

植物生态学报 ›› 2014, Vol. 38 ›› Issue (1): 54-61.DOI: 10.3724/SP.J.1258.2014.00006
收稿日期:2013-08-22
接受日期:2013-11-26
出版日期:2014-08-22
发布日期:2014-01-15
通讯作者:
任安芝
作者简介:*(E-mail:renanzhi@nankai.edu.cn)
ZHOU Yong, ZHENG Lu-Yu, ZHU Min-Jie, LI Xia, REN An-Zhi*( ), GAO Yu-Bao
), GAO Yu-Bao
Received:2013-08-22
Accepted:2013-11-26
Online:2014-08-22
Published:2014-01-15
Contact:
REN An-Zhi
摘要:
以羊草(Leymus chinensis)-内生真菌共生体为研究对象, 分别在野外样地和室内盆栽两种实验条件下研究了内生真菌感染对土壤特性和微生物群落结构的影响。结果显示:在处理时间较长并伴随有枯落物分解的羊草样地中, 内生真菌感染促进了土壤氮(N)的积累, 提高了30天培养时间内土壤初始碳(C)矿化速率和前3天土壤矿化量和土壤矿化总量; 而在处理时间较短且没有地上枯落物分解的盆栽羊草中, 内生真菌感染对土壤的C、N含量及C矿化均无显著影响。无论是野外样地还是室内盆栽实验, 内生真菌感染均未引起土壤微生物磷脂脂肪酸种类的变化, 但内生真菌感染均有提高土壤微生物生物量的趋势, 内生真菌显著增加了盆栽羊草土壤中细菌、革兰氏阴性细菌、真菌磷脂脂肪酸含量和磷脂脂肪酸总量, 增加了羊草样地土壤中革兰氏阳性细菌和放线菌的磷脂脂肪酸含量。总体看来, 内生真菌感染能够改变土壤N积累和C矿化率, 并且改变土壤中微生物群落的结构, 这有助于进一步认识内生真菌与羊草之间的共生关系及其在生态系统C、N循环中所起的作用。
周勇, 郑璐雨, 朱敏杰, 李夏, 任安芝, 高玉葆. 内生真菌感染对禾草宿主生境土壤特性和微生物群落的影响. 植物生态学报, 2014, 38(1): 54-61. DOI: 10.3724/SP.J.1258.2014.00006
ZHOU Yong, ZHENG Lu-Yu, ZHU Min-Jie, LI Xia, REN An-Zhi, GAO Yu-Bao. Effects of fungal endophyte infection on soil properties and microbial communities in the host grass habitat. Chinese Journal of Plant Ecology, 2014, 38(1): 54-61. DOI: 10.3724/SP.J.1258.2014.00006

图1 内生真菌感染对盆栽土壤和样地土壤总C (A)、总N (B)和C:N比值(C)的影响(平均值±标准误差, n = 5)。E+, 染菌; E-, 不染菌。不同小写字母表示差异显著(p < 0.05)。
Fig. 1 Effects of fungal endophyte infection on total C (A), total N (B), and C:N (C) in pot and field soils (mean ± SE, n = 5). E+, endophyte-infected; E-, endophyte-free. Different lower-case letters indicate significant differences at p < 0.05.
