

Chin J Plant Ecol ›› 2012, Vol. 36 ›› Issue (7): 671-680.DOI: 10.3724/SP.J.1258.2012.00671
Previous Articles Next Articles
ZHOU Shuai1, LIN Fu-Ping1, WANG Yu-Kui3, SHEN Ying-Bai2, ZHANG Ru-Min1, GAO Rong-Fu2, GAO Yan1,*( )
)
Published:2012-07-10
Contact:
GAO Yan
ZHOU Shuai, LIN Fu-Ping, WANG Yu-Kui, SHEN Ying-Bai, ZHANG Ru-Min, GAO Rong-Fu, GAO Yan. Effects of mechanical damage of leaves on volatile organic compounds and chlorophyll fluorescence parameters in seedlings of Cinnamomum camphora[J]. Chin J Plant Ecol, 2012, 36(7): 671-680.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.3724/SP.J.1258.2012.00671
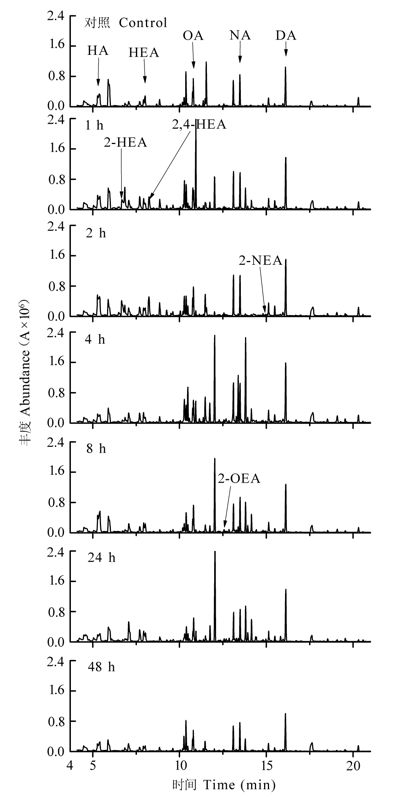
Fig. 1 Total ion current of volatile organic compounds in mechanically damaged leaves of Cinnamomum camphora seedlings. A, peak area; DA, decanal; FA, furfural; HA, hexanal; HEA, heptanal; 2-HEA, (E)-2-hexenal; 2,4-HEA, (E,E)- 2,4-hexadienal; 2-NEA, (E)-2-nonenal; NA, nonanal; OA, octanal; 2-OEA, (E)-2-octenal.
| C6-C10醛类化合物 C6-C10 aldehydes | 时间 Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | 48 | |
| 糠醛 Furfural | 1.58 ± 0.54 | 2.61 ± 0.59* | 1.18 ± 0.42 | 0.83 ± 0.25 | 1.30 ± 0.35 | 1.08 ± 0.36 | 1.32 ± 0.34 |
| 己醛 Hexanal | 9.53 ± 1.02 | 32.53 ± 4.92** | 33.05 ± 1.62** | 29.03 ± 3.19** | 18.39 ± 0.86** | 14.44 ± 3.01* | 7.06 ± 1.55 |
| 庚醛 Heptanal | 6.23 ± 0. 97 | 8.82 ± 1.21* | 11.22 ± 0.84** | 12.53 ± 2.80** | 9.45 ± 0.87* | 7.67 ± 0.95 | 6.56 ± 0.84 |
| 辛醛 Octanal | 10.11 ± 2.65 | 13.34 ± 3.67* | 23.66 ± 2.31** | 17.37 ± 3.33* | 16.43 ± 3.01* | 14.32 ± 2.95* | 11.03 ± 2.55 |
| 壬醛 Nonanal | 14.26 ± 3.43 | 22.02 ± 4.07** | 27.24 ± 3.34** | 22.91 ± 3.90* | 19.97 ± 2.85* | 18.97 ± 3.56 | 15.59 ± 2.77 |
| 癸醛 Decanal | 1.72 ± 0.67 | 44.67 ± 9.35** | 50.53 ± 4.94** | 48.84 ± 3.31** | 45.57 ± 4.06** | 36.43 ± 2.18** | 4.76 ± 1.23* |
| (E)-2-己烯醛 (E)-2-hexenal | - | 20.75 ± 2.72 | 22.05 ± 1.25 | 5.37 ± 1.63 | 1.85 ± 0.04 | 1.61 ± 0.41 | 1.22 ± 0.23 |
| (E,E)-2,4-己二烯醛 (E,E)-2,4-hexadienal | - | 12.54 ± 3.46 | 16.43 ± 2.89 | 3.81 ± 1.06 | 1.32 ± 0.36 | 0.43 ± 0.16 | - |
| (E)-2-壬烯醛 (E)-2-nonenal | - | - | 0.43 ± 0.05 | 0.51 ± 0.10 | 0.64 ± 0.14 | 0.69 ± 0.13 | 0.30 ± 0.10 |
| (E)-2-辛烯醛 (E)-2-octenal | - | - | - | - | 0.68 ± 0.08 | 0.55 ± 0.06 | 0.30 ± 0. 10 |
Table 1 Variation of the release amount of C6-C10 aldehydes in mechanically damaged leaves of Cinnamomum camphora seedlings (mean ± SE)
| C6-C10醛类化合物 C6-C10 aldehydes | 时间 Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | 48 | |
| 糠醛 Furfural | 1.58 ± 0.54 | 2.61 ± 0.59* | 1.18 ± 0.42 | 0.83 ± 0.25 | 1.30 ± 0.35 | 1.08 ± 0.36 | 1.32 ± 0.34 |
| 己醛 Hexanal | 9.53 ± 1.02 | 32.53 ± 4.92** | 33.05 ± 1.62** | 29.03 ± 3.19** | 18.39 ± 0.86** | 14.44 ± 3.01* | 7.06 ± 1.55 |
| 庚醛 Heptanal | 6.23 ± 0. 97 | 8.82 ± 1.21* | 11.22 ± 0.84** | 12.53 ± 2.80** | 9.45 ± 0.87* | 7.67 ± 0.95 | 6.56 ± 0.84 |
| 辛醛 Octanal | 10.11 ± 2.65 | 13.34 ± 3.67* | 23.66 ± 2.31** | 17.37 ± 3.33* | 16.43 ± 3.01* | 14.32 ± 2.95* | 11.03 ± 2.55 |
| 壬醛 Nonanal | 14.26 ± 3.43 | 22.02 ± 4.07** | 27.24 ± 3.34** | 22.91 ± 3.90* | 19.97 ± 2.85* | 18.97 ± 3.56 | 15.59 ± 2.77 |
| 癸醛 Decanal | 1.72 ± 0.67 | 44.67 ± 9.35** | 50.53 ± 4.94** | 48.84 ± 3.31** | 45.57 ± 4.06** | 36.43 ± 2.18** | 4.76 ± 1.23* |
| (E)-2-己烯醛 (E)-2-hexenal | - | 20.75 ± 2.72 | 22.05 ± 1.25 | 5.37 ± 1.63 | 1.85 ± 0.04 | 1.61 ± 0.41 | 1.22 ± 0.23 |
| (E,E)-2,4-己二烯醛 (E,E)-2,4-hexadienal | - | 12.54 ± 3.46 | 16.43 ± 2.89 | 3.81 ± 1.06 | 1.32 ± 0.36 | 0.43 ± 0.16 | - |
| (E)-2-壬烯醛 (E)-2-nonenal | - | - | 0.43 ± 0.05 | 0.51 ± 0.10 | 0.64 ± 0.14 | 0.69 ± 0.13 | 0.30 ± 0.10 |
| (E)-2-辛烯醛 (E)-2-octenal | - | - | - | - | 0.68 ± 0.08 | 0.55 ± 0.06 | 0.30 ± 0. 10 |
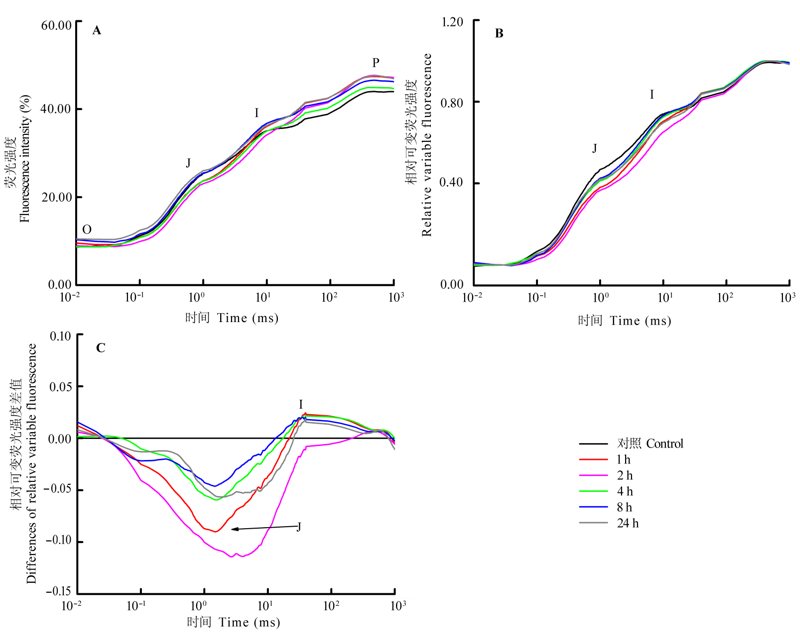
Fig. 2 Variation of chlorophyll fluorescence transients in mechanically damaged leaves of Cinnamomum camphora seedlings. A, O-J-I-P transient recorded in seedlings leaves of C. camphora after mechanical damage (average of three samples). B, relative variable fluorescence (Vt) between Fo and Fm, Vt = (Ft -Fo)/(Fm-Fo), Ft description fluorescence at time t, Fo description minimum fluorescence intensity after dark adaptation, Fm description maximum fluorescence intensity after dark adaptation. C, differences (?Vt) of Vt to the CK, ?Vt = Vt (treatment) - Vt (CK). Meaning of O, J, I and P referred to Li et al. (2005).
| 参数 Parameter | 时间 Time (h) | |||||
|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | |
| ABS/RC | 2.35 ± 0.17 | 2.21 ± 0.15 | 2.05 ± 0.04** | 2.30 ± 0.06 | 2.37 ± 0.07 | 2.40 ± 0.17 |
| TRo/RC | 1.84 ± 0.08 | 1.71 ± 0.05 | 1.66 ± 0.03** | 1.91 ± 0.07 | 1.86 ± 0.06 | 1.96 ± 0.14 |
| ETo/RC | 0.96 ± 0.14 | 1.04 ± 0.09 | 1.01 ± 0.11 | 1.03 ± 0.01 | 1.01 ± 0.01 | 1.15 ± 0.06** |
| ψo | 0.52 ± 0.01 | 0.61 ± 0.01* | 0.64 ± 0.08** | 0.54 ± 0.03 | 0.50 ± 0.06 | 0.51 ± 0.00 |
| ΦEo | 0.45 ± 0.19 | 0.43 ± 0.05 | 0.56 ± 0.01** | 0.41 ± 0.01 | 0.40 ± 0.00 | 0.48 ± 0.06 |
| RC/CS | 3.82 ± 0.37 | 4.26 ± 0.19* | 4.71 ± 0.59** | 3.77 ± 0.37 | 4.21 ± 0.08 | 3.91 ± 0.11 |
| FV/Fm | 0.87 ± 0.01 | 0.89 ± 0.07 | 0.89 ± 0.06 | 0.85 ± 0.01 | 0.75 ± 0.07 | 0.74 ± 0.03 |
| PIABS | 1.86 ± 0.46 | 2.77 ± 1.06** | 3.41 ± 0.83** | 2.27 ± 0.16* | 1.99 ± 0.36 | 1.89 ± 0.51 |
Table 2 Variations of main chlorophyll ?uorescence parameters in mechanically damaged leaves of Cinnamomum camphora seedlings (mean ± SE)
