

Chin J Plant Ecol ›› 2016, Vol. 40 ›› Issue (11): 1208-1217.DOI: 10.17521/cjpe.2015.0470
• Research Articles • Previous Articles
Zi-Piao YE1,*( ), Wen-Hai HU2, Xiao-Hong YAN2,3
), Wen-Hai HU2, Xiao-Hong YAN2,3
Received:2015-12-22
Accepted:2016-07-19
Online:2016-11-10
Published:2016-11-25
Contact:
Zi-Piao YE
Zi-Piao YE, Wen-Hai HU, Xiao-Hong YAN. Comparison on light-response models of actual photochemical efficiency in photosystem II[J]. Chin J Plant Ecol, 2016, 40(11): 1208-1217.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2015.0470
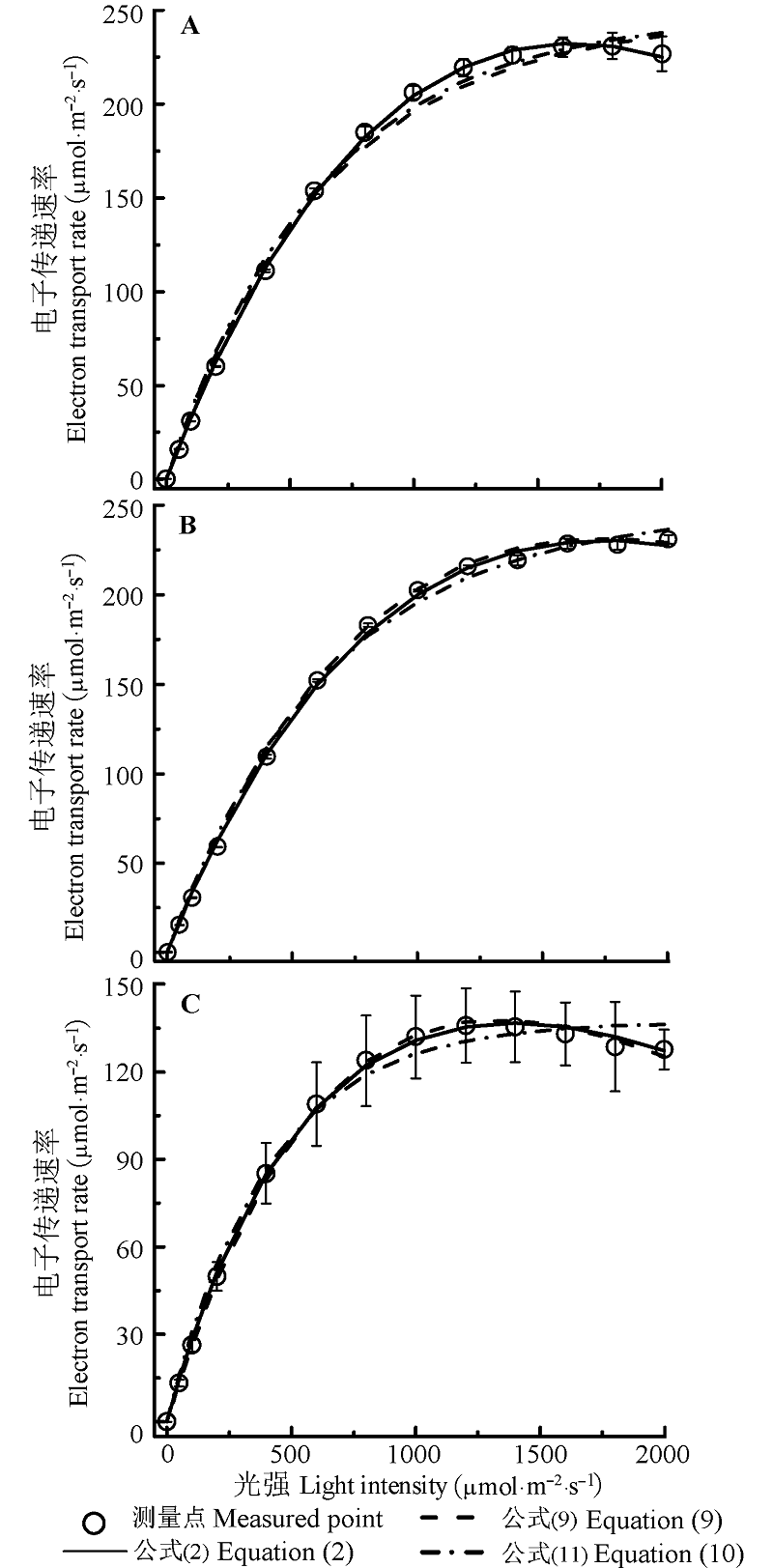
Fig. 1 Light-response curves of electron transport rate (ETR- I) for Coreopsis lanceolata (A), Vitex negundo (B) and Bidens frondosa (C) (mean ± SE). Equation (2) is ETR =α (1-β I) / (1 + γI) I. Here α is the initial slope of ETR-I, β is the photoinhibition term, γ is the saturation term, I is light intensity; Equation (9) is ETR =α'β'IΦPSIImaxe-kwI. Here α' is the light absorption coefficient of leaf, β' is the light energy distribution coefficient between photosystem II and photosystem I, ΦPSIImax is the maximum photochemical quantum efficiency while I = 0, kw is a constant; Equation (11) is ETR = α'β'(Fv/Fm) PARsat [1-exp(-I/PARsat)]. Here Fv/Fm is the maximum quantum of photosystem II, PARsat is the saturation irradiance.
| 光合参数 Photosynthetic parameter | 剑叶金鸡菊 C. lanceolata | 黄荆 V. negundo | 大狼杷草 B. frondosa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 公式(2) Equation (2) | 公式(9) Equation (9) | 公式(11) Equation (11) | 测量值 Measured value | 公式(2) Equation (2) | 公式(9) Equation (9) | 公式(11) Equation (11) | 测量值 Measured value | 公式(2) Equation (2) | 公式(9) Equation (9) | 公式(11) Equation (11) | 测量值 Measured value | ||||||||
| ETR-I的初始斜率 Initial slope of ETR-I (μmol·μmol -1) | 0.347 | ΦPSIImax (0.859) | Fv/Fm (0.954) | - | 0.352 | ΦPSIImax (0.834) | Fv/Fm (0.928) | - | 0.315 | ΦPSIImax (0.675) | Fv/Fm (0.820) | - | |||||||
| 最大电子传递速率 Maximu electron transport rate (μmol·m-2·s-1) | 232.3 | 231.3 | 156.5 | 230.4 | 230.2 | 229.8 | 155.99 | ≈228.8 | 136.7 | 137.28 | 86.67 | ≈135.8 | |||||||
| 饱和光强 Saturation irradiance (μmol·m-2·s-1) | 1 641.7 | 1 742.2 | 617.83 | ≈1 600 | 1 740.9 | 1 782.5 | 633.1 | ≈1 750 | 1 394.5 | 1 326.3 | 395.2 | ≈1 400 | |||||||
| 叶绿素含量 Chlorophyll content (mg·m-2) | 253.68 | 268.18 | 286.33 | ||||||||||||||||
| 本征截面 Eign-absorption cross section (10-21 m2) | 5.41 | - | - | - | 5.19 | - | - | - | 4.35 | - | - | - | |||||||
| 决定系数 Determined coefficient | 1.000 | 0.998 | 0.995 | - | 0.999 | 0.999 | 0.997 | - | 0.998 | 0.997 | 0.995 | - | |||||||
| AIC信息准则 Akaike’s information criterion | 7.40 | 7.34 | 9.22 | - | 8.34 | 7.48 | 8.78 | - | 7.74 | 7.27 | 8.46 | - | |||||||
Table 1 The measured data and fitted values for Coreopsis lanceolata, Vitex negundo and Bidens frondosa using three models
| 光合参数 Photosynthetic parameter | 剑叶金鸡菊 C. lanceolata | 黄荆 V. negundo | 大狼杷草 B. frondosa | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 公式(2) Equation (2) | 公式(9) Equation (9) | 公式(11) Equation (11) | 测量值 Measured value | 公式(2) Equation (2) | 公式(9) Equation (9) | 公式(11) Equation (11) | 测量值 Measured value | 公式(2) Equation (2) | 公式(9) Equation (9) | 公式(11) Equation (11) | 测量值 Measured value | ||||||||
| ETR-I的初始斜率 Initial slope of ETR-I (μmol·μmol -1) | 0.347 | ΦPSIImax (0.859) | Fv/Fm (0.954) | - | 0.352 | ΦPSIImax (0.834) | Fv/Fm (0.928) | - | 0.315 | ΦPSIImax (0.675) | Fv/Fm (0.820) | - | |||||||
| 最大电子传递速率 Maximu electron transport rate (μmol·m-2·s-1) | 232.3 | 231.3 | 156.5 | 230.4 | 230.2 | 229.8 | 155.99 | ≈228.8 | 136.7 | 137.28 | 86.67 | ≈135.8 | |||||||
| 饱和光强 Saturation irradiance (μmol·m-2·s-1) | 1 641.7 | 1 742.2 | 617.83 | ≈1 600 | 1 740.9 | 1 782.5 | 633.1 | ≈1 750 | 1 394.5 | 1 326.3 | 395.2 | ≈1 400 | |||||||
| 叶绿素含量 Chlorophyll content (mg·m-2) | 253.68 | 268.18 | 286.33 | ||||||||||||||||
| 本征截面 Eign-absorption cross section (10-21 m2) | 5.41 | - | - | - | 5.19 | - | - | - | 4.35 | - | - | - | |||||||
| 决定系数 Determined coefficient | 1.000 | 0.998 | 0.995 | - | 0.999 | 0.999 | 0.997 | - | 0.998 | 0.997 | 0.995 | - | |||||||
| AIC信息准则 Akaike’s information criterion | 7.40 | 7.34 | 9.22 | - | 8.34 | 7.48 | 8.78 | - | 7.74 | 7.27 | 8.46 | - | |||||||
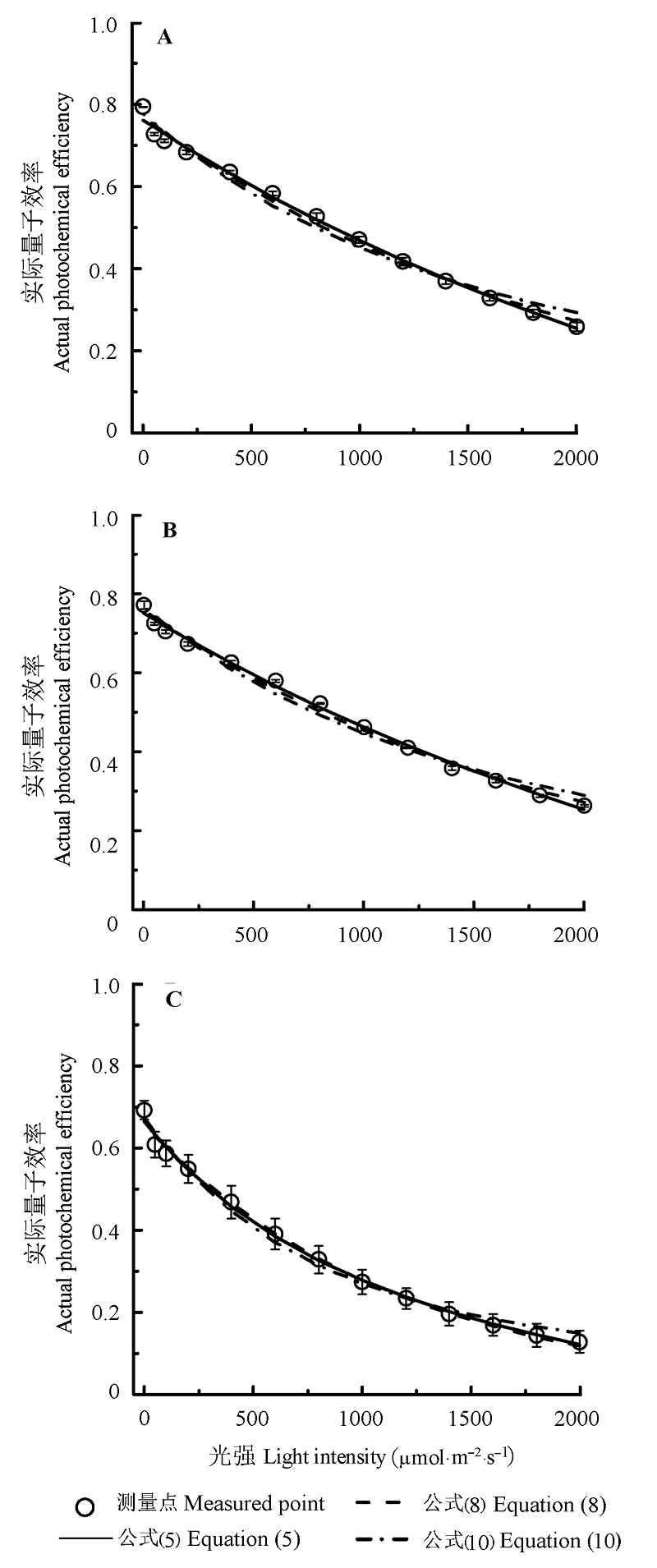
Fig. 2 Light-response curves of ΦPSII for Coreopsis lanceolata (A), Vitex negundo (B) and Bideas frondosa (C)(mean ± SE). Equation (5) is ΦPSII = ω (1-βI) / (1 + γI). Here ω is the initial slope of ΦPSII-I; Equation (8) is ΦPSII = ΦPSIImax e-kwI; Equation (10) is ΦPSII = (Fv/Fm) PARsat / I (1-exp(-I/PARsat)). The definition of β, γ, Fv/Fm, ΦPSIImax, kw and PARsat see Fig. 1.
| 光合参数 Photosynthetic parameter | 剑叶金鸡菊 C. lanceolata | 黄荆 V. negundo | 大狼杷草 B. frondosa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 公式(5) Equation (5) | 公式(8) Equation (8) | 公式(10) Equation (10) | 测量值 Measured value | 公式(5) Equation (5) | 公式(8) Equation (8) | 公式(10) Equation (10) | 测量值 Measured value | 公式(5) Equation (5) | 公式(8) Equation (8) | 公式(10) Equation (10) | 测量值 Measured value | |||
| ΦPSII-I的初始斜率 Initial slope of ΦPSII-I (μmol·μmol -1) | 0.763 | ΦPSIImax (0.771) | Fv/Fm (0.779) | - | 0.752 | ΦPSIImax (0.759) | Fv/Fm (0.767) | - | 0.665 | ΦPSIImax (0.678) | Fv/Fm (0.674) | - | ||
| 饱和光强 Saturation irradiance (μmol·m-2·s-1) | 1 642.9 | 1 744.9 | 824.95 | ≈1 600 | 1 712.7 | 1 763.3 | 830.33 | ≈1 750 | 1 406.7 | 1 331.2 | 448.50 | ≈1 400 | ||
| 决定系数 Determined coefficient | 0.995 | 0.992 | 0.984 | - | 0.996 | 0.994 | 0.986 | - | 0.996 | 0.995 | 0.991 | - | ||
| AIC信息准则 Akaike’s information criterion | -2.49 | -2.75 | -2.03 | - | -2.79 | -3.04 | -2.20 | - | -2.51 | -3.06 | -2.46 | - | ||
Table 2 The measured data and fitted values for Coreopsis lanceolata, Vitex negundo and Bidens frondosa using three models
| 光合参数 Photosynthetic parameter | 剑叶金鸡菊 C. lanceolata | 黄荆 V. negundo | 大狼杷草 B. frondosa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 公式(5) Equation (5) | 公式(8) Equation (8) | 公式(10) Equation (10) | 测量值 Measured value | 公式(5) Equation (5) | 公式(8) Equation (8) | 公式(10) Equation (10) | 测量值 Measured value | 公式(5) Equation (5) | 公式(8) Equation (8) | 公式(10) Equation (10) | 测量值 Measured value | |||
| ΦPSII-I的初始斜率 Initial slope of ΦPSII-I (μmol·μmol -1) | 0.763 | ΦPSIImax (0.771) | Fv/Fm (0.779) | - | 0.752 | ΦPSIImax (0.759) | Fv/Fm (0.767) | - | 0.665 | ΦPSIImax (0.678) | Fv/Fm (0.674) | - | ||
| 饱和光强 Saturation irradiance (μmol·m-2·s-1) | 1 642.9 | 1 744.9 | 824.95 | ≈1 600 | 1 712.7 | 1 763.3 | 830.33 | ≈1 750 | 1 406.7 | 1 331.2 | 448.50 | ≈1 400 | ||
| 决定系数 Determined coefficient | 0.995 | 0.992 | 0.984 | - | 0.996 | 0.994 | 0.986 | - | 0.996 | 0.995 | 0.991 | - | ||
| AIC信息准则 Akaike’s information criterion | -2.49 | -2.75 | -2.03 | - | -2.79 | -3.04 | -2.20 | - | -2.51 | -3.06 | -2.46 | - | ||
| 剑叶金鸡菊 C. lanceolata | 黄荆 V. negundo | 大狼杷草 B. frondosa | ||||||
|---|---|---|---|---|---|---|---|---|
| ΦPSIImax | Fv/Fm | ΦPSIImax | Fv/Fm | ΦPSIImax | Fv/Fm | |||
| 公式(8) Equation (8) | 0.859 ± 0.011a | - | 0.834 ± 0.005a | - | 0.678 ± 0.059a | - | ||
| 公式(9) Equation (9) | 0.771 ± 0.004b | - | 0.759 ± 0.006b | - | 0.675 ± 0.051a | - | ||
| 公式(10) Equation (10) | - | 0.954 ± 0.019a | - | 0.928 ± 0.005a | - | 0.820 ± 0.079a | ||
| 公式(11) Equation (11) | - | 0.779 ± 0.004b | - | 0.767 ± 0.006b | - | 0.674 ± 0.029a | ||
| α/ω | 0.455 ± 0.007* | 0.468 ± 0.008* | 0.474 ± 0.023ns | |||||
Table 3 The fitted values of ΦPSII-I curves for Coreopsis lanceolata, Vitex negundo and Bidens frondosa using three models (mean ± SE)
| 剑叶金鸡菊 C. lanceolata | 黄荆 V. negundo | 大狼杷草 B. frondosa | ||||||
|---|---|---|---|---|---|---|---|---|
| ΦPSIImax | Fv/Fm | ΦPSIImax | Fv/Fm | ΦPSIImax | Fv/Fm | |||
| 公式(8) Equation (8) | 0.859 ± 0.011a | - | 0.834 ± 0.005a | - | 0.678 ± 0.059a | - | ||
| 公式(9) Equation (9) | 0.771 ± 0.004b | - | 0.759 ± 0.006b | - | 0.675 ± 0.051a | - | ||
| 公式(10) Equation (10) | - | 0.954 ± 0.019a | - | 0.928 ± 0.005a | - | 0.820 ± 0.079a | ||
| 公式(11) Equation (11) | - | 0.779 ± 0.004b | - | 0.767 ± 0.006b | - | 0.674 ± 0.029a | ||
| α/ω | 0.455 ± 0.007* | 0.468 ± 0.008* | 0.474 ± 0.023ns | |||||
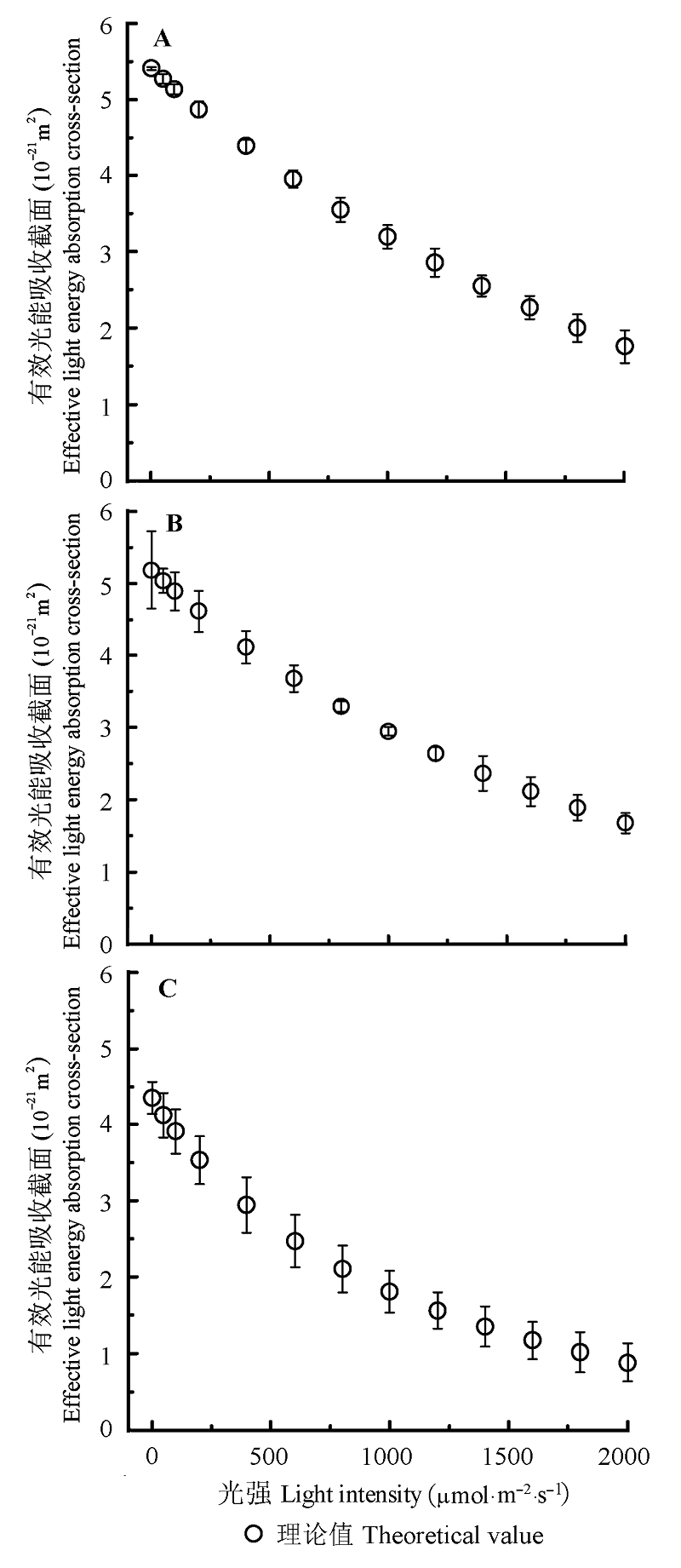
Fig. 3 Light-response curves of effective light energy absorption cross-section (σ'ik-I) for Coreopsis lanceolata (A), Vitex negundo (B) and Bidens frondosa (C)(mean ± SE).
| [1] | Akaike H (1973). A new look at the statistical model identifica- tion.IEEE Translations Automatic Control, 19, 716-723. |
| [2] | An DS, Cao J, Huang XH, Zhou J, Dou MA (2015). Application of Lake-model based indices from chlorophyll fluorescence on sugarcane seedling drought resistance study.Chinese Journal of Plant Ecology, 39, 398-406. (in Chinese with English abstract)[安东升, 曹娟, 黄小华, 周娟, 窦美安 (2015). 基于Lake模型的叶绿素荧光参数在甘蔗苗期抗旱性研究中的应用. 植物生态学报, 39, 398-406.] |
| [3] | Arnon DJ (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24, 1-15. |
| [4] | Baker NR (2008). Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annual of Review Plant Biology, 59, 89-113. |
| [5] | Ehleringer J, Pearcy RW (1983). Variation in quantum yield for CO2 uptake among C3 and C4 plants.Plant Physiology, 73, 555-559. |
| [6] | Feng HQ, Jiao QS, Tian WY, Sun K, Jia LY (2014). Effects of extracellular ATP on the characteristics of photochemical reaction in bean (Phaseolus vulgaris) leaves under differ- ent light intensities. Chinese Journal of Plant Ecology, 38, 1117-1123. (in Chinese with English abstract)[冯汉青, 焦青松, 田武英, 孙坤, 贾凌云 (2014). 不同光强下细胞外三磷酸腺苷对菜豆叶片光化学反应特性的影响. 植物生态学报, 38, 1117-1123.] |
| [7] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence.Biochimica et Biophysica Acta, 990, 87-92. |
| [8] | Goh CH, Ko SM, Koh S, Kim YJ, Bae HJ (2012). Photosyn- thesis and environments: Photoinhibition and repair mech- anisms in plants.Journal of Plant Biology, 55, 93-101. |
| [9] | Govindjee (2002). A role for a light-harvesting antenna complex of photosystem II in photoprotection.The Plant Cell, 14, 1663-1668. |
| [10] | Krall JP, Edward GE (1992). Relationship between photo- system II activity and CO2 fixation in leaves.Physiologia Plantarum, 86,180-187. |
| [11] | Laws E, Sakshaug E, Babin M, Dandonneau Y, Falkowski P, Geider R, Legendre L, Morel A, Sondergaard M, Takahashi M, Williams PJ (2002). Photosynthesis and primary productivity in marine ecosystems: Practical aspects and application of techniques.Joint Global Ocean Flux Study, 36, 1-77. |
| [12] | Lin L, Tang Y, Zhang JT, Yan WL, Xiao JH, Ding C, Dong C, Ji ZS (2015). Effects of different water potentials on leaf gas exchange and chlorophyll fluorescence parameters of cucumber during post-flowering growth stage.Chinese Journal of Applied Ecology, 26, 2030-2040. (in Chinese with English abstract)[林琭, 汤昀, 张纪涛, 闫万丽, 肖建红, 丁超, 董川, 籍增顺 (2015). 不同水势对黄瓜花后叶片气体交换及叶绿素荧光参数的影响. 应用生态学报, 26, 2030-2040.] |
| [13] | Lin MZ, Wang ZW, He LC, Xu K, Cheng DL, Wang GX (2015). Plant photosynthesis-irradiance curve response to pollution show non-competitive inhibited Michaelis kinet- ics.PLOS ONE, 10, e0142712. doi: 10.1371/journal. pone. 0142712. |
| [14] | Major KM, Dunton KH (2002). Variations in light-harvesting characteristics of the seagrass,Thalassia testudinum: Evidence for photoacclimation. Journal of Experimental Marine Biology and Ecology, 275, 173-189. |
| [15] | Murchie EH, Ali A, Herman T (2015). Photoprotection as a trait for rice yield improvement: Status and prospects.Rice, 8, 31-40. |
| [16] | Nelson N, Yocum CF (2006). Structure of function of photo- systems I and II.Annual Review of Plant Biology, 57, 521-565. |
| [17] | Niyogi KK, Truong TB (2013). Evolution of flexible non- photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis.Current Opinion in Plant Biology, 16, 307-314. |
| [18] | Oxborough K (2004). Imaging of chlorophyll a fluorescence: Theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance.Journal of Experimental Botany, 55, 1195-1205. |
| [19] | Panitchayangkoon G, Hayes D, Fransted KA, Caram JR, Harel E, Wen J, Blankenship RE, Engel GS (2010). Long-lived quantum coherence in photosynthetic complexes at physiological temperature.Proceedings of the National Academy Sciences of the United States of America, 107, 12766-12770. |
| [20] | Ralph PJ, Gademann R (2005). Rapid light curves: A powerful tool to assess photosynthetic activity.Aquatic Botany, 82, 222-237. |
| [21] | Richter M, Renger T, Knorr A (2008). A Block equation approach to intensity dependent optical spectra of light harvesting complex II. Excitation dependence of light harvesting complex II pump-probe spectra.Photosynthesis Research, 95, 119-127. |
| [22] | Ritchie RJ (2008). Fitting light saturation curves measured using modulated fluorometry.Photosynthesis Research, 96, 201-215. |
| [23] | Ritchie RJ, Bunthawin S (2010). The use of pulse amplitude modulation (PAM) fluorometry to measure photosynthesis in a cam orchid,Dendrobium spp.(D. cv. Viravuth Pink). International Journal of Plant Sciences, 171, 575-585. |
| [24] | Rochaix JD (2014). Regulation and dynamics of the light- harvesting system. Annual of Review Plant Biology, 65, 287-309. |
| [25] | Sarovar M, Ishizaki A, Fleming G, Whaley B (2010). Quantum entanglement in photosynthetic light-harvesting com- plexes.Nature Physics, 6, 462-467. |
| [26] | Shi SB, Li TC, Li M, Liu SZ, Li AD, Ma JP (2015). Interaction effect analysis of soil drought and strong light on PSII nonphotochemical quenching inKobresia pygmaea leaves. Plant Physiology Journal, 51, 1678-1686. (in Chinese with English abstract)[师生波, 李天才, 李妙, 刘世增, 李爱德, 马剑平 (2015). 土壤干旱和强光对高山嵩草叶片PSII反应中心非光化学猝灭的交互影响分析. 植物生理学报, 51, 1678-1686.] |
| [27] | Silsbe GM, Kromkamp JC (2012). Modeling the irradiance dependency of the quantum efficiency of photosynthesis.Limnology and Oceanography: Methods, 10, 645-652. |
| [28] | Smyth TJ, Pemberton KL, Aiken J, Geider RJ (2004). A meth- odology to determine primary production and phytoplank- ton photosynthetic parameters from fast repetition rate fluorometry.Journal of Plankton Research, 26, 1337-1350. |
| [29] | Takahashi S, Badger MR (2011). Photoprotection in plants: A new light on photosystem II damage.Trends in Plant Sciences, 16, 53-59. |
| [30] | Vijayan P, Browse J (2002). Photoinhibition in mutants of arabidopsis deficient in thylakoid unsaturation.Plant Physiology, 129, 876-885. |
| [31] | Ware MA, Belgio E, Ruban AV (2015). Photoprotective capacity of non-photochemical quenching in plants acclimated to different light intensities.Photosynthesis Research, 126, 261-274. |
| [32] | Webb WL, Newton M, Starr D (1974). Carbon dioxide ex- change of Alnus rubra: A mathematical model. Oecologia, 17, 281-291. |
| [33] | White AJ, Critchley C (1999). Rapid light curves: A new fluo- rescence method to asses the state of the photosynthetic apparatus.Photosynthesis Research, 59, 63-72. |
| [34] | Ye ZP, Hu WH, Xiao YA, Fan DY, Yin JH, Duan SH, Yan XH, He L, Zhang SS (2014). A mechanistic model of light-response of photosynthetic electron flow and its application.Chinese Journal of Plant Ecology, 38, 1241-1249. (in Chinese with English abstract)[叶子飘, 胡文海, 肖宜安, 樊大勇, 尹建华, 段世华, 闫小红, 贺俐, 张斯斯 (2014). 光合电子流对光响应的机理模型及其应用. 植物生态学报, 38, 1241-1249.] |
| [35] | Ye ZP, Robakowski P, Suggett DJ (2013a). A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules.Planta, 237, 837-847. |
| [36] | Ye ZP, Suggett DJ, Robakowski P, Kang HJ (2013b). A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of PSII in C3 and C4 species.New Phytologist, 199, 110-120. |
| [1] | WANG Fu-Biao, YE Zi-Piao. A review on light response models of electron transport rates of plant [J]. Chin J Plant Ecol, 2024, 48(3): 287-305. |
| [2] | SHI Sheng-Bo, ZHOU Dang-Wei, LI Tian-Cai, DE Ke-Jia, GAO Xiu-Zhen, MA Jia-Lin, SUN Tao, WANG Fang-Lin. Responses of photosynthetic function of Kobresia pygmaea to simulated nocturnal low temperature on the Qingzang Plateau [J]. Chin J Plant Ecol, 2023, 47(3): 361-373. |
| [3] | LIU Hai-Yan, ZANG Sha-Sha, ZHANG Chun-Xia, ZUO Jin-Cheng, RUAN Zuo-Xi, WU Hong-Yan. Photochemical reaction of photosystem II in diatoms under phosphorus starvation and its response to high light intensity [J]. Chin J Plant Ecol, 2023, 47(12): 1718-1727. |
| [4] | SHI Sheng-Bo, SHI Rui, ZHOU Dang-Wei, ZHANG Wen. Effects of low temperature on photochemical and non-photochemical energy dissipation of Kobresia pygmaea leaves [J]. Chin J Plant Ecol, 2023, 47(10): 1441-1452. |
| [5] | LI Qun, ZHAO Cheng-Zhang, WANG Ji-Wei, WEN Jun, LI Zi-Qin, MA Jun-Yi. Morphological and photosynthetic physiological characteristics of Saussurea salsa in response to flooding in salt marshes of Xiao Sugan Lake, Gansu, China [J]. Chin J Plant Ecol, 2019, 43(8): 685-696. |
| [6] | Zi-Piao YE, Shi-Hua DUAN, Ting AN, Hua-Jing KANG. Determination of maximum electron transport rate and its impact on allocation of electron flow [J]. Chin J Plant Ecol, 2018, 42(4): 498-507. |
| [7] | LI Li-Yuan, LI Jun, TONG Xiao-Juan, MENG Ping, ZHANG Jin-Song, ZHANG Jing-Ru. Simulation on the light-response curves of electron transport rate of Quercus variabilis and Robinia pseudoacacia leaves in the Xiaolangdi area, China [J]. Chin J Plant Ecol, 2018, 42(10): 1009-1021. |
| [8] | YE Zi-Piao, DUAN Shi-Hua, AN Ting, KANG Hua-Jing. Construction of CO2-response model of electron transport rate in C4 crop and its application [J]. Chin J Plant Ecol, 2018, 42(10): 1000-1008. |
| [9] | LI Zhi-Zhen, LIU Dong-Huan, ZHAO Shi-Wei, JIANG Chuang-Dao, SHI Lei. Mechanisms of photoinhibition induced by high light in Hosta grown outdoors [J]. Chin J Plant Ecol, 2014, 38(7): 720-728. |
| [10] | XUE Wei, LI Xiang-Yi, LIN Li-Sha, WANG Ying-Ju, LI Lei. Effects of short time heat stress on photosystem II, Rubisco activities and oxidative radicals in Alhagi sparsifolia [J]. Chin J Plant Ecol, 2011, 35(4): 441-451. |
| [11] | ZHANG Xu-Cheng, YU Xian-Feng, GAO Shi-Ming. Effects of nitrogen application rates on photosynthetic energy utilization in wheat leaves under elevated atmospheric CO2 concentration [J]. Chin J Plant Ecol, 2010, 34(10): 1196-1203. |
| [12] | ZU Yuan-Gang, ZHANG Zhong-Hua, WANG Wen-Jie, YANG Feng-Jian, HE Hai-Sheng. DIFFERENT CHARACTERISTICS OF PHOTOSYNTHESIS IN STEMS AND LEAVES OF MIKANIA MICRANTH [J]. Chin J Plant Ecol, 2006, 30(6): 998-1004. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2026 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
![]()