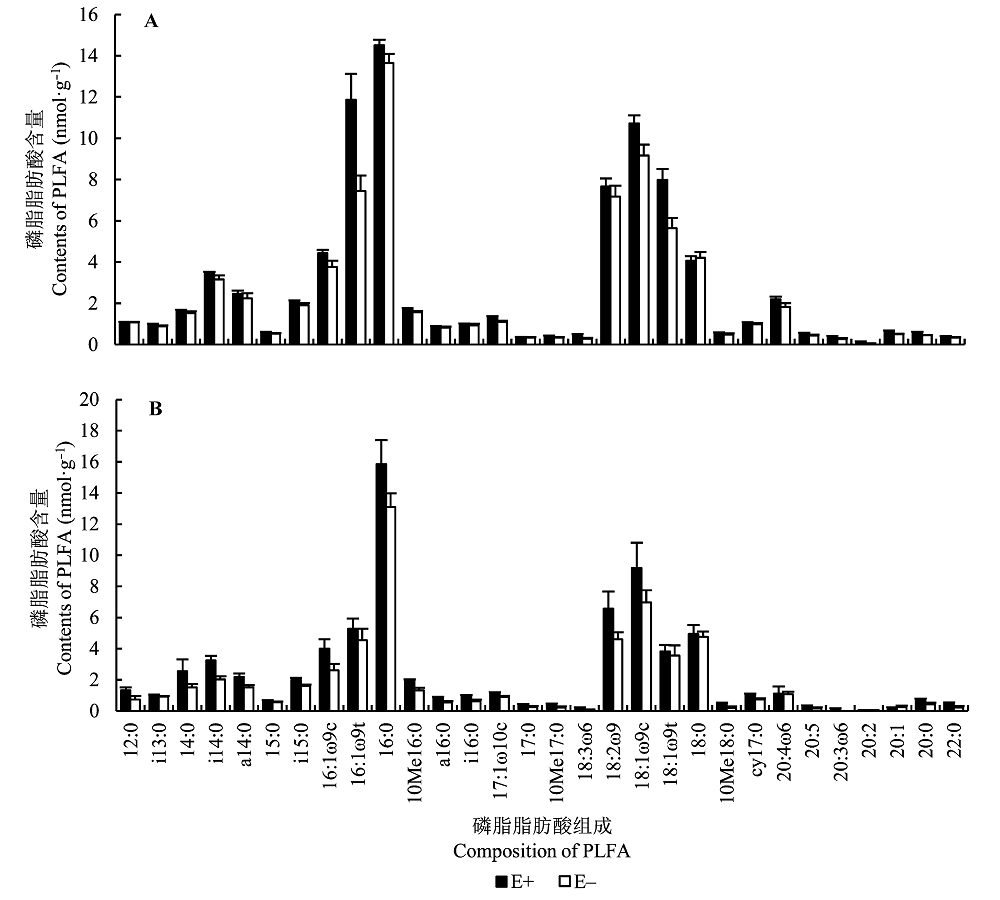
图2 不同染菌条件下土壤微生物磷脂脂肪酸(PLFA)图谱(平均值±标准误差, n = 5)。A, 盆栽土壤。B, 样地土壤。E+, 染菌; E-, 不染菌。
Fig. 2 Soil microbial phospholipid fatty acid (PLFA) profiles with different fungal endophyte infections (mean ± SE, n = 5). A, Pot soil. B, Field soil. E+, endophyte-infected; E-, endophyte-free.
| 盆栽土壤 Pot soil | 样地土壤 Plot soil | ||||
|---|---|---|---|---|---|
| E+ | E- | E+ | E- | ||
| 细菌 Bacteria | 37.91 ± 1.12 a | 31.85 ± 1.12 b | 33.15 ± 2.62 a | 27.22 ± 2.32 a | |
| 革兰氏阳性细菌 Gram-positive bacteria G+ | 1.60 ± 0.05 a | 1.47 ± 0.11 a | 1.60 ± 0.11 a | 1.21 ± 0.13 b | |
| 革兰氏阴性细菌 Gram-negative bacteria G- | 17.37 ± 1.26 a | 12.20 ± 0.76 b | 10.32 ± 1.12 a | 7.89 ± 1.15 a | |
| 真菌 Fungi | 19.19 ± 0.63 a | 15.09 ± 0.65 b | 13.16 ± 2.11 a | 10.58 ± 1.38 a | |
| 放线菌 Actinomycetes | 2.69 ± 0.08 a | 2.39 ± 0.14 a | 2.83 ± 0.17 a | 1.74 ± 0.26 b | |
| 原生动物 Protozoa | 2.59 ± 0.15 a | 2.09 ± 0.23 a | 1.21 ± 0.55 a | 1.08 ± 0.18 a | |
| G+:G- | 0.10 ± 0.01 a | 0.12 ± 0.01 a | 0.16 ± 0.01 a | 0.16 ± 0.01 a | |
| 真菌:细菌比值 Bacteria:fungi ratio | 0.51 ± 0.02 a | 0.48 ± 0.02 a | 0.39 ± 0.04 a | 0.38 ± 0.02 a | |
| PLFAs总量 Total PLFAs | 86.27 ± 1.14 a | 73.06 ± 1.91 b | 72.88 ± 7.31 a | 56.11 ± 5.27 a | |
表1 不同染菌条件下盆栽和样地土壤微生物磷脂脂肪酸(PLFAs)含量(nmol·g-1) (平均值±标准误差, n = 5)
Table 1 Contents of soil microbial phospholipid fatty acids (PLFAs) in pots and field plots under different fungal endophyte infections (nmol·g-1) (mean ± SE, n = 5)
| 盆栽土壤 Pot soil | 样地土壤 Plot soil | ||||
|---|---|---|---|---|---|
| E+ | E- | E+ | E- | ||
| 细菌 Bacteria | 37.91 ± 1.12 a | 31.85 ± 1.12 b | 33.15 ± 2.62 a | 27.22 ± 2.32 a | |
| 革兰氏阳性细菌 Gram-positive bacteria G+ | 1.60 ± 0.05 a | 1.47 ± 0.11 a | 1.60 ± 0.11 a | 1.21 ± 0.13 b | |
| 革兰氏阴性细菌 Gram-negative bacteria G- | 17.37 ± 1.26 a | 12.20 ± 0.76 b | 10.32 ± 1.12 a | 7.89 ± 1.15 a | |
| 真菌 Fungi | 19.19 ± 0.63 a | 15.09 ± 0.65 b | 13.16 ± 2.11 a | 10.58 ± 1.38 a | |
| 放线菌 Actinomycetes | 2.69 ± 0.08 a | 2.39 ± 0.14 a | 2.83 ± 0.17 a | 1.74 ± 0.26 b | |
| 原生动物 Protozoa | 2.59 ± 0.15 a | 2.09 ± 0.23 a | 1.21 ± 0.55 a | 1.08 ± 0.18 a | |
| G+:G- | 0.10 ± 0.01 a | 0.12 ± 0.01 a | 0.16 ± 0.01 a | 0.16 ± 0.01 a | |
| 真菌:细菌比值 Bacteria:fungi ratio | 0.51 ± 0.02 a | 0.48 ± 0.02 a | 0.39 ± 0.04 a | 0.38 ± 0.02 a | |
| PLFAs总量 Total PLFAs | 86.27 ± 1.14 a | 73.06 ± 1.91 b | 72.88 ± 7.31 a | 56.11 ± 5.27 a | |
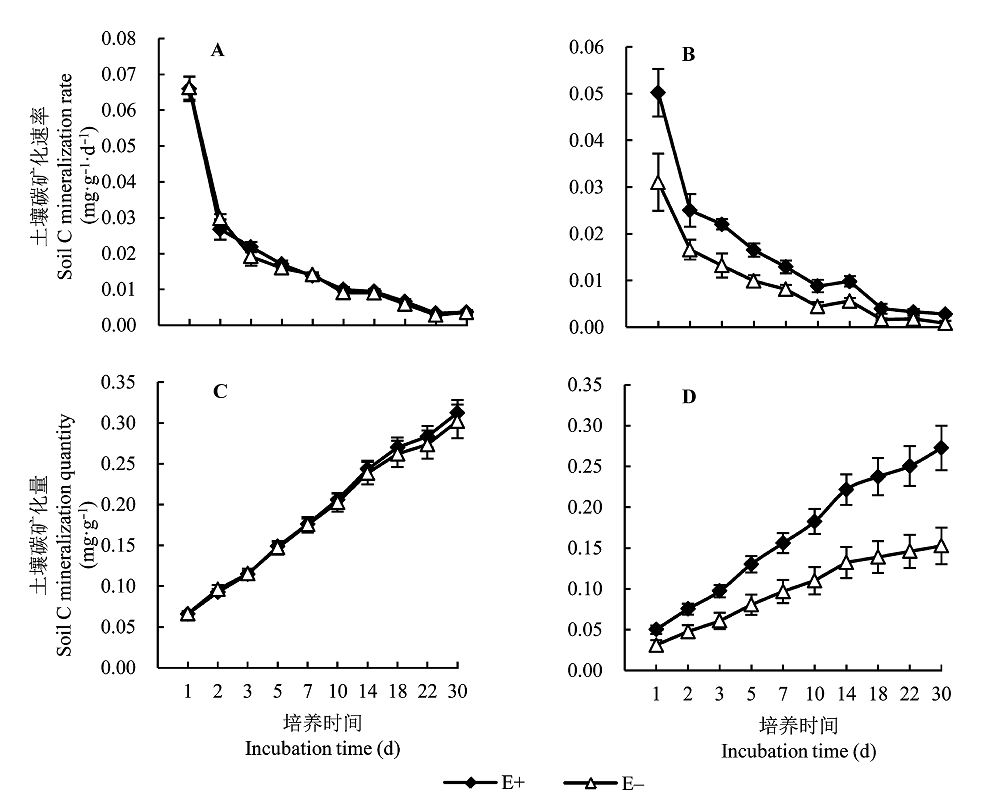
图3 不同染菌条件下土壤C矿化速率和土壤C矿化量的变化(平均值±标准误差, n = 5)。A, 盆栽土壤C矿化速率。B, 样地土壤C矿化速率。C, 盆栽土壤C矿化量。D, 样地土壤C矿化量。E+, 染菌; E-, 不染菌。
Fig. 3 Changes in the rate and quantity of soil C mineralization under different fungal endophyte infections (mean ± SE, n = 5). A, Soil C mineralization rate in pot soil. B, Soil C mineralization rate in field plot. C, Quantity of soil C mineralization in pot soil. D, Quantity of soil C mineralization in field plot. E+, endophyte-infected; E-, endophyte-free.
| [1] | Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA (2000). Are tropical fungal endophytes hyperdiverse? Ecology Letters, 3,267-274. |
| [2] |
Baumann K, Dignac MF, Rumpel C, Bardoux G, Sarr A, Steffens M, Maron PA (2013). Soil microbial diversity affects soil organic matter decomposition in a silty grassland soil. Biogeochemistry, 114,201-212.
DOI URL |
| [3] | Belesky DP, Fedders JM (1995). Tall fescue development in response to Acremonium coenophialum and soil acidity. Crop Science, 35,529-533. |
| [4] | Bultman TL, Bell GD (2003). Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos, 103,182-190. |
| [5] | Burns JC, Fisher DS (2006). Intake and digestion of ‘Jesup’ tall fescue hays with a novel fungal endophyte, without an endophyte, or with a wild-type endophyte. Crop Science, 46,216-223. |
| [6] | Casas C, Omacini M, Montecchia MS, Correa OS (2011). Soil microbial community responses to the fungal endophyte neotyphodium in Italian ryegrass. Plant and Soil, 340,347-355. |
| [7] | Chen XQ, Li YQ, Jia FS, Chen JL (1989). A study on Aneurolepidium chinense. Pratacultural Science, 6,7-12. (in Chinese with English abstract) |
| [ 陈孝泉, 李艳芹, 贾丰生, 陈吉琳 (1989). 羊草植物的研究. 草业科学, 6,7-12.] | |
| [8] | Christensen MJ (1996). Antifungal activity in grasses infected with Acremonium and Epichloë endophytes. Australasian Plant Pathology, 25,186-191. |
| [9] | Clay K (1990). Fungal endophytes of grasses. Annual Review of Ecology and Systematics, 21,275-297. |
| [10] |
Clay K, Holah J (1999). Fungal endophyte symbiosis and plant diversity in successional fields. Science, 285,1742-1744.
URL PMID |
| [11] | Clay K, Marks S, Cheplick GP (1993). Effects of insect herbivory and fungal endophyte infection on competitive interactions among grasses. Ecology, 74,1767-1777. |
| [12] | Dou JX, Liu JS, Wang Y (2009). Effects of amendment C/N ratio on soil organic carbon mineralization of meadow marshes in Sanjiang Plain. Scientia Geographica Sinica, 29,773-778. (in Chinese with English abstract) |
| [ 窦晶鑫, 刘景双, 王洋 (2009). 三江平原草甸湿地土壤有机碳矿化对C/N的响应. 地理科学, 29,773-778.] | |
| [13] |
Franzluebbers AJ, Hill NS (2005). Soil carbon, nitrogen, and ergot alkaloids with short- and long-term exposure to endophyte-infected and endophyte-free tall fescue. Soil Science Society of America Journal, 69,404-412.
DOI URL |
| [14] | Franzluebbers AJ, Nazih N, Stuedemann JA, Fuhrmann JJ, Schomberg HH, Hartel PG (1999). Soil carbon and nitrogen pools under low- and high-endophyte-infected tall fescue. Soil Science Society of America Journal, 63,1687-1694. |
| [15] | Franzluebbers AJ, Stuedemann JA (2005). Soil carbon and nitrogen pools in response to tall fescue endophyte infection, fertilization, and cultivar. Soil Science Society of America Journal, 69,396-403. |
| [16] |
Giardina CP, Ryan MG, Hubbard RM, Binkley D (2001). Tree species and soil textural controls on carbon and nitrogen mineralization rates. Soil Science Society of America Journal, 65,1272-1279.
DOI URL |
| [17] |
Handayani IP, Coyne MS, Phillips TD (2011). Soil organic carbon fractions differ in two contrasting tall fescue systems. Plant and Soil, 338,43-50.
DOI URL |
| [18] |
Hesse U, Schöberlein W, Wittenmayer L, Förster K, Warnstorff K, Diepenbrock W, Merbach W (2003). Ef- fects of neotyphodium endophytes on growth, reproduc- tion and drought-stress tolerance of three Lolium perenne L. genotypes. Grass and Forage Science, 58,407-415.
DOI URL |
| [19] | Iqbal J, Siegrist JA, Nelson JA, Mcculley RL (2012). Fungal endophyte infection increases carbon sequestration potential of southeastern USA tall fescue stands. Soil Biology & Biochemistry, 44,81-92. |
| [20] | Jenkins MB, Franzluebbers AJ, Humayoun SB (2006). Assessing short-term responses of prokaryotic communities in bulk and rhizosphere soils to tall fescue endophyte infection. Plant and Soil, 289,309-320. |
| [21] | Latch GCM (1993). Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes. Agriculture, Ecosystems & Environment, 44,143-156. |
| [22] |
Lemons A, Clay K, Rudgers JA (2005). Connecting plant-microbial interactions above- and below-ground: a fungal endophyte affects decomposition. Oecologia, 145,595-604.
URL PMID |
| [23] | Lewis GC (2004). Effects of biotic and abiotic stress on the growth of three genotypes of Lolium perenne with and without infection by the fungal endophyte Neotyphodium lolii. Annals of Applied Biology, 144,53-63. |
| [24] |
Mack KML, Rudgers JA (2008). Balancing multiple mutualists: asymmetric interactions among plants, arbuscular mycorrhizal fungi, and fungal endophytes. Oikos, 117,310-320.
DOI URL |
| [25] | Marks S, Clay K (1996). Physiological responses of Festuca arundinacea to fungal endophyte infection. New Phytologist, 133,727-733. |
| [26] | Núñeza S, Martínez-Yrízara A, Búrqueza A, García-Oliva F (2001). Carbon mineralization in the southern Sonoran Desert. Acta Oecologica, 22,269-276. |
| [27] | Omacini M, Chaneton EJ, Ghersa CM, Otero P (2004). Do foliar endophytes affect grass litter decomposition? A microcosm approach using Lolium multiflorum. Oikos, 104,581-590. |
| [28] | Rasmussen S, Parsons AJ, Bassett S, Christensen MJ, Hume DE, Johnson LJ, Johnson RD, Simpson WR, Stacke C, Voisey CR, Xue H, Newman JA (2007). High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytologist, 173,787-797. |
| [29] |
Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA (2008). Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiology, 146,1440-1453.
URL PMID |
| [30] | Schutter ME, Dick RP (2000). Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Science Society of America Journal, 64,1659-1668. |
| [31] | Siegrist JA, McCulley RL, Bush LP, Phillips TD (2010). Alkaloids may not be responsible for endophyte- associated reductions in tall fescue decomposition rates. Functional Ecology, 24,460-468. |
| [32] | van Hecke MM, Treonis AM, Kaufman JR (2005). How does the fungal endophyte Neotyphodium coenophialum affect tall fescue ( Festuca arundinacea) rhizodeposition and soil microorganisms? Plant and Soil, 275,101-109. |
| [33] |
Zhu MJ, Ren AZ, Wen W, Gao YB (2013). Diversity and taxonomy of endophytes from Leymus chinensis in the Inner Mongolia Steppe of China. FEMS Microbiology Letters, 340,135-145.
URL PMID |
| [1] | 刘瑶 钟全林 徐朝斌 程栋梁 郑跃芳 邹宇星 张雪 郑新杰 周云若. 不同大小刨花楠细根功能性状与根际微环境关系[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 白皓然 侯盟 刘艳杰. 少花蒺藜草入侵与干旱对羊草草原生产力的影响机制[J]. 植物生态学报, 2024, 48(5): 577-589. |
| [3] | 代景忠, 白玉婷, 卫智军, 张楚, 辛晓平, 闫玉春, 闫瑞瑞. 羊草功能性状对施肥的动态响应[J]. 植物生态学报, 2023, 47(7): 943-953. |
| [4] | 李冠军, 陈珑, 余雯静, 苏亲桂, 吴承祯, 苏军, 李键. 固体培养内生真菌对土壤盐胁迫下木麻黄幼苗渗透调节和抗氧化系统的影响[J]. 植物生态学报, 2023, 47(6): 804-821. |
| [5] | 李雪, 董杰, 韩广轩, 张奇奇, 谢宝华, 李培广, 赵明亮, 陈克龙, 宋维民. 黄河三角洲典型滨海盐沼湿地土壤CO2和CH4排放对水盐变化的响应[J]. 植物生态学报, 2023, 47(3): 434-446. |
| [6] | 甘子莹, 王浩, 丁驰, 雷梅, 杨晓刚, 蔡敬琰, 丘清燕, 胡亚林. 亚热带森林不同植物及器官来源的可溶性有机质输入对土壤激发效应的影响及其作用机理[J]. 植物生态学报, 2022, 46(7): 797-810. |
| [7] | 石新建, 张靖歆, 秦天姿, 刘金铭, 高玉葆, 任安芝. 内生真菌感染对宿主羽茅及邻生植物抗病性的影响[J]. 植物生态学报, 2021, 45(8): 860-869. |
| [8] | 董利军, 李金花, 陈珊, 张瑞, 孙建, 马妙君. 若尔盖湿地高寒草甸退化过程中土壤有机碳含量变化及成因分析[J]. 植物生态学报, 2021, 45(5): 507-515. |
| [9] | 代景忠, 白玉婷, 卫智军, 张楚, 闫瑞瑞. 切根对羊草营养生长期内植物功能性状的影响[J]. 植物生态学报, 2021, 45(12): 1292-1302. |
| [10] | 罗林, 黄艳, 梁进, 汪恩涛, 胡君, 贺合亮, 赵春章. 西南亚高山针叶林主要树种互作及增温对根区土壤微生物群落的影响[J]. 植物生态学报, 2020, 44(8): 875-884. |
| [11] | 秦天姿, 任安芝, 樊晓雯, 高玉葆. 内生真菌种类和母本基因型对内生真菌-禾草共生体叶形状和叶面积的影响[J]. 植物生态学报, 2020, 44(6): 654-660. |
| [12] | 李颖, 龚吉蕊, 刘敏, 侯向阳, 丁勇, 杨波, 张子荷, 王彪, 朱趁趁. 不同放牧强度下内蒙古温带典型草原优势种植物防御策略[J]. 植物生态学报, 2020, 44(6): 642-653. |
| [13] | 吴曼, 李娟娟, 刘金铭, 任安芝, 高玉葆. 刈割干扰和养分添加条件下Epichloë内生真菌感染对羽茅所在群落多样性和生产力的影响[J]. 植物生态学报, 2019, 43(2): 85-93. |
| [14] | 李春杰, 姚祥, 南志标. 醉马草内生真菌共生体研究进展[J]. 植物生态学报, 2018, 42(8): 793-805. |
| [15] | 张璐, 郝匕台, 齐丽雪, 李艳龙, 徐慧敏, 杨丽娜, 宝音陶格涛. 草原群落生物量和土壤有机质含量对改良措施的动态响应[J]. 植物生态学报, 2018, 42(3): 317-326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19