| 参数 Parameter | 时间 Time (h) | |||||
|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | |
| ABS/RC | 2.35 ± 0.17 | 2.21 ± 0.15 | 2.05 ± 0.04** | 2.30 ± 0.06 | 2.37 ± 0.07 | 2.40 ± 0.17 |
| TRo/RC | 1.84 ± 0.08 | 1.71 ± 0.05 | 1.66 ± 0.03** | 1.91 ± 0.07 | 1.86 ± 0.06 | 1.96 ± 0.14 |
| ETo/RC | 0.96 ± 0.14 | 1.04 ± 0.09 | 1.01 ± 0.11 | 1.03 ± 0.01 | 1.01 ± 0.01 | 1.15 ± 0.06** |
| ψo | 0.52 ± 0.01 | 0.61 ± 0.01* | 0.64 ± 0.08** | 0.54 ± 0.03 | 0.50 ± 0.06 | 0.51 ± 0.00 |
| ΦEo | 0.45 ± 0.19 | 0.43 ± 0.05 | 0.56 ± 0.01** | 0.41 ± 0.01 | 0.40 ± 0.00 | 0.48 ± 0.06 |
| RC/CS | 3.82 ± 0.37 | 4.26 ± 0.19* | 4.71 ± 0.59** | 3.77 ± 0.37 | 4.21 ± 0.08 | 3.91 ± 0.11 |
| FV/Fm | 0.87 ± 0.01 | 0.89 ± 0.07 | 0.89 ± 0.06 | 0.85 ± 0.01 | 0.75 ± 0.07 | 0.74 ± 0.03 |
| PIABS | 1.86 ± 0.46 | 2.77 ± 1.06** | 3.41 ± 0.83** | 2.27 ± 0.16* | 1.99 ± 0.36 | 1.89 ± 0.51 |
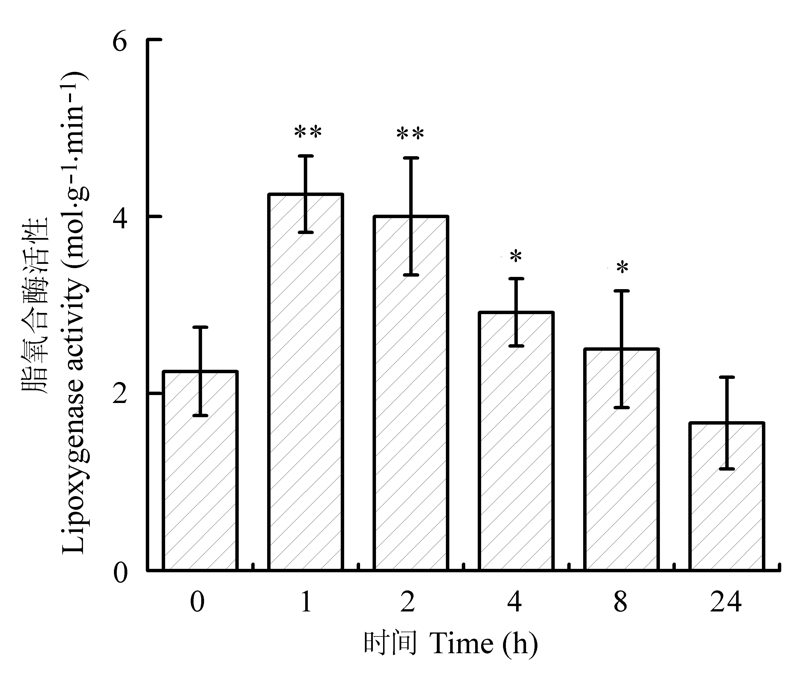
Fig. 3 Activity of lipoxygenase in leaves of Cinnamomum camphora seedlings at different time after mechanical damage (mean ± SE). *, p < 0.05; **, p < 0.01.
| [1] |
Apel K, Hirt H (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373-399.
DOI URL PMID |
| [2] |
Appenroth KJ, Stöckel J, Srivastava A, Strasser RJ (2001). Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environmental Pollution, 115, 49-64.
URL PMID |
| [3] |
Bi JL, Felton GW (1995). Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. Journal of Chemical Ecology, 21, 1511-1529.
URL PMID |
| [4] |
Bown AW, Hall DE, MacGregor KB (2002). Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiology, 129, 1430-1434.
DOI URL PMID |
| [5] |
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004). Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781-1785.
DOI URL PMID |
| [6] |
Feussner I, Wasternack C (2002). The lipoxygenase pathway. Annual Review of Plant Biology, 53, 275-297.
DOI URL PMID |
| [7] | Gao Y, Jin YJ, Li HD, Chen HJ (2005). Volatile organic compounds and their roles in bacteriostasis in five conifer species. Journal of Integrated Plant Biology, 47, 499-507. |
| [8] |
Gouinguené SP, Turlings TCJ (2002). The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiology, 129, 1296-1307.
DOI URL PMID |
| [9] |
Graus M, Schnitzler JP, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J (2004). Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiology, 135, 1967-1975.
URL PMID |
| [10] | Grote R, Keenan T, Lavoir AV, Staudt M (2010). Process- based simulation of seasonality and drought stress in monoterpene emission models. Biogeosciences, 7, 257-274. |
| [11] |
Hartikainen K, Nerg A, Kivimäenpää M, Kontunen-soppela S, Mäenpää M, Oksanen E, Rousi M, Holopainen T (2009). Emissions of volatile organic compounds and leaf structural characteristics of European aspen ( Populus tremula) grown under elevated ozone and temperature. Tree Physiology, 29, 1163-1173.
URL PMID |
| [12] | Hatanaka A (1993). The biogeneration of green odour by green leaves. Phytochemistry, 34, 1201-1218. |
| [13] |
Holopainen JK (2011). Can forest trees compensate for stress-generated growth losses by induced production of volatile compounds? Tree Physiology, 31, 1356-1377.
DOI URL PMID |
| [14] | Hu ZH, Shen YB, Luo YQ, Shen FY, Gao HB, Gao RF (2008). Aldehyde volatiles emitted in succession from mechanically damaged leaves of poplar cuttings. Plant Biology, 51, 269-275. |
| [15] | Hu ZH, Shen YB, Su XH (2009). Saturated aldehydes C6-C10 emitted from ashleaf maple ( Acer negundo L.) leaves at different levels of light intensity, O2, and CO2. Journal of Plant Biology, 52, 289-297. |
| [16] |
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2005). Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against botrytis cinerea in Arabidopsis thaliana. Plant and Cell Physiology, 46, 1093-1102.
URL PMID |
| [17] |
León J, Rojo E, Sánchez-Serrano JJ (2001). Wound signaling in plants. Journal of Experimental Botany, 52, 1-9.
DOI URL PMID |
| [18] | Li PM (李鹏民), Gao HY (高辉远), Strasser RJ (2005). Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Journal of Plant Physiology and Molecular Biology (植物生理与分子生物学学报), 31, 559-566. (in Chinese with English abstract) |
| [19] |
Loreto F, Barta C, Brilli F, Nogues I (2006). On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell & Environment, 29, 1820-1828.
DOI URL PMID |
| [20] |
Loreto F, Schnitzler JP (2010). Abiotic stresses and induced BVOCs. Trends in Plant Science, 15, 154-166.
DOI URL PMID |
| [21] |
Matsui K, Kurishita S, Hisamitsu A, Kajiwara T (2000). A lipid-hydrolysing activity involved in hexenal formation. Biochemical Society Transactions, 28, 857-860.
URL PMID |
| [22] |
Niinemets Ü, Loreto F, Reichstein M (2004). Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science, 9, 180-186.
URL PMID |
| [23] |
Pegoraro E, Rey A, Bobich EG, Barron-Gafford G, Grieve KA, Malhi Y, Murthy R (2004). Effect of elevated CO2 concentration and vapour pressure deficit on isoprene emission from leaves of Populus deltoides during drought. Functional Plant Biology, 31, 1137-1147.
DOI URL PMID |
| [24] |
Peñuelas J, Staudt M (2010). BVOCs and global change. Trends in Plant Science, 15, 133-144.
DOI URL PMID |
| [25] |
Piesik D, Pańka D, Delaney KJ, Skoczek A, Lamparski R, Weaver DK (2011). Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.) Journal of Plant Physiology, 168, 878-886.
URL PMID |
| [26] | Ping LY (平立岩), Shen YB (沈应柏), Jin YJ (金幼菊), Hao JH (郝建华) (2001). Leaf volatiles induced by mechanical damage from diverse taxonomic tree species. Acta Botanica Sinica (植物学报), 43, 261-266. (in Chinese with English abstract) |
| [27] | Sallas L, Kainulainen P, Utriainen J, Holopainen T, Holopainen JK (2001). The influence of elevated O3 and CO2 concentrations on secondary metabolites of Scots pine ( Pinus sylvestris L.) seedlings. Global Change Biology, 7, 303-311. |
| [28] |
Sallas L, Luomala EM, Ultriainen J, Kainulainen P, Holopainen JK (2003). Contrasting effects of elevated carbon dioxide concentration and temperature on rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiology, 23, 97-108.
DOI URL PMID |
| [29] |
Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A (2010). Real-time monitoring of herbivore induced volatile emissions in the field. Physiologia Plantarum, 138, 123-133.
DOI URL PMID |
| [30] | Sharkey TD, Wiberley AE, Donohue AR (2008). Isoprene emission from plants: why and how. Annals of the Missouri Botanical Garden, 101, 5-18. |
| [31] | Shen YB (沈应柏) (2008). Responses of Populus simonii × Populus pyramidalis ‘Opera 8277’ Cuttings to Wounding and Airborne Defensive Signals . (合作杨苗木对伤害和气体防御信号的响应) PhD dissertation, Beijing Forestry University, Beijing, 7-35. (in Chinese with English abstract) |
| [32] |
Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J (2006). Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences of the United States of America, 103, 16672-16676.
URL PMID |
| [33] | Strasser RJ, Srivastava A, Tsimilli-Michael M (2000). The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P eds. Probing Photosynthesis: Mechanisms, Regulation and Adaptation. Taylor and Francis, London. 445-483. |
| [34] | Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal, 11, 1187-1194. |
| [35] | van Heerden PDR, Strasser RJ, Krüger GHJ (2004). Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiolo- gia Plantarum, 121, 239-249. |
| [36] |
van Heerden PDR, Tsimilli-Michael M, Krüger GHJ, Strasser RJ (2003). Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiologia Plantarum, 117, 476-491.
DOI URL PMID |
| [37] |
Wildt J, Kobel K, Schuh-Thomas G, Heiden AC (2003). Emissions of oxygenated volatile organic compounds from plants. Part II: Emissions of saturated aldehydes. Journal of Atmospheric Chemistry, 45, 173-196.
DOI URL |
| [38] |
Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R (2009). Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Global Change Biology, 15, 1189-1200.
DOI URL |
| [39] | Wojtaszek P (1997). Oxidative burst: an early plant response to pathogen infection. Biochemical Journal, 322, 681-692. |
| [40] | Xue W (薛伟), Li XY (李向义), Lin LS (林丽莎), Wang YJ (王迎菊), Li L (李磊) (2011). Effects of short time heat stress on photosystem II, rubisco activities and oxidative radicals in Alhagi sparsifolia. Chinese Journal of Plant Ecology (植物生态学报), 35, 441-451. (in Chinese with English abstract) |
| [41] |
Zeringue HJ (1991). Effect of C6 to C9 alkenals on aflatoxin production in corn, cottonseed, and peanuts. Applied and Environmental Microbiology, 57, 2433-2434.
DOI URL PMID |
| [42] | Zhang FJ (张风娟), Li JQ (李继泉), Xu XY (徐兴友), Meng XD (孟宪东), Chen FJ (陈发菊) (2007). The volatiles of two greening tree species and the antimicrobial activity. Acta Horticulturae Sinica (园艺学报), 34, 4973-4978. (in Chinese with English abstract) |
| [43] | Zuo ZJ (左照江), Zhang RM (张汝民), Wang Y (王勇), Hou P (侯平), Wen GS (温国胜), Gao Y (高岩) (2010). Analysis of main volatile organic compounds and study of aboveground structures in Artemisia frigida. Chinese Journal of Plant Ecology (植物生态学报), 34, 462-468. (in Chinese with English abstract) |
| [1] | REN Pei-Xin, LI Peng, PENG Chang-Hui, ZHOU Xiao-Lu, YANG Ming-Xia. Temporal and spatial variation of vegetation photosynthetic phenology in Dongting Lake basin and its response to climate change [J]. Chin J Plant Ecol, 2023, 47(3): 319-330. |
| [2] | SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(3): 361-373. |
| [3] | SHI Sheng-Bo, SHI Rui, ZHOU Dang-Wei, ZHANG Wen. Effects of low temperature on photochemical and non-photochemical energy dissipation of Kobresia pygmaea leaves [J]. Chin J Plant Ecol, 2023, 47(10): 1441-1452. |
| [4] | XUE Jin-Ru, LÜ Xiao-Liang. Assessment of vegetation productivity under the implementation of ecological programs in the Loess Plateau based on solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2022, 46(10): 1289-1304. |
| [5] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [6] | ZHOU Wen, CHI Yong-Gang, ZHOU Lei. Vegetation phenology in the Northern Hemisphere based on the solar-induced chlorophyll fluorescence [J]. Chin J Plant Ecol, 2021, 45(4): 345-354. |
| [7] | DING Jian-Xi, ZHOU Lei, WANG Yong-Lin, ZHUANG Jie, CHEN Ji-Jing, ZHOU Wen, ZHAO Ning, SONG Jun, CHI Yong-Gang. Application prospects for combining active and passive observations of chlorophyll fluorescence [J]. Chin J Plant Ecol, 2021, 45(2): 105-118. |
| [8] | GUO Qing-Hua, HU Tian-Yu, MA Qin, XU Ke-Xin, YANG Qiu-Li, SUN Qian-Hui, LI Yu-Mei, SU Yan-Jun. Advances for the new remote sensing technology in ecosystem ecology research [J]. Chin J Plant Ecol, 2020, 44(4): 418-435. |
| [9] | LIU Xiao-Ming, YANG Xiao-Fang, WANG Xuan, ZHANG Shou-Ren. Effects of simulated nitrogen deposition on growth and photosynthetic characteristics of Quercus wutaishanica and Acer pictum subsp. mono in a warm-temperate deciduous broad- leaved forest [J]. Chin J Plant Ecol, 2019, 43(3): 197-207. |
| [10] | Jian-Guo CAI, Meng-Qi WEI, Yi ZHANG, Yun-Long WEI. Effects of shading on photosynthetic characteristics and chlorophyll fluorescence parameters in leaves of Hydrangea macrophylla [J]. Chin J Plan Ecolo, 2017, 41(5): 570-576. |
| [11] | Meng-Meng LIU, Li JIA, Lu-Yun CHENG, Hong-Qin ZHANG, Xiao-Lin ZANG, Taogetao BAOYIN, Ru-Min ZHANG, Yan GAO. Responses of phenolic acid and defensive enzyme activities to mechanical damage in Artemisia frigida [J]. Chin J Plant Ecol, 2017, 41(2): 219-230. |
| [12] | Da-Yong FAN, Zeng-Juan FU, Zong-Qiang XIE, Rong-Gui LI, Shu-Min ZHANG. A new technology of modulated Chl a fluorescence image: In vivo measurement of the PSII maximum photochemical efficiency and its heterogeneity within leaves [J]. Chin J Plant Ecol, 2016, 40(9): 942-951. |
| [13] | Zi-Jun WANG, Guang-Rong SHEN, Yun ZHU, Yu-Jie HAN, Chun-Jiang LIU, Zhe WANG, Chun-Yan XUE. Research on characteristics of biomass distribution in urban forests of Shanghai metropolis based on remote sensing and spatial analysis [J]. Chin J Plant Ecol, 2016, 40(4): 385-394. |
| [14] | LIU Chang,SUN Peng-Sen,LIU Shi-Rong. A review of plant spectral reflectance response to water physiological changes [J]. Chin J Plan Ecolo, 2016, 40(1): 80-91. |
| [15] | AN Dong-Sheng,CAO Juan,HUANG Xiao-Hua,ZHOU Juan,DOU Mei-An. Application of Lake-model based indices from chlorophyll fluorescence on sugarcane seedling drought resistance study [J]. Chin J Plan Ecolo, 2015, 39(4): 398-406. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn