

Chin J Plan Ecolo ›› 2016, Vol. 40 ›› Issue (9): 933-941.DOI: 10.17521/cjpe.2015.0261
• Research Articles • Previous Articles Next Articles
Jia SHEN, Ya-He LI, Lin ZHANG, Xue SUN*( )
)
Received:2015-07-08
Accepted:2016-04-25
Online:2016-09-10
Published:2016-09-29
Contact:
Xue SUN
Jia SHEN, Ya-He LI, Lin ZHANG, Xue SUN. Response of growth and inorganic carbon utilization to different light and CO2 levels in Chlorella pyrenoidosa[J]. Chin J Plan Ecolo, 2016, 40(9): 933-941.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2015.0261
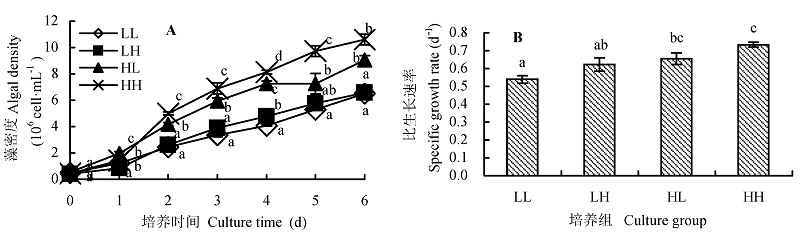
Fig. 1 Effect of different light intensity and CO2 concentration conditions on the growth of Chlorella pyrenoidosa (mean ± SD). A, Growth curve. B, Specific growth rate. LL, LH, HL and HH represent low-light intensity and low-CO2 concentration, low-light intensity and high-CO2 concentration, high-light intensity and low-CO2 concentration, high-light intensity and high-CO2 concentration conditions, respectively. Different lowercase letters indicate significant difference (p < 0.05).
| 处理组 Treatment group | 总碱度 Total alkalinity (µmol•L-1) | pH | DIC (µmol•L-1) | CO2 (µmol•L-1) | HCO3- (µmol•L-1) | CO32- (µmol•L-1) |
|---|---|---|---|---|---|---|
| LL | 1 387.8 ± 55.1b | 9.35 ± 0.00c | 765.3 ± 36.8ab | 0.22 ± 0.18a | 383.6 ± 18.44a | 381.5 ± 18.3c |
| LH | 1 388.4 ± 57.3b | 8.48 ± 0.03a | 1 163.3 ± 58.8c | 4.37 ± 0.46b | 1 022.1 ± 57.4c | 136.8 ± 3.6a |
| HL | 1 275.0 ± 16.5ab | 9.41 ± 0.04c | 665.8 ± 22.6a | 0.16 ± 0.44a | 311.7 ± 24.2a | 354.0 ± 5.8c |
| HH | 1 190.1 ± 0.0a | 8.95 ± 0.04b | 803.4 ± 17.2b | 0.84 ± 0.12a | 574.9 ± 27.2b | 227.6 ± 10.2b |
Table 1 Comparison of the parameters of carbonate system under different light intensity and CO2 concentration conditions in Chlorella pyrenoidosa (mean ± SD, n = 3)
| 处理组 Treatment group | 总碱度 Total alkalinity (µmol•L-1) | pH | DIC (µmol•L-1) | CO2 (µmol•L-1) | HCO3- (µmol•L-1) | CO32- (µmol•L-1) |
|---|---|---|---|---|---|---|
| LL | 1 387.8 ± 55.1b | 9.35 ± 0.00c | 765.3 ± 36.8ab | 0.22 ± 0.18a | 383.6 ± 18.44a | 381.5 ± 18.3c |
| LH | 1 388.4 ± 57.3b | 8.48 ± 0.03a | 1 163.3 ± 58.8c | 4.37 ± 0.46b | 1 022.1 ± 57.4c | 136.8 ± 3.6a |
| HL | 1 275.0 ± 16.5ab | 9.41 ± 0.04c | 665.8 ± 22.6a | 0.16 ± 0.44a | 311.7 ± 24.2a | 354.0 ± 5.8c |
| HH | 1 190.1 ± 0.0a | 8.95 ± 0.04b | 803.4 ± 17.2b | 0.84 ± 0.12a | 574.9 ± 27.2b | 227.6 ± 10.2b |
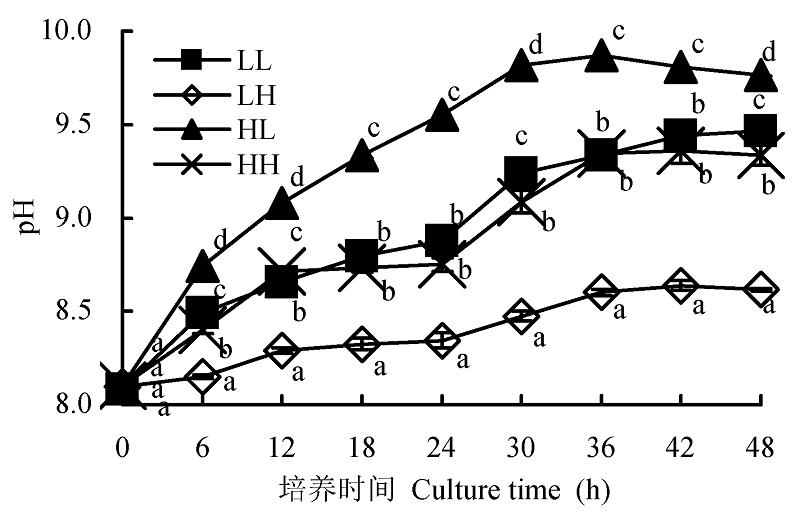
Fig. 2 Effect of different light intensity and CO2 concentration conditions on pH compensation point of Chlorella pyrenoidosa (mean ± SD). LL, LH, HL and HH represent low-light intensity and low-CO2 concentration, low-light intensity and high-CO2 concentration, high-light intensity and low-CO2 concentration, high-light intensity and high-CO2 concentration conditions, respectively. Different lowercase letters indicate significant difference (p < 0.05).
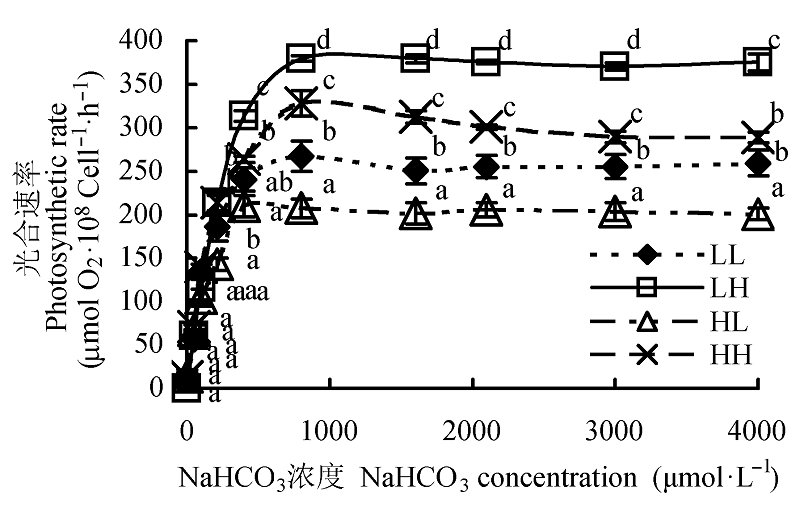
Fig. 3 Effect of different light intensity and CO2 concentration conditions on P-C curve of Chlorella pyrenoidosa (mean ± SD). LL, LH, HL and HH represent low-light intensity and low-CO2 concentration, low-light intensity and high-CO2 concentration, high-light intensity and low-CO2 concentration, high-light intensity and high-CO2 concentration conditions, respectively. Different lowercase letters indicate significant difference (p < 0.05).
| 处理组 Treatmeat group | Vmax (µmol O2• 108cell·h-1) | K0.5 (µmol•L-1) | ||
|---|---|---|---|---|
| DIC | CO2 | HCO3- | ||
| LL | 275.93 ± 16.83b | 107.20 ± 2.49a | 2.43 ± 0.06a | 104.78 ± 2.43a |
| LH | 415.44 ± 4.23d | 188.33 ± 2.04b | 4.26 ± 0.45b | 184.07 ± 19.59b |
| HL | 218.03 ± 10.16a | 94.20 ± 7.03a | 2.13 ± 0.16a | 92.07 ± 6.87a |
| HH | 324.05 ± 1.34c | 116.83 ± 6.89a | 2.64 ± 0.16a | 114.19 ± 6.73a |
Table 2 Effect of different light intensity and CO2 concentration conditions on Vmax and K0.5 of Chlorella pyrenoidosa (mean ± SD, n = 3)
| 处理组 Treatmeat group | Vmax (µmol O2• 108cell·h-1) | K0.5 (µmol•L-1) | ||
|---|---|---|---|---|
| DIC | CO2 | HCO3- | ||
| LL | 275.93 ± 16.83b | 107.20 ± 2.49a | 2.43 ± 0.06a | 104.78 ± 2.43a |
| LH | 415.44 ± 4.23d | 188.33 ± 2.04b | 4.26 ± 0.45b | 184.07 ± 19.59b |
| HL | 218.03 ± 10.16a | 94.20 ± 7.03a | 2.13 ± 0.16a | 92.07 ± 6.87a |
| HH | 324.05 ± 1.34c | 116.83 ± 6.89a | 2.64 ± 0.16a | 114.19 ± 6.73a |
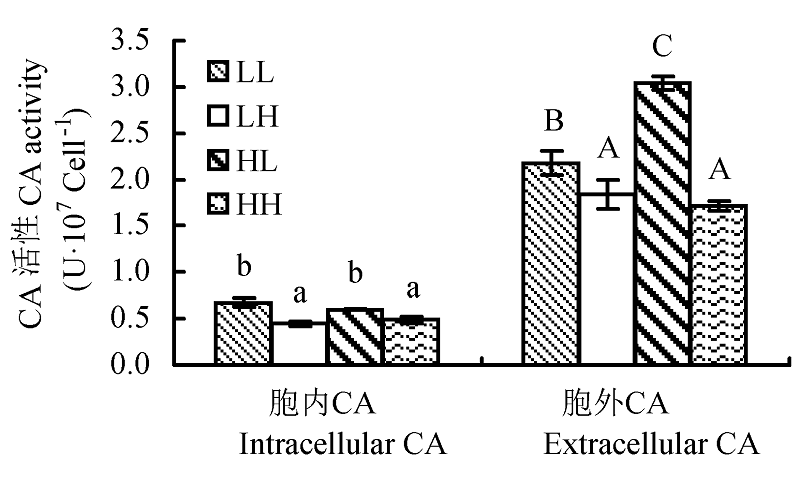
Fig. 4 Effect of different light intensity and CO2 concentration conditions on carbonic anhydrase (CA) activity of Chlorella pyrenoidosa (mean ± SD). LL, LH, HL and HH represent low-light intensity and low-CO2 concentration, low-light intensity and high-CO2 concentration, high-light intensity and low-CO2 concentration, high-light intensity and high-CO2 concentration conditions, respectively. Different letters indicate significant difference (p < 0.05).
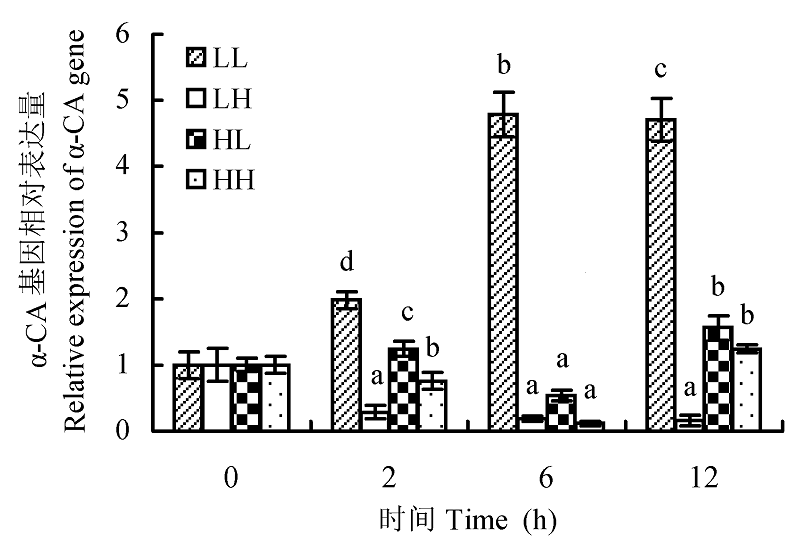
Fig. 5 The effect of different light intensity and CO2 concentration conditions on α-CA gene expression of Chlorella pyrenoidosa (mean ± SD). LL, LH, HL and HH represent low-light intensity and low-CO2 concentration, low-light intensity and high-CO2 concentration, high-light intensity and low-CO2 concentration, high-light intensity and high-CO2 concentration conditions, respectively. Different lowercase letters indicate significant difference (p < 0.05).
| 1 | Badger MR (1980). Internal inorganic carbon pool of Chlamydomonas reinhardtii: Evidence for a carbon dioxide- concentrating mechanism.Plant Physiology, 66, 407-413. |
| 2 | Badger MR (1987). The CO2-concentrating mechanism in aquatic phototrophs. In: Hatch MD, Boardman NK eds. The Biochemistry of Plants: A Comprehensive Treatise, Vol 10, Photosynthesis. Academic Press, San Diego, USA. 219-274. |
| 3 | Bozzo GG, Colman B, Matsuda Y (2000). Active transport of CO2 and bicarbonate is induced in response to external CO2 concentration in the green alga Chlorella kessleri.Journal of Experimental Botany, 51, 1341-1348. |
| 4 | Chen XW, Gao KS (2003). Effect of CO2 concentrations on the activity of photosynthetic CO2 fixation and extracellular carbonic anhydrase in the marine diatom Skeletonema costatum.Chinese Science Bulletin, 48, 2275-2279. (in Chinese with English abstract)[陈雄文, 高坤山 (2003). CO2浓度对中肋骨条藻的光合无机碳吸收和胞外碳酸酐酶活性的影响. 科学通报, 48, 2275-2279.] |
| 5 | Elrad D, Niyogi KK, Grossman AR (2002). A major light- harvesting polypeptide of photosystem II functions in thermal dissipation.Plant Cell, 14, 1801-1816. |
| 6 | Gao K, Aruga Y, Asada K, Kiyohara M (1993). Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis.Journal of Applied Phycology, 5, 563-571. |
| 7 | Gao KS (1999). Research techniques and methods in characterizing photosynthetic carbon fixation by algae.Marine Sciences, 23, 37-41. (in Chinese with English abstract)[高坤山 (1999). 藻类光合固碳的研究技术与解析方法. 海洋科学, 23, 37-41.] |
| 8 | Goyal A, Shiraiwa Y, Husic HD, Tolbert NE (1992). External and internal carbonic anhydrases in Dunaliella species.Marine Biology, 113, 349-355. |
| 9 | Guillard RRL, Ryther JH (1962). Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confer- vacea (Cleve) Gran.Canadian Journal of Microbiology, 8, 229-240. |
| 10 | Harada H, Nakatsuma D, Ishida M, Matsuda Y (2005). Regulation of the expression of intracellular β-carbonic anhydrase in response to CO2 and light in the marine diatom Phaeodactylum tricornutum.Plant Physiology, 139, 1041-1050. |
| 11 | Hu HH, Gao KS (2003). Optimization of growth and fatty acid composition of a unicellular marine picoplankton, Nanno chloropsis sp., with enriched carbon sources.Biotechnology Letters, 25, 421-425. |
| 12 | Kucho K, Yoshioka S, Taniguchi F, Ohyama K, Fukuzawa H (2003). Cis-acting elements and DNA-binding proteins involved in CO2-responsive transcriptional activation of Cah1 encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii.Plant Physiology, 133, 783-793. |
| 13 | Li L, Fu ML, Zhao YH, Zhu YT (2012). Characterization of carbonic anhydrase II from Chlorella vulgaris in bio-CO2 capture.Environmental Science and Pollution Research, 19, 4227-4232. |
| 14 | Li N, Bi YH, Gao DW, Hu ZY, Ren NQ (2011). Effects of elevated CO2 concentration on growth of microcystis aeruginosa.Acta Hydrobiologica Sinica, 35, 698-702. (in Chinese with English abstract)[李娜, 毕永红, 高大文, 胡征宇, 任南琪 (2011). 大气CO2浓度变化对铜绿微囊藻生长的影响. 水生生物学报, 35, 698-702.] |
| 15 | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method.Methods, 25, 402-408. |
| 16 | Maberly SC (1990). Exogenous sources of inorganic carbon for photosynthesis by marine macroalgae.Journal of Phycology, 26, 439-499. |
| 17 | Millero FJ, Graham TB, Huang F, Bustos-Serrano H, Pierrot D (2006). Dissociation constants of carbonic acid in seawater as a function of salinity and temperature.Marine Chemistry, 100, 80-94. |
| 18 | Miura K, Kohinata T, Yoshioka S, Ohyama K, Fukuzawa H (2002). Regulation of a carbon concentrating mechanism through CCM1 in Chlamydomonas reinhardtii.Functional Plant Biology, 29, 211-219. |
| 19 | Moroney JV, Ynalvez RA (2007). Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii.Eukaryotic Cell, 6, 1251-1259. |
| 20 | Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B (2011). The car- bonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles.Photosynthesis Research, 109, 133-149. |
| 21 | Ochiai T, Colman B, Matsuda Y (2007). Acclimation of wild-type cells and CO2-insensitive mutants of the green alga Chlorella ellipsoidea to elevated [CO2].Plant, Cell & Environment, 30, 944-951. |
| 22 | Raven JA (2010). Inorganic carbon acquisition by eukaryotic algae: Four current questions.Photosynthesis Research, 106, 123-134. |
| 23 | Tachibana M, Allen AE, Kikutani S, Endo Y, Bowler C, Matsuda Y (2011). Localization of putative carbonic anhy- drases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana.Photosynthesis Research, 109, 205-221. |
| 24 | Wang SG, Yang R, Zhou XQ, Song DD, Sun X, Luo QJ (2013). Utilization of inorganic carbon in Pyropia haitanensis (Rhodophyta) under heat stress.Oceanologia et Limnologia Sinica, 44, 1378-1385. (in Chinese with English abstract)[王淑刚, 杨锐, 周新倩, 宋丹丹, 孙雪, 骆其君 (2013). 高温胁迫下坛紫菜(Pyropia haitanensis)对无机碳的利用. 海洋与湖沼, 44, 1378-1385.] |
| 25 | Wang SS, Liu YD, Zou YD, Li DH (2006). Modulation and adaptation of carbonic anhydrase activity in Microcystis spp. under different environmental factors.Acta Ecologica Sinica, 26, 2443-2448. (in Chinese with English abstract)[王山杉, 刘永定, 邹永东, 李敦海 (2006). 微囊藻碳酸酐酶活性在不同环境因素下的调节与适应. 生态学报, 26, 2443-2448.] |
| 26 | Wang WW, Sun X, Wang DM, Shen J, Xu NJ (2014). Effects of salinity and inorganic carbon on the growth, extracellu- lar carbonic anhydrase activity and ca gene expression of Chlorella pyrenoidosa.Journal of Fisheries of China, 38, 920-928. (in Chinese with English abstract)[王玮蔚, 孙雪, 王冬梅, 沈佳, 徐年军 (2014). 盐度和无机碳对蛋白核小球藻生长、胞外碳酸酐酶活性及其基因表达的影响. 水产学报, 38, 920-928.] |
| 27 | Wang Y, Spalding MH (2006). An inorganic carbon transport system responsible for acclimation specific to air levels of CO2 in Chlamydomonas reinhardtii.Proceedings of the National Academy of Sciences of the United States of America, 103, 10110-10115. |
| 28 | Wang Y, Sun Z, Horken KM, Im CS, Xiang YB, Grossman, Arthur R, Weeks DP (2005). Analyses of CIA5, the master regulator of the carbon-concentrating mechanism in Chlamydomonas reinhardtii, and its control of gene expression.Canadian Journal of Botany, 83, 765-779. |
| 29 | Wilbur KM, Anderson NG (1948). Electrometric and colori- metric determination of carbonic anhydrase.The Journal of Biological Chemistry, 176, 147-154. |
| 30 | Wu Y, Gao K, Riebesell U (2010). CO2 induced seawater acidification affects physiological performance of the marine diatom Phaeodactylyum tricornutum.Biogeosciences, 7, 2915-2923. |
| 31 | Xia JR, Gao KS (2001). Effects of high CO2 concentration on growth and photosynthesis of Spirulina maxima.Acta Hydrobiologica Sinica, 25, 474-480. (in Chinese with English abstract)[夏建荣, 高坤山 (2001). 高浓度CO2对极大螺旋藻生长和光合作用的影响. 水生生物学报, 25, 474-480.] |
| 32 | Xia JR, Gao KS (2002). Advances in research on CO2 con- centrating mechanism of green algae.Chinese Journal of Applied Ecology, 11, 1507-1510. (in Chinese with English abstract)[夏建荣, 高坤山 (2002). 绿藻CO2浓缩机制的研究进展. 应用生态学报, 11, 1507-1510.] |
| 33 | Xia JR, Yu JL (2009). Effects of high CO2 concentration on ex- tracellular carbonic anhydrase activity and photosynthesis in Nitzschia closterium var. minutissima. Journal of Guangzhou University (Natural Science Edition), 8, 49-53. (in Chinese with English abstract)[夏建荣, 余锦兰 (2009). 高浓度CO2对小新月菱形藻胞外碳酸酐酶活性和光合作用的影响. 广州大学学报(自然科学版), 8, 49-53.] |
| 34 | Yamano T, Miura K, Fukuzawa H (2008). Expression analysis of genes associated with the induction of the carbon- concentrating mechanism in Chlamydomonas reinhardtii.Plant Physiology, 147, 340-354. |
| 35 | Yoshioka S, Taniguchi F, Miura K, Inoue T, Yamano T, Fukuzawa H (2004). The novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1, encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii.Plant Cell, 16, 1466-1477. |
| [1] | ZHANG Jin-Yan, CUN Zhu, SHUANG Sheng-Pu, HONG Jie, MENG Zhen-Gui, CHEN Jun-Wen. Steady-state and dynamic photosynthetic characteristics of shade-tolerant species Panax notoginseng in response to nitrogen levels [J]. Chin J Plant Ecol, 2023, 47(3): 331-347. |
| [2] | HAO Qing, HUANG Chang. A review of forest aboveground biomass estimation based on remote sensing data [J]. Chin J Plant Ecol, 2023, 47(10): 1356-1374. |
| [3] | ZHENG Zhou-Tao, ZHANG Yang-Jian. Variation in ecosystem water use efficiency and its attribution analysis during 1982-2018 in Qingzang Plateau [J]. Chin J Plant Ecol, 2022, 46(12): 1486-1496. |
| [4] | WANG Jing-Yuan, WEI Jie, WEN Xue-Fa. Progress in the theory, hypothesis and application of the methods measuring soil CO2 flux gradient [J]. Chin J Plant Ecol, 2022, 46(12): 1523-1536. |
| [5] | WANG Jia-Tong, NIU Chun-Yue, HU Tian-Yu, LI Wen-Kai, LIU Ling-Li, GUO Qing-Hua, SU Yan-Jun. Three-dimensional radiative transfer modeling of forest: recent progress, applications, and future opportunities [J]. Chin J Plant Ecol, 2022, 46(10): 1200-1218. |
| [6] | Li-Ting YANG, Yan-Yan XIE, Ke-Yi ZUO, Sen XU, Rui GU, Shuang-Lin CHEN, Zi-Wu GUO. Effects of ramet ratio on photosynthetic physiology of Indocalamus decorus clonal system under heterogeneous light environment [J]. Chin J Plant Ecol, 2022, 46(1): 88-101. |
| [7] | WU Hong-Min, SHUANG Sheng-Pu, ZHANG Jin-Yan, CUN Zhu, MENG Zhen-Gui, LI Long-Gen, SHA Ben-Cai, CHEN Jun-Wen. Photodamage to photosystem in a typically shade-tolerant species Panax notoginseng exposed to a sudden increase in light intensity [J]. Chin J Plant Ecol, 2021, 45(4): 404-419. |
| [8] | YE Zi-Piao, YU Feng, AN Ting, WANG Fu-Biao, KANG Hua-Jing. Investigation on CO2-response model of stomatal conductance for plants [J]. Chin J Plant Ecol, 2021, 45(4): 420-428. |
| [9] | YI Hai-Yan, ZENG Yuan, ZHAO Yu-Jin, ZHENG Zhao-Ju, XIONG Jie, ZHAO Dan. Forest species diversity mapping based on clustering algorithm [J]. Chin J Plant Ecol, 2020, 44(6): 598-615. |
| [10] | FENG Zhao-Zhong, LI Pin, ZHANG Guo-You, LI Zheng-Zhen, PING Qin, PENG Jin-Long, LIU Shuo. Impacts of elevated carbon dioxide concentration on terrestrial ecosystems: problems and prospective [J]. Chin J Plant Ecol, 2020, 44(5): 461-474. |
| [11] | LI Xin-Hao, YAN Hui-Juan, WEI Teng-Zhou, ZHOU Wen-Jun, JIA Xin, ZHA Tian-Shan. Relative changes of resource use efficiencies and their responses to environmental factors in Artemisia ordosica during growing season [J]. Chin J Plant Ecol, 2019, 43(10): 889-898. |
| [12] | LI Qi, HU Fei. Effects of light quality on circumnutation of Vigna unguiculata seedlings [J]. Chin J Plant Ecol, 2018, 42(12): 1192-1199. |
| [13] | Ling HAN, Cheng-Zhang ZHAO, Wei FENG, Ting XU, Hui-Ling ZHENG, Bei-Bei DUAN. Trade-off relationship between vein density and vein diameter of Achnatherum splendens in response to habitat changes in Zhangye wetland [J]. Chin J Plant Ecol, 2017, 41(8): 872-881. |
| [14] | Jing CHEN, Cheng-Zhang ZHAO, Ji-Wei WANG, Lian-Chun ZHAO. Canopy structure and radiation interception of Salix matsudana: Stand density dependent relationships [J]. Chin J Plan Ecolo, 2017, 41(6): 661-669. |
| [15] | Hong-Tao XIE, Mu-Kui YU, Xiang-Rong CHENG. Effects of light intensity variation on nitrogen and phosphorus contents, allocation and limitation in five shade-enduring plants [J]. Chin J Plant Ecol, 2017, 41(5): 559-569. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn