

植物生态学报 ›› 2011, Vol. 35 ›› Issue (9): 965-972.DOI: 10.3724/SP.J.1258.2011.00965
时鹏1, 王淑平1,*( ), 贾书刚2, 高强3, 孙晓强4
), 贾书刚2, 高强3, 孙晓强4
收稿日期:2011-01-24
接受日期:2011-06-28
出版日期:2011-01-24
发布日期:2011-09-01
通讯作者:
王淑平
作者简介:*(E-mail:wshuping@gucas.ac.cn)
SHI Peng1, WANG Shu-Ping1,*( ), JIA Shu-Gang2, GAO Qiang3, SUN Xiao-Qiang4
), JIA Shu-Gang2, GAO Qiang3, SUN Xiao-Qiang4
Received:2011-01-24
Accepted:2011-06-28
Online:2011-01-24
Published:2011-09-01
Contact:
WANG Shu-Ping
摘要:
土壤微生物是表征土壤质量变化的敏感指标之一。借助长期定位试验, 采用磷脂脂肪酸分析方法研究了3种种植方式(玉米(Zea mays)连作、玉米非连作和撂荒)对土壤微生物群落组成的影响。结果表明, 在不同的种植方式下, 土壤微生物群落组成有明显的差异。玉米连作的土壤中总磷脂脂肪酸和细菌磷脂脂肪酸含量最低, 分别为33.12 nmol·g-1和18.09 nmol·g-1。非连作的土壤真菌磷脂脂肪酸和真菌/细菌分别为0.61 nmol·g-1和3.06%, 显著低于撂荒和连作(p < 0.05), 非连作方式下, 革兰氏阳性细菌/革兰氏阴性细菌增大。撂荒土壤的总磷脂脂肪酸和细菌磷脂脂肪酸分别为42.98和24.68 nmol·g-1, 高于耕作处理。 同时, 在撂荒方式下, 革兰氏阳性细菌和革兰氏阴性细菌的含量增加, 革兰氏阳性细菌/革兰氏阴性细菌降低。主成分分析结果表明: 耕作处理(玉米连作和非连作)分布第一主成分负方向上, 第一主成分得分系数分别为-2.48和-1.84; 撂荒分布第一主成分正方向上, 第一主成分得分系数为2.31, 与连作和非连作差异显著(p < 0.05)。冗余分析(RDA)表明: 土壤pH、总氮、有效磷和土壤>0.25 mm水稳性团聚体含量与磷脂脂肪酸呈正相关, 并且土壤pH和土壤>0.25 mm水稳性团聚体含量对土壤微生物群落的影响最大。
时鹏, 王淑平, 贾书刚, 高强, 孙晓强. 三种种植方式对土壤微生物群落组成的影响. 植物生态学报, 2011, 35(9): 965-972. DOI: 10.3724/SP.J.1258.2011.00965
SHI Peng, WANG Shu-Ping, JIA Shu-Gang, GAO Qiang, SUN Xiao-Qiang. Effects of three planting patterns on soil microbial community composition. Chinese Journal of Plant Ecology, 2011, 35(9): 965-972. DOI: 10.3724/SP.J.1258.2011.00965
| 处理 Treatment | 连作 Continuous cropping | 非连作 Non-continuous cropping | 撂荒 Uncultivated |
|---|---|---|---|
| pH (水土比 water : soil 1 : 2.5) | 6.84 ± 0.05b | 7.01 ± 0.15b | 7.56 ± 0.09a |
| 总碳 Total C (g·kg-1) | 14.9 ± 0.2b | 17.9 ± 0.3a | 15.4 ± 0.5b |
| 总氮 Total N (g·kg-1) | 1.3 ± 0.0b | 1.5 ± 0.0a | 1.3 ± 0.0ab |
| 碱解氮 Alkali-hydrolyzable N (mg·kg-1) | 96.1 ± 6.7a | 94.9 ± 0.5a | 96.0 ± 0.7a |
| 硝态氮 NO3--N (mg·kg-1) | 5.78 ± 0.09a | 9.29 ± 2.40a | 9.91 ± 2.35a |
| 铵态氮 NH4+-N (mg·kg-1) | 0.12 ± 0.05b | 0.45 ± 0.02a | 0.18 ± 0.10b |
| 有效磷 Available P (mg·kg-1) | 10.0 ± 0.5c | 22.3 ± 0.4b | 24.5 ± 1.6a |
| 土壤>0.25 mm水稳性团聚体含量 Contents of soil-water stable aggragate > 0.25 mm (%) | 21.4 ± 1.0a | 23.3 ± 1.9a | 28.0 ± 2.4a |
表1 土壤基本理化性质(平均值±标准误差)
Table 1 Soil basic physical and chemical properties (mean ± SE)
| 处理 Treatment | 连作 Continuous cropping | 非连作 Non-continuous cropping | 撂荒 Uncultivated |
|---|---|---|---|
| pH (水土比 water : soil 1 : 2.5) | 6.84 ± 0.05b | 7.01 ± 0.15b | 7.56 ± 0.09a |
| 总碳 Total C (g·kg-1) | 14.9 ± 0.2b | 17.9 ± 0.3a | 15.4 ± 0.5b |
| 总氮 Total N (g·kg-1) | 1.3 ± 0.0b | 1.5 ± 0.0a | 1.3 ± 0.0ab |
| 碱解氮 Alkali-hydrolyzable N (mg·kg-1) | 96.1 ± 6.7a | 94.9 ± 0.5a | 96.0 ± 0.7a |
| 硝态氮 NO3--N (mg·kg-1) | 5.78 ± 0.09a | 9.29 ± 2.40a | 9.91 ± 2.35a |
| 铵态氮 NH4+-N (mg·kg-1) | 0.12 ± 0.05b | 0.45 ± 0.02a | 0.18 ± 0.10b |
| 有效磷 Available P (mg·kg-1) | 10.0 ± 0.5c | 22.3 ± 0.4b | 24.5 ± 1.6a |
| 土壤>0.25 mm水稳性团聚体含量 Contents of soil-water stable aggragate > 0.25 mm (%) | 21.4 ± 1.0a | 23.3 ± 1.9a | 28.0 ± 2.4a |
| 处理 Treatment | 总磷脂脂肪酸 Total phospholipid fatty acids (nmol·g-1) | 细菌磷脂脂肪酸 Bacterial phospholipid fatty acids (nmol·g-1) | 真菌磷脂脂肪酸 Fungal phospholipid fatty acids (nmol·g-1) | 真菌/细菌 Fungi/Bacteria (%) |
|---|---|---|---|---|
| 连作 Continuous cropping | 33.12 ± 2.90a | 18.09 ± 1.55a | 2.91 ± 0.41a | 15.95 ± 1.14a |
| 非连作 Non-continuous cropping | 33.81 ± 5.28a | 19.26 ± 2.73a | 0.61 ± 0.17b | 3.06 ± 0.44b |
| 撂荒 Uncultivated | 42.98 ± 8.81a | 24.68 ± 4.86a | 2.62 ± 0.67a | 11.01 ± 2.22a |
表2 土壤微生物总磷脂脂肪酸、细菌磷脂脂肪酸、真菌磷脂脂肪酸和真菌/细菌(平均值±标准误差)
Table 2 Soil microbial total phospholipid fatty acids, bacterial phospholipid fatty acids, fungal phospholipid fatty acids and fungi/ bacteria (mean ± SE)
| 处理 Treatment | 总磷脂脂肪酸 Total phospholipid fatty acids (nmol·g-1) | 细菌磷脂脂肪酸 Bacterial phospholipid fatty acids (nmol·g-1) | 真菌磷脂脂肪酸 Fungal phospholipid fatty acids (nmol·g-1) | 真菌/细菌 Fungi/Bacteria (%) |
|---|---|---|---|---|
| 连作 Continuous cropping | 33.12 ± 2.90a | 18.09 ± 1.55a | 2.91 ± 0.41a | 15.95 ± 1.14a |
| 非连作 Non-continuous cropping | 33.81 ± 5.28a | 19.26 ± 2.73a | 0.61 ± 0.17b | 3.06 ± 0.44b |
| 撂荒 Uncultivated | 42.98 ± 8.81a | 24.68 ± 4.86a | 2.62 ± 0.67a | 11.01 ± 2.22a |
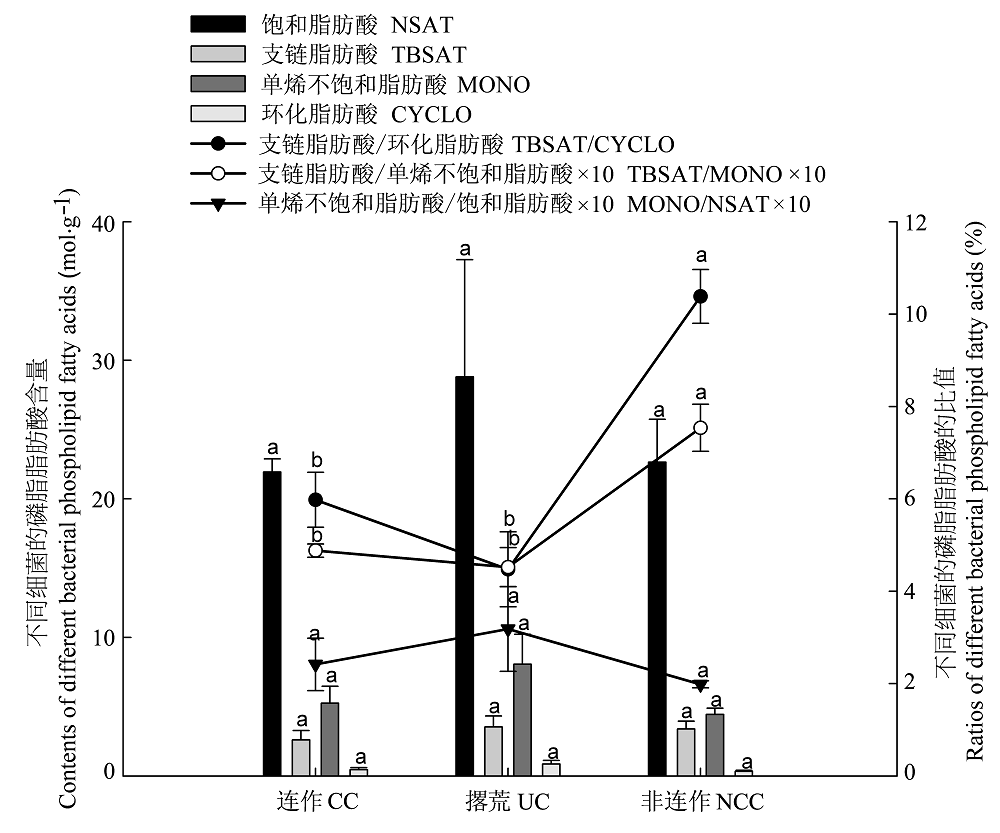
图1 不同种植方式细菌的磷脂脂肪酸含量和比值(平均值±标准误差)。 同一指标下不同字母表示差异显著(p < 0.05)。
Fig. 1 Contents of bacterial phospholipid fatty acids and their ratios in different planting patterns (mean ± SE). Values within same index with different letters are significantly different at p < 0.05 level. CC, continuous cropping; CYCLO, cyclopropyl fatty acids; MONO, monounsaturated fatty acids; NCC, non-continuous cropping. NSAT, normal saturated fatty acids; TBSAT, terminally branched saturated fatty acids; UC, uncultivated.
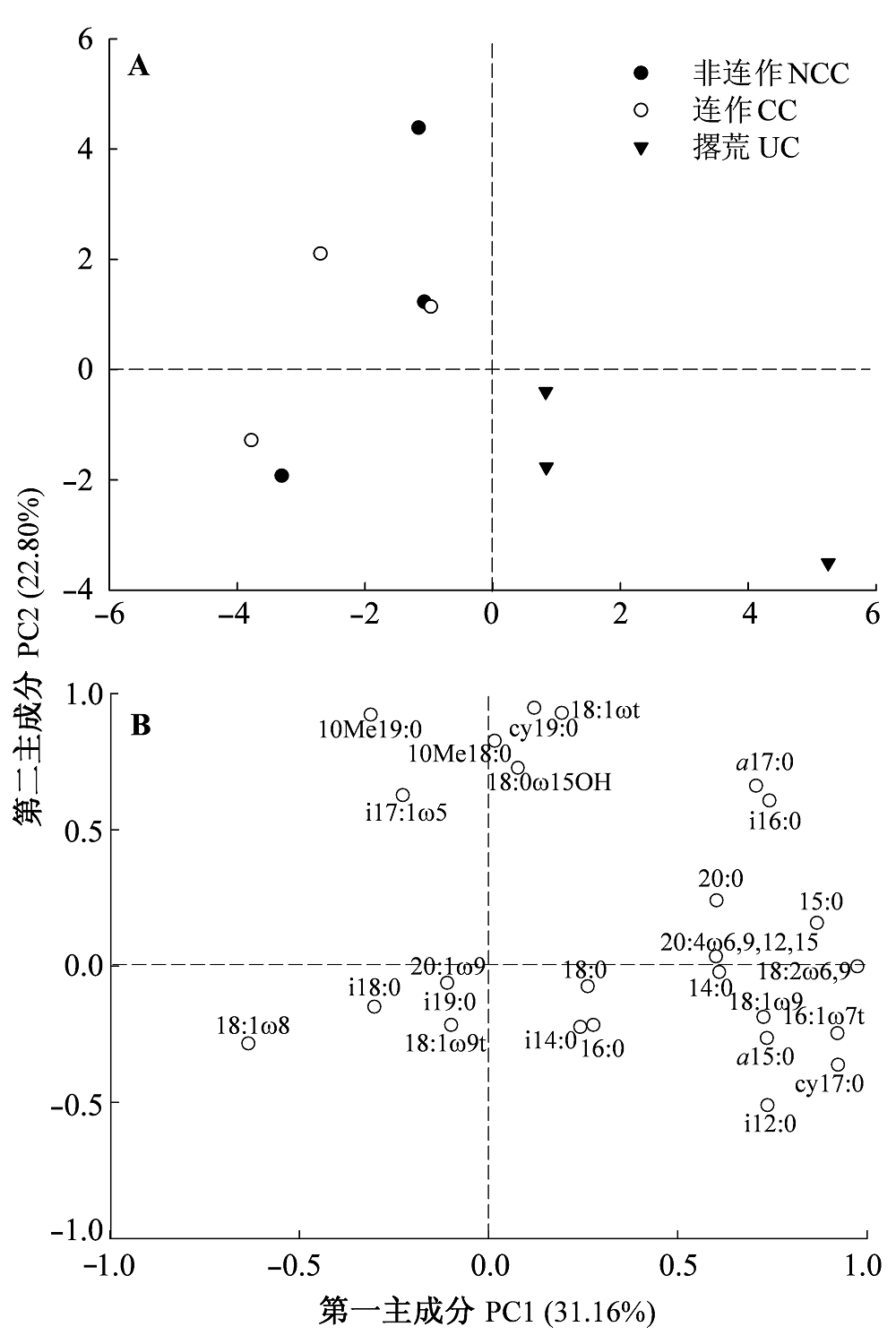
图2 土壤微生物群落结构主成分分析(A)和磷脂脂肪酸初始载荷因子主成分分析(B)。
Fig. 2 Principal component analysis of soil microbial community structure (A) and eigenvector of phospholipid fatty acids contributing to soil microbial communities ordination pattern (B). CC, continuous cropping; NCC, non-continuous cropping; PC1, first principal component; PC2, second principal component; UC, uncultivated.
| 处理 Treatment | 第一主成分 First principal component | 第二主成分 Second principal component |
|---|---|---|
| 连作 Continuous cropping | -2.48 ± 0.82b | 0.66 ± 1.01a |
| 非连作 Non-continuous cropping | -1.84 ± 0.73b | 1.23 ± 1.82a |
| 撂荒 Uncultivated | 2.31 ± 1.47a | -1.89 ± 0.90a |
表3 不同处理主成分得分系数(平均值±标准误差)
Table 3 Principal component scores for different treatments (mean ± SE)
| 处理 Treatment | 第一主成分 First principal component | 第二主成分 Second principal component |
|---|---|---|
| 连作 Continuous cropping | -2.48 ± 0.82b | 0.66 ± 1.01a |
| 非连作 Non-continuous cropping | -1.84 ± 0.73b | 1.23 ± 1.82a |
| 撂荒 Uncultivated | 2.31 ± 1.47a | -1.89 ± 0.90a |
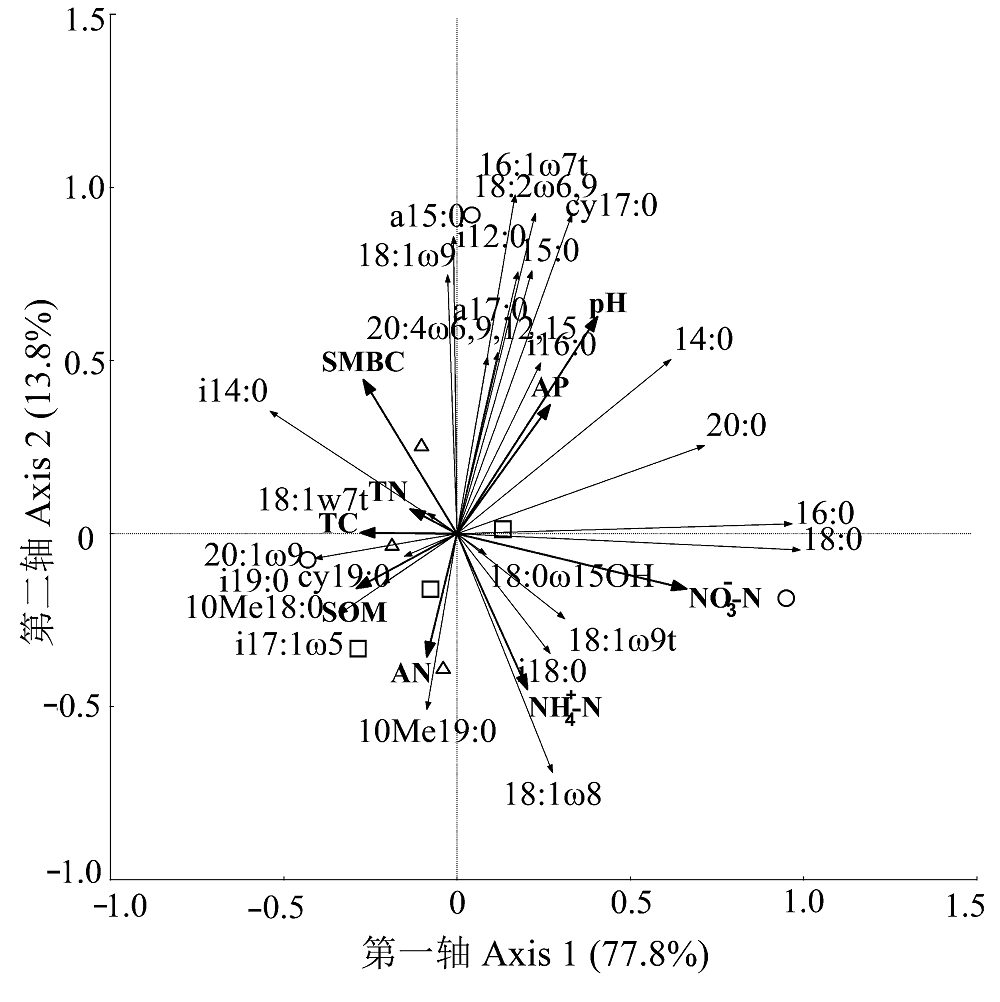
图3 土壤微生物群落结构与环境因子的冗余分析。 △, 连作; □, 非连作; ○, 撂荒。AN, 碱解氮; AP, 有效磷; NO3--N, 硝态氮; NH4+-N, 铵态氮; pH, 酸碱度; SOM, 有机质; SWSA, 土壤>0.25 mm水稳性团聚体含量; TC, 总碳; TN, 总氮。
Fig. 3 Redundancy analysis of soil microbial community structure and environmental variables.
| [1] | Bai Z ( 白震), Zhang M ( 张明), Song DY ( 宋斗妍), Zhang XD ( 张旭东) (2008). Effect of different fertilization on microbial community in an arable mollisol. Acta Ecologica Sinica (生态学报), 28, 3244-3253. (in Chinese with English abstract) |
| [2] | Bligh EG, Dyer WJ (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Physiology and Pharmacology, 37, 911-917. |
| [3] |
Boehm MJ, Wu TY, Stone AG, Kraakman B, Iannotti DA, Wilson GE, Madden LV, Hoitink HAJ (1997). Cross- polarized magic-angle spinning 13C nuclear magnetic resonance spectroscopic characterization of soil organic matter relative to culturable bacterial species composition and sustained biological control of Pythium root rot. Applied and Environmental Microbiology, 63, 162-168.
URL PMID |
| [4] | Böehme L, Langer U, Böehme F (2005). Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agriculture, Ecosystems & Environment, 109, 141-152. |
| [5] |
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998). Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipid fatty acid profiles. Microbial Ecology, 36, 1-12.
URL PMID |
| [6] |
Bünemann EK, Bossio DA, Smithson PC, Frossard E, Oberson A (2004). Microbial community composition and substrate use in a highly weathered soil as affected by crop rotation and P fertilization. Soil Biology & Biochemistry, 36, 889-901.
DOI URL |
| [7] |
Calderón FJ, Jackson LE, Scow KM, Rolston DE (2000). Microbial responses to simulated tillage in cultivated and uncultivated soils. Soil Biology & Biochemistry, 32, 1547-1559.
DOI URL |
| [8] |
Cookson WR, Murphy DV, Roper MM (2008). Characterizing the relationships between soil organic matter components and microbial function and composition along a tillage disturbance gradient. Soil Biology & Biochemistry, 40, 763-777.
DOI URL |
| [9] |
Franzluebbers AJ, Arshad MA (1996). Water-stable aggrega- tion and organic matter in four soils under conventional and zero tillage. Canadian Journal of Soil Science, 76, 387-393.
DOI URL |
| [10] |
Franzluebbers AJ, Arshad MA (1997). Soil microbial biomass and mineralizable carbon of water-stable aggregates affected by texture and tillage. Soil Science Society of America Journal, 61, 1090-1097.
DOI URL |
| [11] |
Frostegård Å, Bååth E (1996). The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils, 22, 59-65.
DOI URL |
| [12] |
Frostegård Å, Bååth E, Tunlio A (1993). Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biology & Biochemistry, 25, 723-730.
DOI URL |
| [13] |
Green CT, Scow KM (2000). Analysis of phospholipid fatty acids (PLFA) to characterize microbial communities in aquifers. Hydrogeology Journal, 8, 126-141.
DOI URL |
| [14] | Kaur A, Chaudhary A, Kaur A, Choudhary R, Kaushik R (2005). Phospholipid fatty acid―a bioindicator of environment monitoring and assessment in soil ecosystem. Current Science, 89, 1103-1112. |
| [15] |
Kennedy N, Brodie E, Connolly J, Clipson N (2004). Impact of lime, nitrogen and plant species on bacterial community structure in grassland microcosms. Environmental Microbiology, 6, 1070-1080.
DOI URL PMID |
| [16] |
Kieft TL, Ringelberg DB, White DC (1994). Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium. Applied and Environmental Microbiology, 60, 3292-3299.
URL PMID |
| [17] | Lu RK ( 鲁如坤) (2000). Analytical Method of Soil Agrochemistry (土壤农业化学分析方法). Chinese Agriculture Science and Technology Press, Beijing. (in Chinese) |
| [18] |
Lupwayi NZ, Arshad MA, Rice WA, Clayton GW (2001). Bacterial diversity in water-stable aggregates of soils under conventional and zero tillage management. Applied Soil Ecology, 16, 251-261.
DOI URL |
| [19] |
McKinley VL, Peacock AD, White DC (2005). Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biology & Biochemistry, 37, 1946-1958.
DOI URL |
| [20] | Meng QJ ( 孟庆杰), Xu YL ( 许艳丽), Li CJ ( 李春杰), Han XZ ( 韩晓增), Pei XC ( 裴希超) (2008). Effects of different vegetation coverage on microbial functional diversity in black soil. Chinese Journal of Ecology (生态学杂志), 27, 1134-1140. (in Chinese with English abstract) |
| [21] | National Bureau of Statistics of China(中华人民共和国国家统计局) (2009). China Statistical Yearbook 2009 (中国统计年鉴2009). China Statistics Press, Beijing. (in Chinese) |
| [22] |
Nayyar A, Hamel C, Lafond G, Gossen BD, Hanson K, Germida J (2009). Soil microbial quality associated with yield reduction in continuous-pea. Applied Soil Ecology, 43, 115-121.
DOI URL |
| [23] |
Nusslein K, Tiedje JM (1999). Soil bacterial community shift correlated with change from forest to pasture vegetation in a tropical soil. Applied and Environmental Microbiology, 65, 3622-3626.
DOI URL PMID |
| [24] | Olsen SR, Cole CV, Watanabe FS, Dean LA (1954). Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. Circulation, 939. Washington, D. C.:US Govt. Printing Office. |
| [25] | Schloter M, Dilly IO, Muncha JC (2003). Indicators for evaluating soil quality. Agriculture, Ecosystems & Environment, 98, 255-262. |
| [26] | Shi P ( 时鹏), Gao Q ( 高强), Wang SP ( 王淑平), Zhang Y ( 张妍) (2010). Effects of continuous cropping of corn and fertilization on soil microbial community functional diversity. Acta Ecologica Sinica (生态学报), 30, 6173-6182. (in Chinese with English abstract) |
| [27] |
Staley TE, Edwards WM, Scott CL, Owens LB (1988). Soil microbial biomass and organic component alterations in a no-tillage chronosequence. Soil Science Society of America Journal, 52, 998-1005.
DOI URL |
| [28] |
Steenwerth KL, Jackson LE, Calderón FJ, Stromberg MR, Scow KM (2002). Soil microbial community composi- tion and land use history in cultivated and grassland ecosystems of coastal California. Soil Biology & Biochemistry, 34, 1599-1611.
DOI URL |
| [29] |
Waid JS (1999). Does soil biodiversity depend upon metabiotic activity and influences? Applied Soil Ecology, 13, 151-158.
DOI URL |
| [30] |
White PA, Rice CW (2009). Tillage effects on microbial and carbon dynamics during plant residue decomposition. Soil Science Society of America Journal, 73, 138-145.
DOI URL |
| [31] |
Zelles L (1999). Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biology and Fertility of Soils, 29, 111-129.
DOI URL |
| [32] |
Zelles L, Bai QY, Beck T, Beese F (1992). Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biology & Biochemistry, 24, 317-323.
DOI URL |
| [33] | Zhang L ( 张蕾), Yi YL ( 依艳丽), He ZK ( 贺忠科), Jia ZW ( 贾振文), Du AJ ( 杜安军) (2008). Compositions of organic matter in soil in continuous corn cropping soil in Shenyang suburban and affect on soil structure. Chinese Journal of Soil Science (土壤通报), 39, 756-760. (in Chinese with English abstract) |
| [34] | Zhao LP ( 赵兰坡), Wang HB ( 王鸿斌), Liu HQ ( 刘会青), Wang YL ( 王艳玲), Liu SX ( 刘淑霞), Wang Y ( 王宇) (2006). Mechanism of fertility degradation of black soil in corn belt of Songliao Plain. Acta Pedologica Sinica (土壤学报), 43, 79-84. (in Chinese with English abstract) |
| [1] | 刘瑶 钟全林 徐朝斌 程栋梁 郑跃芳 邹宇星 张雪 郑新杰 周云若. 不同大小刨花楠细根功能性状与根际微环境关系[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 冯继广, 张秋芳, 袁霞, 朱彪. 氮磷添加对土壤有机碳的影响: 进展与展望[J]. 植物生态学报, 2022, 46(8): 855-870. |
| [3] | 聂秀青, 王冬, 周国英, 熊丰, 杜岩功. 三江源地区高寒湿地土壤微生物生物量碳氮磷及其化学计量特征[J]. 植物生态学报, 2021, 45(9): 996-1005. |
| [4] | 裴广廷, 孙建飞, 贺同鑫, 胡宝清. 长期人为干扰对桂西北喀斯特草地土壤微生物多样性及群落结构的影响[J]. 植物生态学报, 2021, 45(1): 74-84. |
| [5] | 罗林, 黄艳, 梁进, 汪恩涛, 胡君, 贺合亮, 赵春章. 西南亚高山针叶林主要树种互作及增温对根区土壤微生物群落的影响[J]. 植物生态学报, 2020, 44(8): 875-884. |
| [6] | 王军, 王冠钦, 李飞, 彭云峰, 杨贵彪, 郁建春, 周国英, 杨元合. 短期增温对紫花针茅草原土壤微生物群落的影响[J]. 植物生态学报, 2018, 42(1): 116-125. |
| [7] | 贺同鑫, 李艳鹏, 张方月, 王清奎. 林下植被剔除对杉木林土壤呼吸和微生物群落结构的影响[J]. 植物生态学报, 2015, 39(8): 797-806. |
| [8] | 周勇, 郑璐雨, 朱敏杰, 李夏, 任安芝, 高玉葆. 内生真菌感染对禾草宿主生境土壤特性和微生物群落的影响[J]. 植物生态学报, 2014, 38(1): 54-61. |
| [9] | 王爱丽. 应用磷脂脂肪酸和聚合酶链式反应-变性梯度凝胶电泳分析技术研究湿地植物根际微生物群落多样性[J]. 植物生态学报, 2013, 37(8): 750-757. |
| [10] | 张乃莉, 郭继勋, 王晓宇, 马克平. 土壤微生物对气候变暖和大气N沉降的响应[J]. 植物生态学报, 2007, 31(2): 252-261. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19