis PE, Gómez-Dans J, Kuze A, Suto H, Grainger RG (2012). Retrieval and global assessment of terrestrial chlorophyll fluorescence from GOSAT space measurements. Remote Sensing of Environment, 121,236-251.
DOI URL |
| [41] | Guanter L, Zhang Y, Jung M, Joiner J, Voigt M, Berry JA, Frankenberg C, Huete AR, Zarco-Tejada P, Lee JE, Moran MS, Ponce-Campos G, Beer C, Camps-Valls G, Buchmann N, Gianelle D, Klumpp K, Cescatti A, Baker JM, Griffis TJ (2014). Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proceedings of the National Academy of Sciences of the United States of American, 111,1327-1333. |
| [42] |
Hasegawa M, Shiina T, Terazima M, Kumazaki S (2010). Selective excitation of photosystems in chloroplasts inside plant leaves observed by near-infrared laser-based fluorescence spectral microscopy. Plant and Cell Physiology, 51,225-238.
DOI URL |
| [43] |
Hendrickson L, Furbank RT, Chow WS (2004). A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynthesis Research, 82,73-81.
PMID |
| [44] | Hmimina G, Dufrêne E, Soudani K (2014). Relationship between photochemical reflectance index and leaf ecophysiological and biochemical parameters under two different water statuses: towards a rapid and efficient correction method using real-time measurements. Plant, Cell & Environment, 37,473-487. |
| [45] |
Hu JC, Liu LY, Guo J, Du SS, Liu XJ (2018). Upscaling solar- induced chlorophyll fluorescence from an instantaneous to daily scale gives an improved estimation of the gross primary productivity. Remote Sensing, 10, 1663. DOI: 10.3390/rs10101663.
DOI |
| [46] |
Huang D, Knyazikhin Y, Dickinson RE, Rautiainen M, Stenberg P, Disney M, Lewis P, Cescatti A, Tian YH, Verhoef W, Martonchik JV, Myneni RB (2007). Canopy spectral invariants for remote sensing and model applications. Remote Sensing of Environment, 106,106-122.
DOI URL |
| [47] | Ji MH, Tang BH, Li ZL (2019). Review of solar-induced chlorophyll fluorescence retrieval methods from satellite data. Remote Sensing Technology and Application, 34,455-466. |
| [ 纪梦豪, 唐伯惠, 李召良 (2019). 太阳诱导叶绿素荧光的卫星遥感反演方法研究进展. 遥感技术与应用, 34,455-466.] | |
| [48] |
Joiner J, Guanter L, Lindstrot R, Voigt M, Vasilkov AP, Middleton EM, Huemmrich KF, Yoshida Y, Frankenberg C (2013). Global monitoring of terrestrial chlorophyll fluorescence from moderate-spectral-resolution near-infrared satellite measurements: methodology, simulations, and application to GOME-2. Atmospheric Measurement Techniques, 6,2803-2823.
DOI URL |
| [49] |
Joiner J, Yoshida Y, Vasilkov AP, Yoshida Y, Corp LA, Middleton EM (2011). First observations of global and seasonal terrestrial chlorophyll fluorescence from space. Biogeosciences, 8,637-651.
DOI URL |
| [50] |
Kolber Z, Klimov D, Ananyev G, Rascher U, Berry J, Osmond B (2005). Measuring photosynthetic parameters at a distance: laser induced fluorescence transient (LIFT) method for remote measurements of photosynthesis in terrestrial vegetation. Photosynthesis Research, 84,121-129.
DOI URL |
| [51] |
Konanz S, Kocsányi L, Buschmann C (2014). Advanced multi-color fluorescence imaging system for detection of biotic and abiotic stresses in leaves. Agriculture, 4,79-95.
DOI URL |
| [52] |
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004). New fluorescence parameters for the determination of QARedox state and excitation energy fluxes. Photosynthesis Research, 79,209-218.
DOI URL |
| [53] |
Kromdijk J, Glowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016). Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science, 354,857-861.
DOI URL |
| [54] |
Lambrev PH, Nilkens M, Miloslavina Y, Jahns P, Holzwarth AR (2010). Kinetic and spectral resolution of multiple nonphotochemical quenching components in Arabidopsis leaves. Plant Physiology, 152,1611-1624.
DOI PMID |
| [55] |
Lee JE, Berry JA, van der Tol C, Yang X, Guanter L, Damm A, Baker I, Frankenberg C (2015). Simulations of chlorophyll fluorescence incorporated into the Community Land Model version 4. Global Change Biology, 21,3469-3477.
DOI URL |
| [56] |
Lee JE, Frankenberg C, van der Tol C, Berry JA, Guanter L, Boyce CK, Fisher JB, Morrow E, Worden JR, Asefi S, Badgley G, Saatchi S (2013). Forest productivity and water stress in Amazonia: observations from GOSAT chlorophyll fluorescence. Proceedings of The Royal Society B: Biological Sciences, 280,20130171. DOI: 10.1098/rspb. 2013.0171.
DOI URL |
| [57] | Li QF, Li ZM, Ji JW, Zou QY, Yu H (2013). Applications of chlorophyll fluorescence kinetics in the physiological resistance studies of plant. Hubei Agricultural Sciences, 52,5399-5402. |
| [ 李钦夫, 李征明, 纪建伟, 邹秋滢, 于辉 (2013). 叶绿素荧光动力学及在植物抗逆生理研究中的应用. 湖北农业科学, 52,5399-5402.] | |
| [58] | Li SL, Gao MF, Li ZL, Li FJ, Gao Y, Liao QY (2018). Retrieval of chlorophyll fluorescence from Tansat in Northeast China. China Agricultural Informatics, (6),53-62. |
| [ 李石磊, 高懋芳, 李召良, 李方杰, 高雅, 廖前瑜 (2018). 基于碳卫星的中国东北地区叶绿素荧光反演. 中国农业信息, (6),53-62.] | |
| [59] | Liang Y, Li JY, Zhang YW (2013). Research advances of the remote sensing of solar-induced chlorophyll fluorescence. Chinese Agricultural Science Bulletin, 29,107-112. |
| [ 梁寅, 李军营, 张云伟 (2013). 日光诱导叶绿素荧光遥感探测的研究进展. 中国农学通报, 29,107-112.] | |
| [60] |
Liu LY, Guan LL, Liu XJ (2017). Directly estimating diurnal changes in GPP for C3 and C4 crops using far-red sun-induced chlorophyll fluorescence. Agricultural and Forest Meteorology, 232,1-9.
DOI URL |
| [61] |
Liu XJ, Liu LY, Hu JC, Guo J, Du SS (2020). Improving the potential of red SIF for estimating GPP by downscaling from the canopy level to the photosystem level. Agricultural and Forest Meteorology, 281,107846. DOI: 10.1016/j.agrformet.2019.107846.
DOI URL |
| [62] | Magney TS, Bowling DR, Logan BA, Grossmann K, Stutz J, Blanken PD, Burns SP, Cheng R, Garcia MA, Kӧhler P, Lopez S, Parazoo NC, Raczka B, Schimel D, Frankenberg C (2019a). Mechanistic evidence for tracking the seasonality of photosynthesis with solar-induced fluorescence. Proceedings of the National Academy of Sciences of the United States of American, 116,11640-11645. |
| [63] | Magney TS, Frankenberg C, Fisher JB, Sun Y, North GB, Davis TS, Kornfeld A, Siebke K (2017). Connecting active to passive fluorescence with photosynthesis :a method for evaluating remote sensing measurements of Chl fluorescence. New Phytologist, 215,1594-1608. |
| [64] |
Magney TS, Frankenberg C, Köhler P, North G, Davis TS, Dold C, Dutta D, Fisher JB, Grossmann K, Harrington A, Hatfield J, Stutz J, Sun Y, Porcar-Castell A (2019b). Disentangling changes in the spectral shape of chlorophyll fluorescence: implications for remote sensing of photosynthesis. Journal of Geophysical Research: Biogeosciences, 124,1491-1507.
DOI URL |
| [65] |
Maguire AJ, Eitel JUH, Griffin KL, Magney TS, Long RA, Vierling LA, Schmiege SC, Jennewein JS, Weygint WA, Boelman NT, Bruner SG (2020). On the functional relationship between fluorescence and photochemical yields in complex evergreen needleleaf canopies. Geophysical Research Letters, 47, e2020GL087858. DOI: 10.1029/2020GL087858.
DOI |
| [66] |
Marrs JK, Reblin JS, Logan BA, Allen DW, Reinmann AB, Bombard DM, Tabachnik D, Hutyra LR (2020). Solar- induced fluorescence does not track photosynthetic carbon assimilation following induced stomatal closure. Geophysical Research Letters, 47, e2020GL087956. DOI: 10.1111/nph.16984.
DOI |
| [67] |
Maxwell K, Johnson GN (2000). Chlorophyll fluorescence—A practical guide. Journal of Experimental Botany, 51,659-668.
DOI URL |
| [68] | McMurtrey JE, Middleton EM, Corp LA, Entcheva Campbell PK, Butcher LM, Chappelle EW, Cook WB (2002). Fluorescence responses from nitrogen plant stress in 4 Fraunhofer band regions. IEEE International Geoscience and Remote Sensing Symposium, 3,1538-1540. |
| [69] |
Meroni M, Rossini M, Guanter L, Alonso L, Rascher U, Colombo R, Moreno J (2009). Remote sensing of solar- induced chlorophyll fluorescence: review of methods and applications. Remote Sensing of Environment, 113,2037-2051.
DOI URL |
| [70] |
Miao GF, Guan KY, Yang X, Bernacchi CJ, Berry JA, DeLucia EH, Wu J, Moore CE, Meacham K, Cai YP, Peng B, Kimm H, Masters MD (2018). Sun-induced chlorophyll fluorescence, photosynthesis, and light use efficiency of a soybean field from seasonally continuous measurements. Journal of Geophysical Research: Biogeosciences, 123,610-623.
DOI URL |
| [71] |
Middleton E, Rascher U, Corp L, Huemmrich K, Cook B, Noormets A, Schickling A, Pinto F, Alonso L, Damm A, Guanter L, Colombo R, Campbell P, Landis D, Zhang QY, Rossini M, Schuettemeyer D, Bianchi RM (2017). The 2013 FLEX—US airborne campaign at the parker tract loblolly pine plantation in North Carolina, USA. Remote Sensing, 9,612. DOI: 10.3390/rs9060612.
DOI URL |
| [72] |
Mohammed GH, Colombo R, Middleton EM, Rascher U, van der Tol C, Nedbal L, Goulas Y, Pérez-Priego O, Damm A, Meroni M, Joiner J, Cogliati S, Verhoef W, Malenovský Z, Gastellu-Etchegorry JP, et al. (2019). Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress. Remote Sensing of Environment, 231,111177. DOI: 10.1016/j.rse.2019.04.030.
DOI URL |
| [73] |
Murchie EH, Lawson T (2013). Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. Journal of Experimental Botany, 64,3983-3998.
DOI PMID |
| [74] |
Nakajima M, Suto H, Yotsumoto K, Shiomi K, Hirabayashi T (2017). Fourier transform spectrometer on GOSAT and GOSAT-2//Sodniks, CugnyB, KarafolasN. Proc SPIE 10563, International Conference on Space Optics—ICSO 2014. SPIE, Canary Islans, Spain. DOI: 10.1117/12.2304062.
DOI |
| [75] |
O’Brien DM, Polonsky IN, Utembe SR, Rayner PJ (2016). Potential of a geostationary GeoCARB mission to estimate surface emissions of CO2, CH4 and CO in a polluted urban environment: case study Shanghai. Atmospheric Measurement Techniques, 9,4633-4654.
DOI URL |
| [76] | Pinto F, Damm A, Schickling A, Panigada C, Cogliati S, Müller-Linow M, Balvora A, Rascher U (2016). Sun- induced chlorophyll fluorescence from high-resolution imaging spectroscopy data to quantify spatio-temporal patterns of photosynthetic function in crop canopies. Plant, Cell & Environment, 39,1500-1512. |
| [77] |
Plascyk JA, Gabriel FC (1975). The Fraunhofer line discriminator MKII—An airborne instrument for precise and standardized ecological luminescence measurement. IEEE Transactions on Instrumentation and Measurement, 24,306-313.
DOI URL |
| [78] | Porcar-Castell A, Mac Arthur A, Rossini M, Eklundh L, Pacheco-Labrador J, Anderson K, Balzarolo M, Martín MP, Jin H, Tomelleri E, Cerasoli S, Sakowska K, Hueni A, Julitta T, Nichol CJ, Vescovo L (2015). EUROSPEC: at the interface between remote sensing and ecosystem CO2 flux measurements in Europe. Biogeosciences Discussions, 12,13069-13121. |
| [79] |
Porcar-Castell A, Tyystjärvi E, Atherton J, van der Tol C, Flexas J, Pfündel EE, Moreno J, Frankenberg C, Berry JA (2014). Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: mechanisms and challenges. Journal of Experimental Botany, 65,4065-4095.
DOI PMID |
| [80] |
Raczka B, Porcar-Castell A, Magney T, Lee JE, Köhler P, Frankenberg C, Grossmann K, Logan BA, Stutz J, Blanken PD, Burns SP, Duarte H, Yang X, Lin JC, Bowling DR (2019). Sustained nonphotochemical quenching shapes the seasonal pattern of solar-induced fluorescence at a high-elevation evergreen forest. Journal of Geophysical Research: Biogeosciences, 124,2005-2020.
DOI URL |
| [81] |
Rahimzadeh-Bajgiran P, Tubuxin B, Omasa K (2017). Estimating chlorophyll fluorescence parameters using the joint Fraunhofer line depth and laser-induced saturation pulse (FLD-LISP) method in different plant species. Remote Sensing, 9,599. DOI: 10.3390/rs9060599.
DOI URL |
| [82] |
Ryu Y, Berry JA, Baldocchi DD (2019). What is global photosynthesis? History, uncertainties and opportunities. Remote Sensing of Environment, 223,95-114.
DOI URL |
| [83] |
Stirbet A, Govindjee (2011). On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B: Biology, 104,236-257.
DOI URL |
| [84] |
Sun Y, Frankenberg C, Jung M, Joiner J, Guanter L, Köhler P, Magney T (2018). Overview of solar-induced chlorophyll fluorescence (SIF) from the Orbiting Carbon Observatory-2: retrieval, cross-mission comparison, and global monitoring for GPP. Remote Sensing of Environment, 209,808-823.
DOI URL |
| [85] |
Sun Y, Frankenberg C, Wood JD, Schimel DS, Jung M, Guanter L, Drewry DT, Verma M, Porcar-Castell A, Griffis TJ, Gu L, Magney TS, Köhler P, Evans B, Yuen K (2017). OCO-2 advances photosynthesis observation from space via solar-induced chlorophyll fluorescence. Science,358, eaam5747. DOI: 10.1126/science.aam5747.
DOI |
| [86] |
van der Tol C, Berry JA, Campbell PKE, Rascher U (2014). Models of fluorescence and photosynthesis for interpreting measurements of solar-induced chlorophyll fluorescence. Journal of Geophysical Research: Biogeosciences, 119,2312-2327.
DOI URL |
| [87] |
van der Tol C, Vilfan N, Yang PQ, Bayat B, Verhoef W (2018). Modeling reflectance, fluorescence and photosynthesis: development of the SCOPE model//IEEE. IEEE International Geoscience and Remote Sensing Symposium. IEEE, Beijing. DOI: 10.1109/IGARSS.2018.8517517.
DOI |
| [88] |
Verma M, Schimel D, Evans B, Frankenberg C, Beringer J, Drewry DT, Magney T, Marang I, Hutley L, Moore C, Eldering A (2017). Effect of environmental conditions on the relationship between solar-induced fluorescence and gross primary productivity at an OzFlux grassland site. Journal of Geophysical Research: Biogeosciences, 122,716-733.
DOI URL |
| [89] |
Verrelst J, van der Tol C, Magnani F, Sabater N, Rivera JP, Mohammed G, Moreno J (2016). Evaluating the predictive power of sun-induced chlorophyll fluorescence to estimate net photosynthesis of vegetation canopies: a SCOPE modeling study. Remote Sensing of Environment, 176,139-151.
DOI URL |
| [90] |
Vilfan N, van der Tol C, Muller O, Rascher U, Verhoef W (2016). Fluspect-B: a model for leaf fluorescence, reflectance and transmittance spectra. Remote Sensing of Environment, 186,596-615.
DOI URL |
| [91] |
Vilfan N, van der Tol C, Verhoef W (2019). Estimating photosynthetic capacity from leaf reflectance and Chl fluorescence by coupling radiative transfer to a model for photosynthesis. New Phytologist, 223,487-500.
DOI URL |
| [92] | Wang R, Liu ZG, Yang PQ (2012). Principle and progress in remote sensing of vegetation solar-induced chlorophyll fluorescence. Advances in Earth Science, 27,1221-1228. |
| [ 王冉, 刘志刚, 杨沛琦 (2012). 植物日光诱导叶绿素荧光的遥感原理及研究进展. 地球科学进展, 27,1221-1228.] | |
| [93] |
Wang SH, Zhang LF, Huang CP, Qiao N (2017). Ground-based long-term remote sensing of solar-induced chlorophyll fluorescence: methods, challenges and opportunities. IEEE International Geoscience and Remote Sensing Symposium. DOI: 10.1109/IGARSS.2017.8127845.
DOI |
| [94] |
Wohlfahrt G, Gerdel K, Migliavacca M, Rotenberg E, Tatarinov F, Müller J, Hammerle A, Julitta T, Spielmann FM, Yakir D (2018). Sun-induced fluorescence and gross primary productivity during a heat wave. Scientific Reports, 8,14169. DOI: 10.1038/s41598-018-32602-z.
DOI PMID |
| [95] |
Wolanin A, Rozanov VV, Dinter T, Noël S, Vountas M, Burrows JP, Bracher A (2015). Global retrieval of marine and terrestrial chlorophyll fluorescence at its red peak using hyperspectral top of atmosphere radiance measurements: feasibility study and first results. Remote Sensing of Environment, 166,243-261.
DOI URL |
| [96] |
Wyber R, Malenovský Z, Ashcroft M, Osmond B, Robinson S (2017). Do daily and seasonal trends in leaf solar induced fluorescence reflect changes in photosynthesis, growth or light exposure? Remote Sensing, 9,604. DOI: 10.3390/rs9060604.
DOI URL |
| [97] |
Yang KG, Ryu Y, Dechant B, Berry JA, Hwang Y, Jiang CY, Kang M, Kim J, Kimm H, Kornfeld A, Yang X (2018). Sun-induced chlorophyll fluorescence is more strongly related to absorbed light than to photosynthesis at half-hourly resolution in a rice paddy. Remote Sensing of Environment, 216,658-673.
DOI URL |
| [98] |
Yang X, Tang JW, Mustard JF, Lee JE, Rossini M, Joiner J, Munger JW, Kornfeld A, Richardson AD (2015). Solar- induced chlorophyll fluorescence that correlates with canopy photosynthesis on diurnal and seasonal scales in a temperate deciduous forest. Geophysical Research Letters, 42,2977-2987.
DOI URL |
| [99] | You X, Gong JR (2012). Significance and application of chlorophyll fluorescence dynamics process parameters. Journal of West China Forestry Science, 41,90-94. |
| [ 尤鑫, 龚吉蕊 (2012). 叶绿素荧光动力学参数的意义及实例辨析. 西部林业科学, 41,90-94.] | |
| [100] |
Zarco-Tejada PJ, González-Dugo MV, Fereres E (2016). Seasonal stability of chlorophyll fluorescence quantified from airborne hyperspectral imagery as an indicator of net photosynthesis in the context of precision agriculture. Remote Sensing of Environment, 179,89-103.
DOI URL |
| [101] |
Zarco-Tejada PJ, Morales A, Testi L, Villalobos FJ (2013). Spatio-temporal patterns of chlorophyll fluorescence and physiological and structural indices acquired from hyperspectral imagery as compared with carbon fluxes measured with eddy covariance. Remote Sensing of Environment, 133,102-115.
DOI URL |
| [102] |
Zeng YL, Badgley G, Dechant B, Ryu Y, Chen M, Berry JA (2019). A practical approach for estimating the escape ratio of near-infrared solar-induced chlorophyll fluorescence. Remote Sensing of Environment, 232,111209. DOI: 10.1016/j.rse.2019.05.028.
DOI URL |
| [103] |
Zhang YG, Zhang Q, Liu LY, Wang SQ, Ju WM, Tang JW, Huang Y, Zhu XD, Wang F, Zhang JS, Zhou GS, Zhou L, Tang XG, Zhang ZY, Qiu B, Zhang XK, Wang SH (2020). ChinaSpec: a network for long-term in situ measurements of solar-induced fluorescence and reflectance in China. Earth and Space Science Open Archive, 30. DOI: 10.1002/essoar.10501911.1.
DOI |
| [104] | Zhang YJ, Liu LY, Hou MY, Liu LT, Li CD (2009). Progress in remote sensing of vegetation chlorophyll fluorescence. Journal of Remote Sensing, 13,963-978. |
| [ 张永江, 刘良云, 侯名语, 刘连涛, 李存东 (2009). 植物叶绿素荧光遥感研究进展. 遥感学报, 13,963-978.] | |
| [105] |
Zhang ZY, Chen JM, Guanter L, He LM, Zhang YG (2019). From canopy-leaving to total canopy far-red fluorescence emission for remote sensing of photosynthesis: first results from TROPOMI. Geophysical Research Letters, 46,12030-12040.
DOI URL |
| [106] | Zhang ZY, Wang SH, Qiu B, Song L, Zhang YG (2019). Retrieval of sun-induced chlorophyll fluorescence and advancements in carbon cycle application. Journal of Remote Sensing, 23,37-52. |
| [ 章钊颖, 王松寒, 邱博, 宋练, 张永光 (2019). 日光诱导叶绿素荧光遥感反演及碳循环应用进展. 遥感学报, 23,37-52.] | |
| [107] |
Zhao F, Guo YQ, Verhoef W, Gu XF, Liu LY, Yang GJ (2014). A method to reconstruct the solar-induced canopy fluorescence spectrum from hyperspectral measurements. Remote Sensing, 6,10171-10192.
DOI URL |
| [108] |
Zoogman P, Liu X, Suleiman RM, Pennington WF, Flittner DE, Al-Saadi JA, Hilton BB, Nicks DK, Newchurch MJ, Carr JL, Janz SJ, Andraschko MR, Arola A, Baker BD, Canova BP, et al. (2017). Tropospheric emissions: monitoring of pollution (TEMPO). Journal of Quantitative Spectroscopy and Radiative Transfer, 186,17-39.
DOI PMID |
| [1] | 杨宇萌, 来全, 刘心怡. 气候变化和人类活动对内蒙古植被总初级生产力的定量影响[J]. 植物生态学报, 2024, 48(3): 306-316. |
| [2] | 刘沛荣, 同小娟, 孟平, 张劲松, 张静茹, 于裴洋, 周宇. 散射辐射对中国东部典型人工林总初级生产力的影响[J]. 植物生态学报, 2022, 46(8): 904-918. |
| [3] | 原媛, 母艳梅, 邓钰洁, 李鑫豪, 姜晓燕, 高圣杰, 查天山, 贾昕. 植被覆盖度和物候变化对典型黑沙蒿灌丛生态系统总初级生产力的影响[J]. 植物生态学报, 2022, 46(2): 162-175. |
| [4] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| [5] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [6] | 黄松宇, 贾昕, 郑甲佳, 杨睿智, 牟钰, 袁和第. 中国典型陆地生态系统波文比特征及影响因素[J]. 植物生态学报, 2021, 45(2): 119-130. |
| [7] | 彭书时, 岳超, 常锦峰. 陆地生物圈模型的发展与应用[J]. 植物生态学报, 2020, 44(4): 436-448. |
| [8] | 冯朝阳, 王鹤松, 孙建新. 中国北方植被水分利用效率的时间变化特征及其影响因子[J]. 植物生态学报, 2018, 42(4): 453-465. |
| [9] | 张素彦, 蒋红志, 王扬, 张艳杰, 鲁顺保, 白永飞. 凋落物去除和添加处理对典型草原生态系统碳通量的影响[J]. 植物生态学报, 2018, 42(3): 349-360. |
| [10] | 靳宇曦, 刘芳, 张军, 韩梦琪, 王忠武, 屈志强, 韩国栋. 不同载畜率处理下短花针茅荒漠草原生态系统净碳交换特征[J]. 植物生态学报, 2018, 42(3): 361-371. |
| [11] | 刘晓, 戚超, 闫艺兰, 袁国富. 不同生态系统水分利用效率指标在黄土高原半干旱草地应用的适宜性评价[J]. 植物生态学报, 2017, 41(5): 497-505. |
| [12] | 王克清, 王鹤松, 孙建新. 遥感GPP模型在中国地区多站点的应用与比较[J]. 植物生态学报, 2017, 41(3): 337-347. |
| [13] | 闫敏, 李增元, 田昕, 陈尔学, 谷成燕. 黑河上游植被总初级生产力遥感估算及其对气候变化的响应[J]. 植物生态学报, 2016, 40(1): 1-12. |
| [14] | 谭正洪, 于贵瑞, 周国逸, 韩士杰, 夏禹九, 前田高尚, 小杉绿子, 山野井克己, 李胜功, 太田岳史, 平田竜一, 安田幸生, 中野隆志, 小南裕志, 北村兼三, 溝口康子, 廖志勇, 赵俊福, 杨廉雁. 亚洲东部森林的小气候特征: 1. 辐射和能量的平衡[J]. 植物生态学报, 2015, 39(6): 541-553. |
| [15] | 李登秋, 周艳莲, 居为民, 王辉民, 柳艺博, 吴小翠. 太阳辐射变化对亚热带人工常绿针叶林总初级生产力影响的模拟分析[J]. 植物生态学报, 2014, 38(3): 219-230. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19