

Chin J Plant Ecol ›› 2010, Vol. 34 ›› Issue (4): 462-468.DOI: 10.3773/j.issn.1005-264x.2010.04.012
• Research Communications • Previous Articles Next Articles
ZUO Zhao-Jiang1,3, ZHANG Ru-Min2, WANG Yong1, HOU Ping2, WEN Guo-Sheng2, GAO Yan2,3,*( )
)
Received:2009-09-16
Accepted:2009-11-24
Online:2010-09-16
Published:2010-04-01
Contact:
GAO Yan
ZUO Zhao-Jiang, ZHANG Ru-Min, WANG Yong, HOU Ping, WEN Guo-Sheng, GAO Yan. Analysis of main volatile organic compounds and study of aboveground structures in Artemisia frigida[J]. Chin J Plant Ecol, 2010, 34(4): 462-468.
| 保留时间 Retention time (min) | 挥发性有机化合物 Volatile organic compounds | 分子式 Chemical formula | 相对含量 Relative content (%) | |||
|---|---|---|---|---|---|---|
| 未损伤 UDa | 损伤Da | 中文名称 Chinese name | 英文名称 English name | 未损伤 UDa | 损伤 Da | |
| 13.51 | 顺-3-己烯醛 | cis-3-Hexenal | C6H10O | - | 1.15 ± 0.13 | |
| 15.57 | 2-己烯醛 | 2-Hentenal | C6H10O | - | 1.34 ± 0.18 | |
| 15.57 | 15.82 | 3-己烯醇 | 3-Hexenol(c,t) | C6H12O | 0.05 ± 0.01 | 2.81 ± 0.23 |
| 16.14 | 己醇 | 1-Hexanol | C6H14O | - | 0.50 ± 0.04 | |
| 16.23 | 乙酸异戊酯 | Isopentyl alcohol,acetate | C7H14O2 | - | 0.17 ± 0.05 | |
| 18.01 | 18.02 | α-蒎烯 | α-Pinene | C10H16 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| 18.38 | 18.40 | β-蒎烯 | β-Pinene | C10H16 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| 19.05 | 19.06 | 檀香三烯 | Santolina triene | C10H16 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 19.71 | 19.71 | 水芹烯 | Phellandrene | C10H16 | 0.15 ± 0.02 | 0.39 ± 0.09 |
| 20.07 | 20.09 | 莰烯 | Camphene | C10H16 | 14.27 ± 1.29 | 6.28 ± 0.58 |
| 20.29 | 1-壬烯-3-醇 | 1-Nonen-3-ol | C9H18O | - | 0.58 ± 0.07 | |
| 20.56 | 20.74 | (E)-乙酸-3-己烯酯 | (E)-3-Hexen-1-ol,acetate | C8H14O2 | 10.85 ± 0.83 | 10.96 ± 1.37 |
| 21.54 | 21.79 | 对-伞花烃 | p-Cymene | C10H14 | 9.05 ± 0.79 | 4.11 ± 0.08 |
| 21.95 | 22.02 | 桉树脑 | Eucalyptol | C10H18O | 39.80 ± 2.24 | 22.43 ± 2.49 |
| 22.20 | 2,4-癸二烯-1-醇 | 2,4-Decadien-1-ol | C10H18O | 0.15 ± 0.03 | - | |
| 22.47 | 22.55 | 萜品烯 | Terpinen | C10H16 | 0.93 ± 0.11 | 1.12 ± 0.27 |
| 23.08 | 23.27 | α-萜品醇 | α-Terpineol | C10H18O | 10.04 ± 0.98 | 11.01 ± 0.99 |
| 23.75 | 里纳醇 | Linalol | C10H18O | - | 0.13 ± 0.07 | |
| 23.90 | 23.94 | 黄瓜醇 | Cucumber alcohol | C9H16O | 0.07 ± 0.01 | 0.23 ± 0.04 |
| 24.07 | 24.22 | β-萜品醇 | β-Terpineol | C10H18O | 2.48 ± 0.45 | 3.56 ± 0.26 |
| 25.67 | 25.76 | 樟脑 | Camphor | C10H18O | 5.66 ± 0.97 | 7.85 ± 0.84 |
| 25.87 | 顺-牻牛儿醇 | cis-Geraniol | C10H18O | - | 2.66 ± 0.47 | |
| 26.45 | (R)-(-)-对-薄荷-1-烯-4-醇 | (R)-(-)-p-Menth-1-en-4-ol | C10H18O | 3.84 ± 0.74 | - | |
| 26.58 | 冰片 | Borneol | C10H18O | - | 4.47 ± 0.33 | |
| 26.82 | 26.94 | (S)-(-)-对-薄荷-1-烯-8-醇 | (S)-(-)-p-menth-1-en-8-ol | C10H18O | 0.88 ± 0.12 | 1.80 ± 0.18 |
| 28.64 | 乙酸橙花酯 | Nerol acetate | C12H20O2 | 1.00 ± 0.21 | - | |
| 28.81 | (1R,4R)-(+)-对-薄荷2,8-二烯 | (1R,4R)-(+)-p-Mentha-2,8-diene | C12H20 | - | 9.15 ± 0.86 | |
| 29.27 | 乙酸冰片酯 | Bornyl acetate | C12H20O2 | - | 1.37 ± 0.29 | |
| 31.71 | 31.75 | 古巴烯 | Copaene | C15H24 | 0.25 ± 0.04 | 1.50 ± 0.15 |
| 32.98 | 33.02 | (Z)-法呢烯 | (Z)-Farnesene | C15H24 | 0.02 ± 0.00 | 0.13 ± 0.02 |
| 34.32 | 柏木烯 | Cedrene | C15H24 | - | 0.33 ± 0.03 | |
| 34.42 | 34.50 | 大根叶香烯D | Germacrene D | C15H24 | 0.12 ± 0.01 | 2.48 ± 0.14 |
| 34.70 | 异石竹烯 | Isocaryophillene | C15H24 | 0.21 ± 0.01 | - | |
| 34.76 | 4(14), 11-桉叶双烯 | Eudesma-4(14),11-diene | C15H24 | - | 1.30 ± 0.13 | |
Table 1 The main components of the volatile organic compounds (VOCs) from Artemisia frigida
| 保留时间 Retention time (min) | 挥发性有机化合物 Volatile organic compounds | 分子式 Chemical formula | 相对含量 Relative content (%) | |||
|---|---|---|---|---|---|---|
| 未损伤 UDa | 损伤Da | 中文名称 Chinese name | 英文名称 English name | 未损伤 UDa | 损伤 Da | |
| 13.51 | 顺-3-己烯醛 | cis-3-Hexenal | C6H10O | - | 1.15 ± 0.13 | |
| 15.57 | 2-己烯醛 | 2-Hentenal | C6H10O | - | 1.34 ± 0.18 | |
| 15.57 | 15.82 | 3-己烯醇 | 3-Hexenol(c,t) | C6H12O | 0.05 ± 0.01 | 2.81 ± 0.23 |
| 16.14 | 己醇 | 1-Hexanol | C6H14O | - | 0.50 ± 0.04 | |
| 16.23 | 乙酸异戊酯 | Isopentyl alcohol,acetate | C7H14O2 | - | 0.17 ± 0.05 | |
| 18.01 | 18.02 | α-蒎烯 | α-Pinene | C10H16 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| 18.38 | 18.40 | β-蒎烯 | β-Pinene | C10H16 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| 19.05 | 19.06 | 檀香三烯 | Santolina triene | C10H16 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 19.71 | 19.71 | 水芹烯 | Phellandrene | C10H16 | 0.15 ± 0.02 | 0.39 ± 0.09 |
| 20.07 | 20.09 | 莰烯 | Camphene | C10H16 | 14.27 ± 1.29 | 6.28 ± 0.58 |
| 20.29 | 1-壬烯-3-醇 | 1-Nonen-3-ol | C9H18O | - | 0.58 ± 0.07 | |
| 20.56 | 20.74 | (E)-乙酸-3-己烯酯 | (E)-3-Hexen-1-ol,acetate | C8H14O2 | 10.85 ± 0.83 | 10.96 ± 1.37 |
| 21.54 | 21.79 | 对-伞花烃 | p-Cymene | C10H14 | 9.05 ± 0.79 | 4.11 ± 0.08 |
| 21.95 | 22.02 | 桉树脑 | Eucalyptol | C10H18O | 39.80 ± 2.24 | 22.43 ± 2.49 |
| 22.20 | 2,4-癸二烯-1-醇 | 2,4-Decadien-1-ol | C10H18O | 0.15 ± 0.03 | - | |
| 22.47 | 22.55 | 萜品烯 | Terpinen | C10H16 | 0.93 ± 0.11 | 1.12 ± 0.27 |
| 23.08 | 23.27 | α-萜品醇 | α-Terpineol | C10H18O | 10.04 ± 0.98 | 11.01 ± 0.99 |
| 23.75 | 里纳醇 | Linalol | C10H18O | - | 0.13 ± 0.07 | |
| 23.90 | 23.94 | 黄瓜醇 | Cucumber alcohol | C9H16O | 0.07 ± 0.01 | 0.23 ± 0.04 |
| 24.07 | 24.22 | β-萜品醇 | β-Terpineol | C10H18O | 2.48 ± 0.45 | 3.56 ± 0.26 |
| 25.67 | 25.76 | 樟脑 | Camphor | C10H18O | 5.66 ± 0.97 | 7.85 ± 0.84 |
| 25.87 | 顺-牻牛儿醇 | cis-Geraniol | C10H18O | - | 2.66 ± 0.47 | |
| 26.45 | (R)-(-)-对-薄荷-1-烯-4-醇 | (R)-(-)-p-Menth-1-en-4-ol | C10H18O | 3.84 ± 0.74 | - | |
| 26.58 | 冰片 | Borneol | C10H18O | - | 4.47 ± 0.33 | |
| 26.82 | 26.94 | (S)-(-)-对-薄荷-1-烯-8-醇 | (S)-(-)-p-menth-1-en-8-ol | C10H18O | 0.88 ± 0.12 | 1.80 ± 0.18 |
| 28.64 | 乙酸橙花酯 | Nerol acetate | C12H20O2 | 1.00 ± 0.21 | - | |
| 28.81 | (1R,4R)-(+)-对-薄荷2,8-二烯 | (1R,4R)-(+)-p-Mentha-2,8-diene | C12H20 | - | 9.15 ± 0.86 | |
| 29.27 | 乙酸冰片酯 | Bornyl acetate | C12H20O2 | - | 1.37 ± 0.29 | |
| 31.71 | 31.75 | 古巴烯 | Copaene | C15H24 | 0.25 ± 0.04 | 1.50 ± 0.15 |
| 32.98 | 33.02 | (Z)-法呢烯 | (Z)-Farnesene | C15H24 | 0.02 ± 0.00 | 0.13 ± 0.02 |
| 34.32 | 柏木烯 | Cedrene | C15H24 | - | 0.33 ± 0.03 | |
| 34.42 | 34.50 | 大根叶香烯D | Germacrene D | C15H24 | 0.12 ± 0.01 | 2.48 ± 0.14 |
| 34.70 | 异石竹烯 | Isocaryophillene | C15H24 | 0.21 ± 0.01 | - | |
| 34.76 | 4(14), 11-桉叶双烯 | Eudesma-4(14),11-diene | C15H24 | - | 1.30 ± 0.13 | |
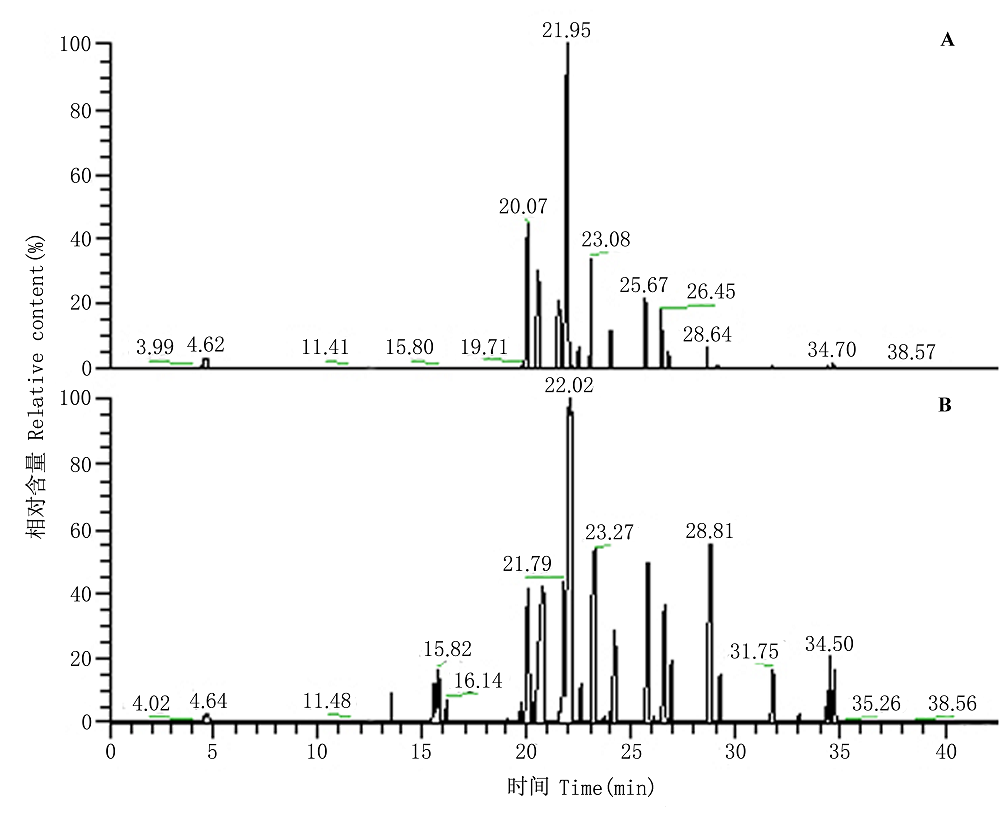
Fig. 1 The total ion current (TIC) of volatile organic compounds (VOCs) from undamaged (A) to damaged (B) Artemisia frigida was analysed by thermal-desorption cold trap/gas chromatography/mass spectrum (TCT/GC/MS).
| [1] | Baldlacchi D, Guenther A, Harley P, Klinger L, Zimmerman P, Lamb B, Westberg H (1995). The fluxes and air chemistry of isoprene above a deciduous hardwood forest. Philosophical Transactions of the Royal Society A, 350, 279-296. |
| [2] |
Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006). Volatile signaling in plant-plant interactions: talking trees in the genomics era. Science, 311, 812-815.
DOI URL PMID |
| [3] |
Dixon RA (2001). Natural products and plant disease resistance. Nature, 411, 843-847.
URL PMID |
| [4] | Du JW (杜家纬) (2001). Plant-insect chemical communication and its behavior control. Acta Photophysiologica Sinica (植物生理学报), 27, 193-200. (in Chinese with English abstract) |
| [5] |
Dudareva N, Pichersky E (2000). Biochemistry and molecular aspects of floral scent. Plant Physiology, 122, 627-634.
URL PMID |
| [6] |
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004). Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781-1785.
DOI URL PMID |
| [7] |
Fall R, Karl T, Hansel A, Jordan A, Lindinger W (1999). Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. Journal of Geophysical Research, 104, 15963-15974.
DOI URL |
| [8] |
Gao Y, Jin YJ, Li HD, Chen HJ (2005). Volatile organic compounds and their roles in bacteriostasis in five conifer species. Journal of Integrative Plant Biology, 47, 499-507.
DOI URL |
| [9] |
Guenther AB, Monson RK, Fall R (1991). Isoprene and monoterpene emission rate variability: observations with Eucalyptus and emission rate algorithm development. Journal of Geophysical Research, 96, 10799-10808.
DOI URL |
| [10] |
Heil M (2007). Indirect defence via tritrophic interactions. New Phytologist, 178, 41-61.
DOI URL |
| [11] | Heil M, Bueno JCS (2007). Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences of the United States of America, 140, 5467-5472. |
| [12] |
Lerdau M, Dilts SB, Westberg H, Lamb BK, Aiiwine EJ (1994). Monoterpene emission from Ponderosa pine. Journal of Geophysical Research, 99, 16609-16615.
DOI URL |
| [13] | Li XG (李新岗), Liu HX (刘惠霞), Liu LP (刘拉平), Ma YM (马养民) (2006). Study on host-plant volatiles affecting the host selection of Dioryctria pryeri. Scientia Silvae Sinicae (林业科学), 42, 71-78. (in Chinese with English abstract) |
| [14] |
Loreto F, Pinelli P, Manes F, Kollist H (2004). Impact of ozone on monoterpene emissions and evidence for an isoprene- like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiology, 24, 361-367.
DOI URL PMID |
| [15] | Ma RJ (马瑞君), Wang ML (王明理), Zhu XT (朱学泰), Lu XW (鲁先文), Sun K (孙坤) (2005). Allelopathy and chemical constituents of Ligularia virgaurea volatile. Chinese Journal of Applied Ecology (应用生态学报), 16, 1826-1829. (in Chinese with English abstract) |
| [16] |
Pàre PW, Tumlinson JH (1997). Denovo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiology, 114, 1161-1167.
DOI URL PMID |
| [17] |
Peñuelas J, Llusià J (2003). BVOCs: plant defense against climate warming. Trends in Plant Science, 8, 105-109.
URL PMID |
| [18] |
Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK (2004). Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biology, 6, 12-21.
DOI URL PMID |
| [19] |
Sharkey TD, Chen X, Yeh S (2001). Isoprene increase thermotolerance of fosmidomycin fed leaves. Plant Physiology, 125, 2001-2006.
URL PMID |
| [20] |
Singsaas L (2000). Terpenes and the thermotolerance of photosynthesis. New Phytologist, 146, 1-16.
DOI URL |
| [21] |
Steinberg S, Dicke M, Vet LEM (1993). Relative importance of info chemicals from first and second trophic level in long range host location by the larval parasitoid Cotesia (= Apanteles) glomerata. Journal of Chemical Ecology, 19, 47-59.
DOI URL PMID |
| [22] |
van den Boom CE, van Beek TA, Posthumus MA, de Groot A, Dicke M (2004). Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. Journal of Chemical Ecology, 30, 69-89.
DOI URL PMID |
| [23] |
Vuorinen T, Nerg AM, Ibrahim MA, Reddy GVP, Holopainen JK (2004). Emission of Plutella xylostella―induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiology, 135, 1984-1992.
DOI URL PMID |
| [24] | Yin J (尹姣), Cao YZ (曹雅忠), Luo LZ (罗礼智), Hu Y (胡毅) (2005). Oviposition preference of the meadow moth, Loxostege sticticalis L., on different host plants and its chemical mechanism. Acta Ecologica Sinica (生态学报), 25, 1844-1852. (in Chinese with English abstract) |
| [25] | Zuo ZJ (左照江), Zhang RM (张汝民), Gao Y (高岩) (2009a). Research advances in volatile signals among plants. Chinese Bulletin of Botany (植物学报), 44, 245-252. (in Chinese with English abstract) |
| [26] | Zuo ZJ (左照江), Zhang RM (张汝民), Zhu JH (朱金胡), Wen GS (温国胜), Hou P (侯平), Gao Y (高岩) (2009b). Effects of volatile organic compounds (VOCs) from Artemisia frigida on germination and growth of four plant types. Journal of Zhejiang Forestry College (浙江林学院学报), 26, 76-82. (in Chinese with English abstract) |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn