

Chin J Plant Ecol ›› 2018, Vol. 42 ›› Issue (6): 681-691.DOI: 10.17521/cjpe.2018.0017
• Research Articles • Previous Articles Next Articles
WANG Jun-Yu,WANG Xiao-Dong,MA Yuan-Dan,FU Lu-Cheng,ZHOU Huan-Huan,WANG Bin,ZHANG Ru-Min,GAO Yan( )
)
Received:2018-01-14
Revised:2018-05-12
Online:2018-06-20
Published:2018-06-20
Contact:
Yan GAO
Supported by:WANG Jun-Yu, WANG Xiao-Dong, MA Yuan-Dan, FU Lu-Cheng, ZHOU Huan-Huan, WANG Bin, ZHANG Ru-Min, GAO Yan. Physiological and ecological responses to drought and heat stresses in Osmanthus fragrans ‘Boyejingui’[J]. Chin J Plant Ecol, 2018, 42(6): 681-691.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2018.0017
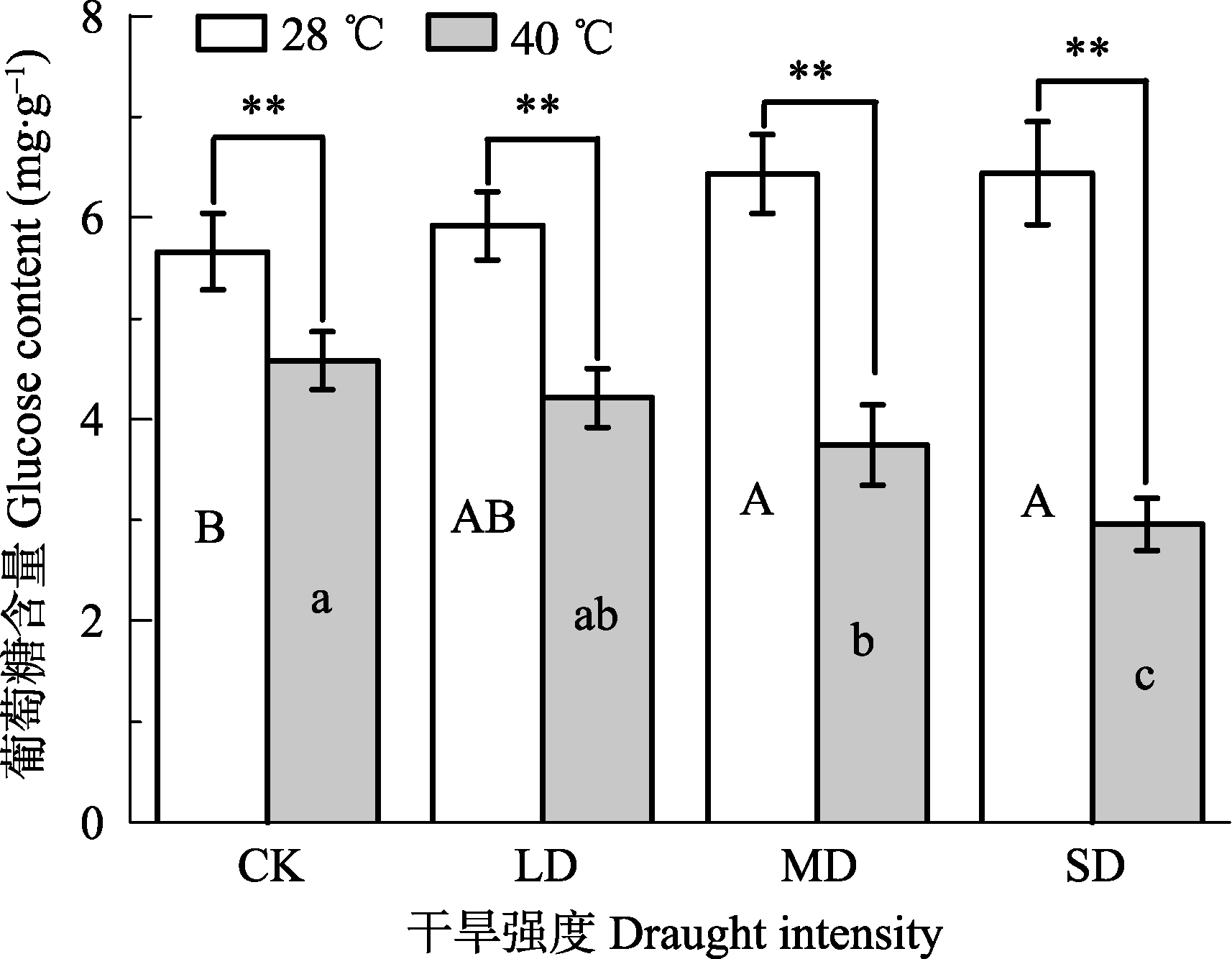
Fig. 1 Effects of drought and heat stress on glucose content in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of drought and different lowercase letters denote statistically significant differences of heat stress treatment (p < 0.05, according to LSD test). p < 0.01. CK, control; LD, light drought; MD, moderate drought; SD, severe drought.
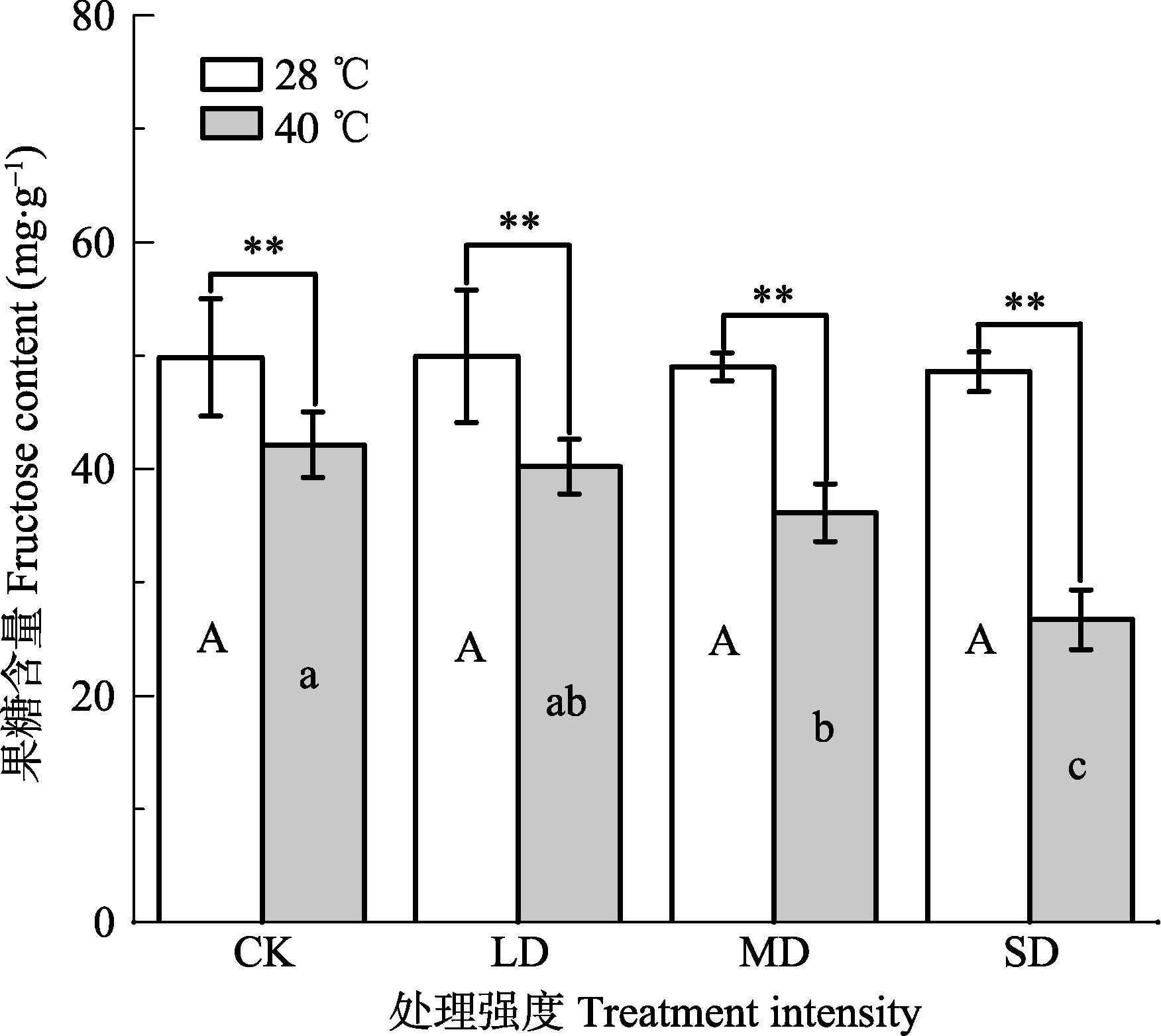
Fig. 2 Effects of drought and heat stress on fructose content in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of drought and different lowercase letters denote statistically significant differences of heat stress treatment (p < 0.05, according to LSD test). **, p < 0.01. CK, control; LD, light processing; MD, moderate drought; SD, severe drought.
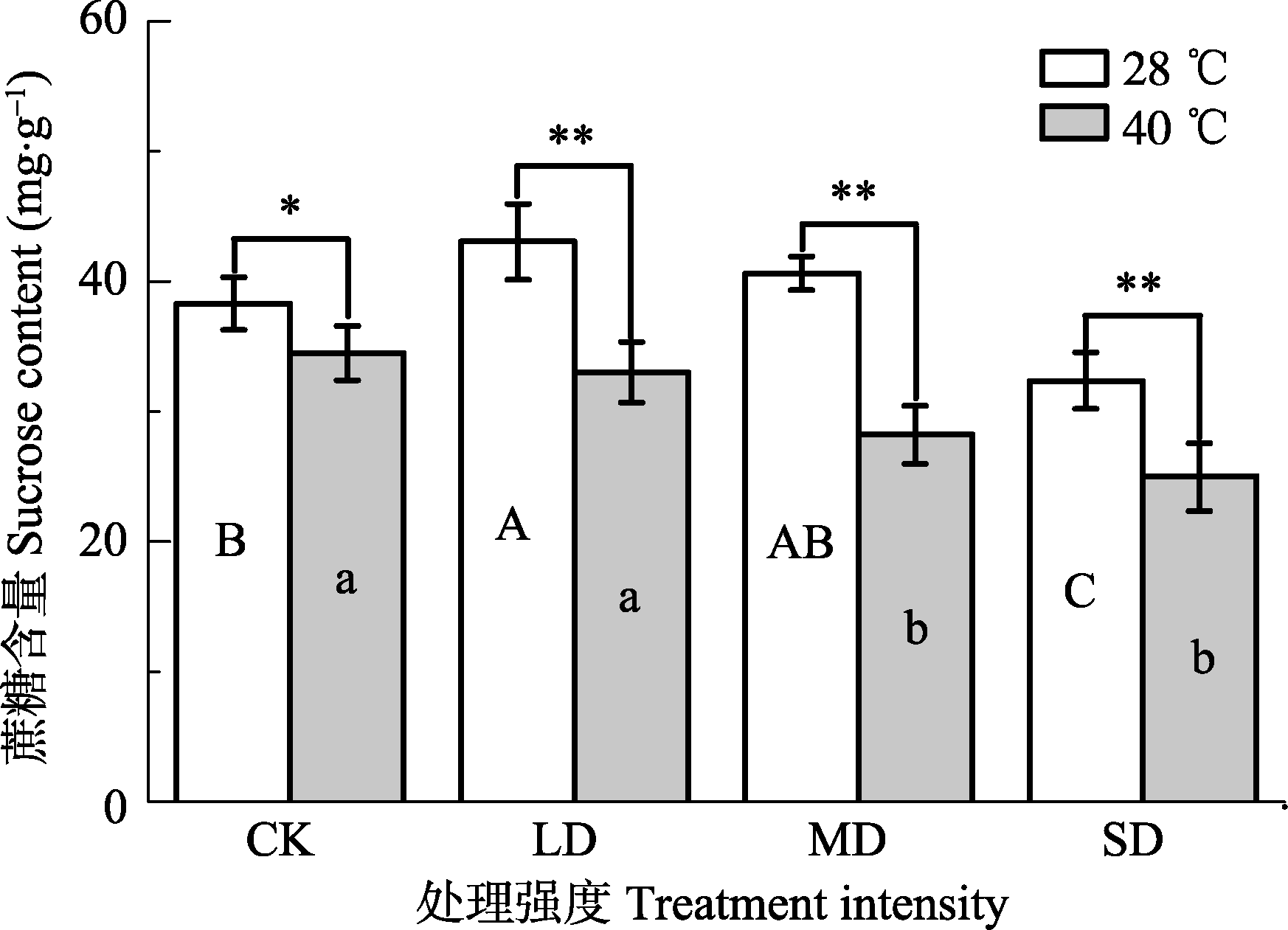
Fig. 3 Effects of drought and heat stress on sucrose content in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of drought and different lowercase letters denote statistically significant differences of heat stress treatment (p < 0.05, according to LSD test). p < 0.01. CK, control; LD, light drought; MD, moderate drought; SD, severe drought.
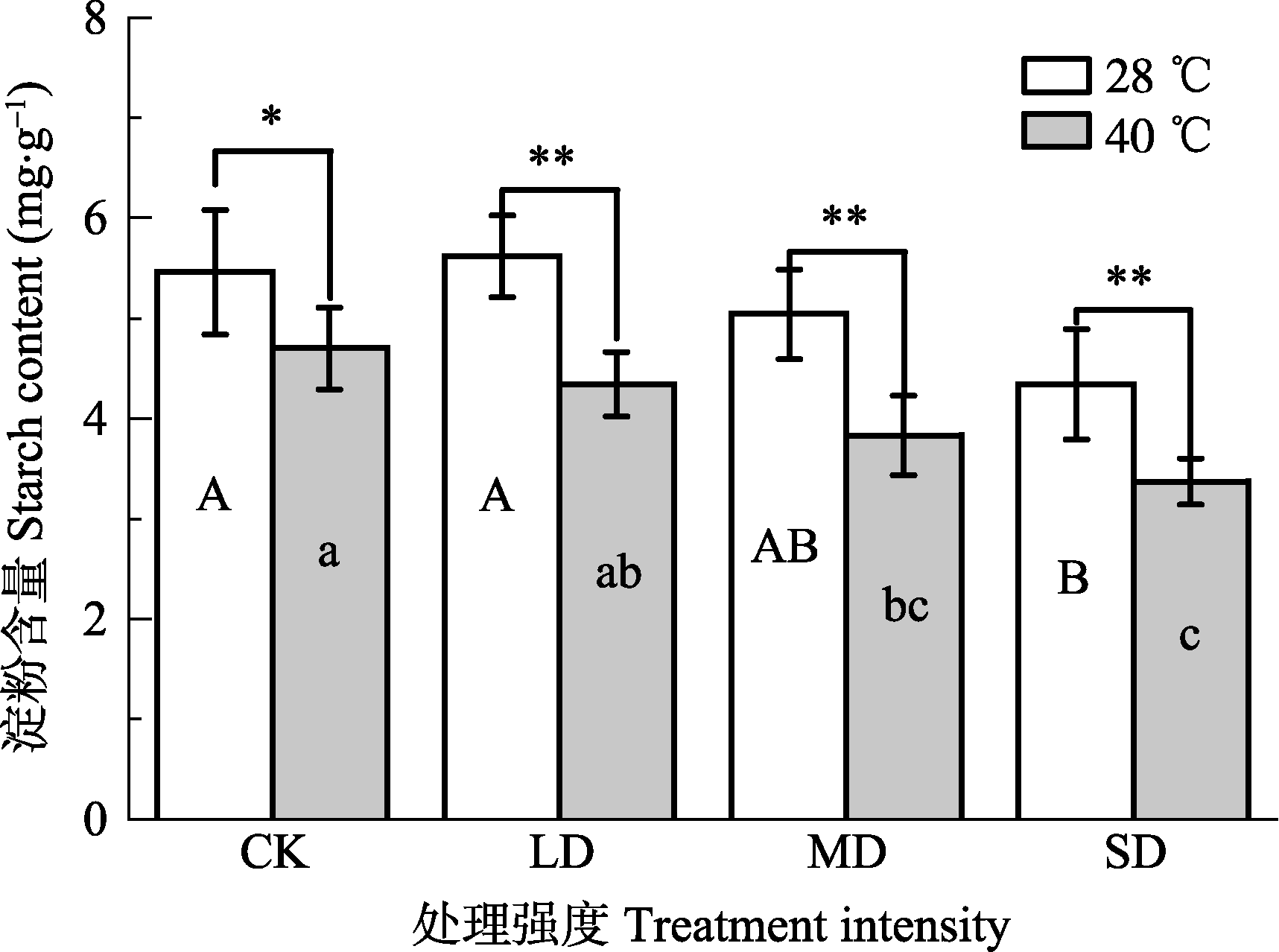
Fig. 4 Effects of drought and heat stress on starch content in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of drought and different lowercase letters denote statistically significant differences of heat stress treatment (p < 0.05, according to LSD test). **, p < 0.01. CK, control; LD, light drought; MD, moderate drought; SD, severe drought.
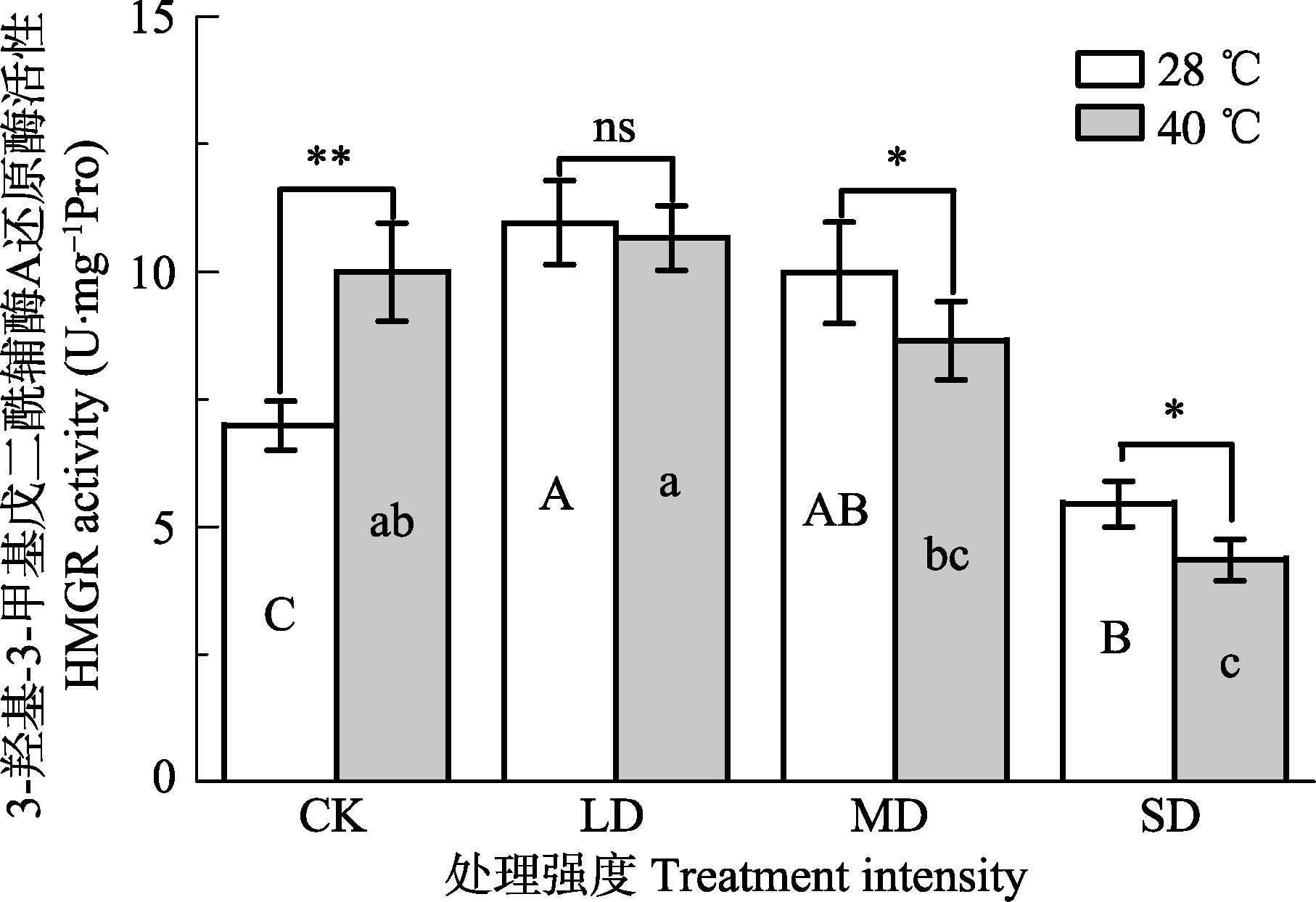
Fig. 5 Effects of drought and heat stress on 3-hydroxy-3- methylglutaryl CoA reductase (HMGR) activity in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of drought and different lowercase letters denote statistically significant differences of heat stress treatment (p < 0.05, according to LSD test). **, p < 0.01. CK, control; LD, light drought; MD, moderate drought; SD, severe drought.
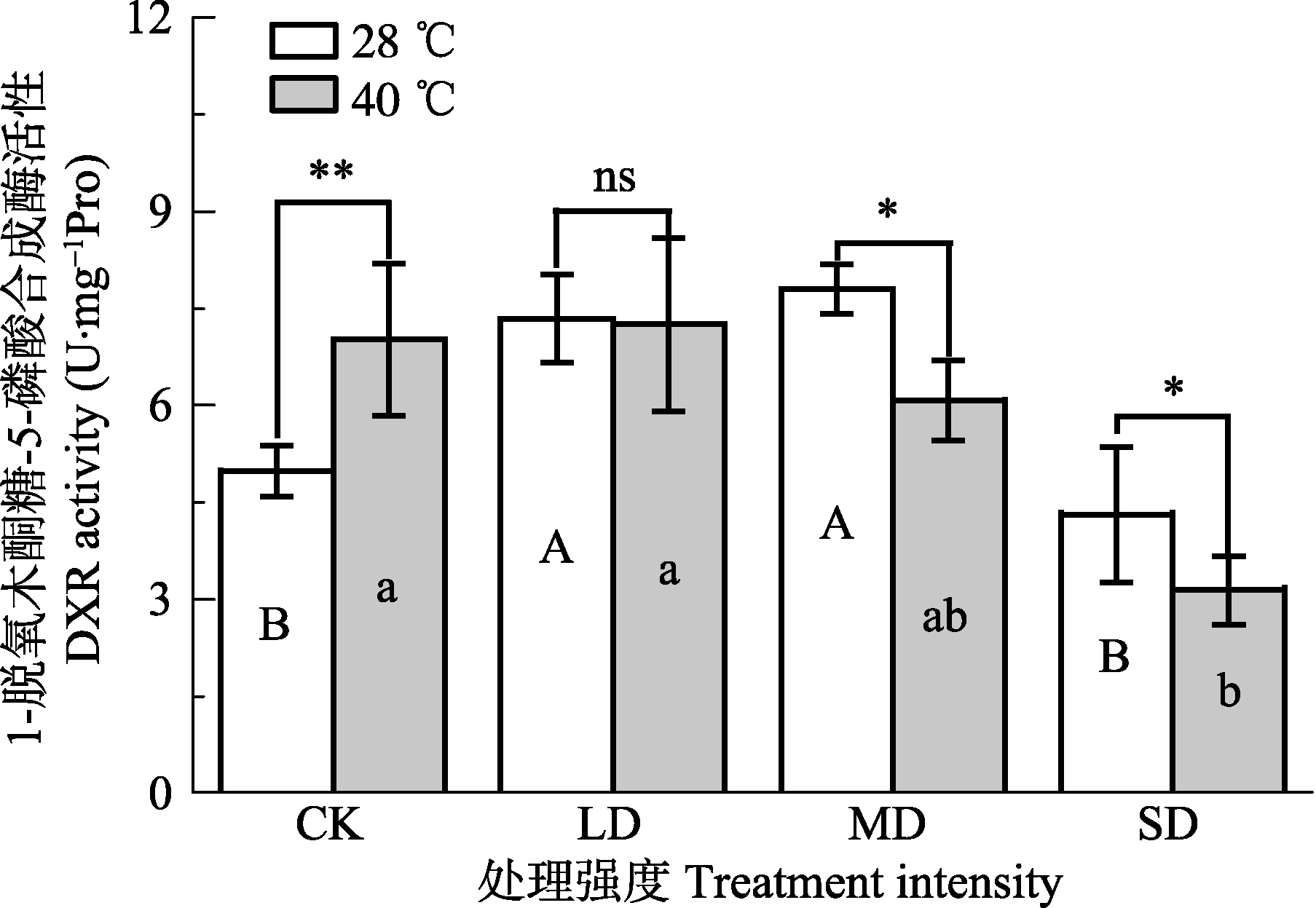
Fig. 6 Effects of drought and heat stress on 1-deoxy- D-xylulose 5-phosphate reductoisomerase (DXR) activity in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of drought and different lowercase letters denote statistically significant differences of d heat stress treatment (p < 0.05, according to LSD test). **, p < 0.01;. CK, control; LD, light drought; MD, moderate drought; SD, severe drought.
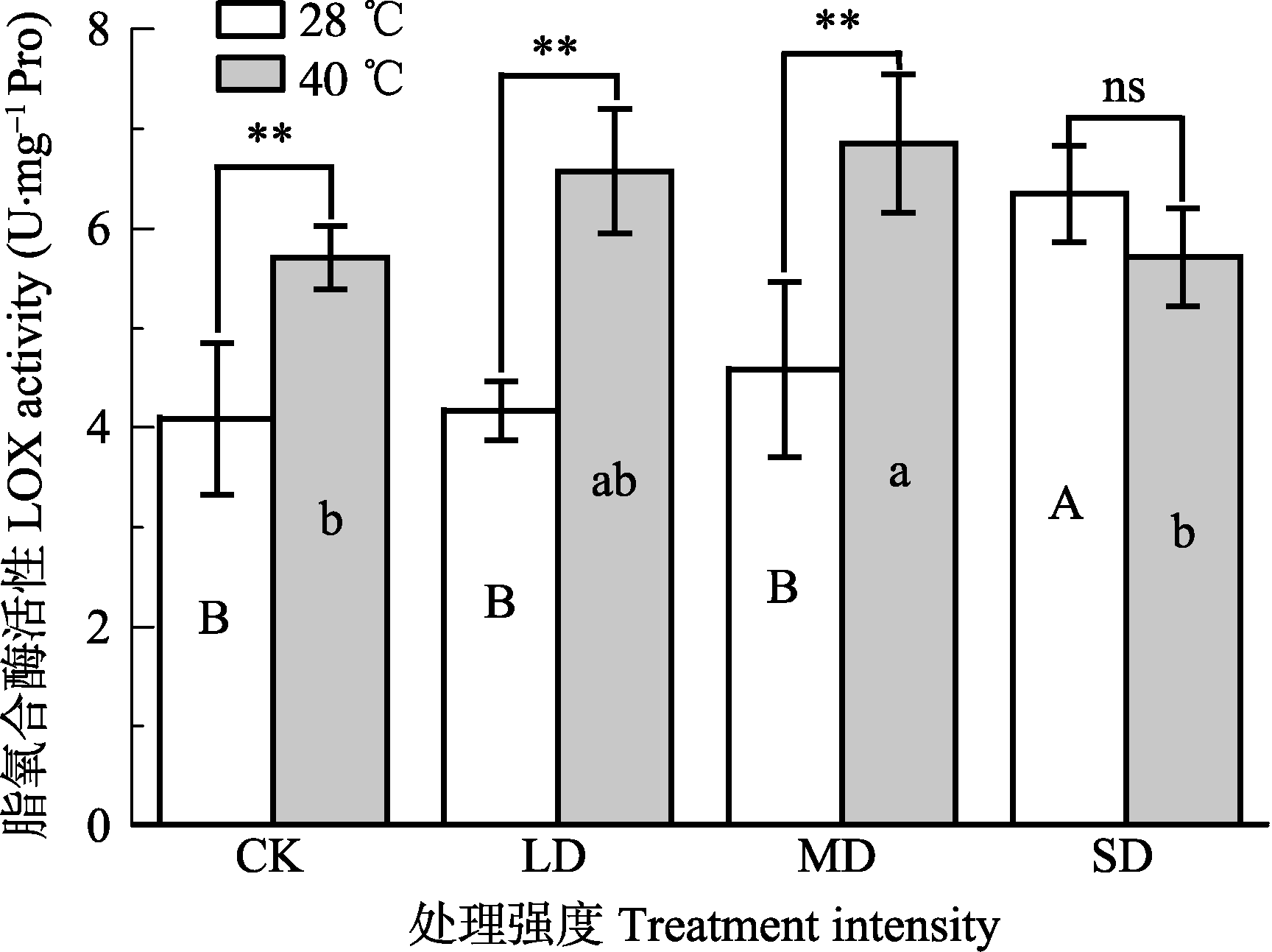
Fig. 7 Effects of drought and heat stress on lipoxygenase (LOX) activity in leaves of Osmanthus fragrans cv. ‘Boyejingui’ (mean ± SE, n = 5). Different uppercase letters indicate significant differences of different lowercase letters denote statistically significant differences of from drought and heat stress treatment (p < 0.05, according to LSD test). **, p < 0.01. CK, control; LD, light drought; MD, moderate drought; SD, severe drought.
| 序号 No. | 挥发性有机物 Volatile organic compounds | 分子式 Chemical formula | 28 ℃ | 40 ℃ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 对照 Control | 轻度干旱 Light drought | 中度干旱 Moderate drought | 重度干旱 Severe drought | 对照 Control | 轻度干旱 Light drought | 中度干旱 Moderate drought | 重度干旱 Severe drought | |||
| 1 | 己烯醛 3-Hexenal | C6H10O | 3.33 ± 0.23 | 0.58 ± 0.65 | 2.27 ± 0.12 | - | 3.61 ± 0.26 | - | - | 1.15 ± 0.05 |
| 2 | 2-甲基-4-戊烯醛 2-Methyl-4-pentenal | C6H10O | 0.99 ± 0.11 | 2.36 ± 0.23 | - | 5.97 ± 0.35 | - | 2.88 ± 0.12 | 3.14 ± 0.16 | 1.69 ± 0.13 |
| 3 | 2-已烯醛 (E)-2-Hexenal | C6H10O | 2.48 ± 0.18 | 2.14 ± 0.23 | 2.54 ± 0.21 | 8.67 ± 1.14 | 4.53 ± 0.54 | 4.35 ± 0.08 | 8.01 ± 0.24 | 7.03 ± 0.19 |
| 4 | 苯基丁醛 4-Phenylbutanal | C10H12O | - | 1.85 ± 0.11 | - | - | - | 5.48 ± 0.13 | 2.58 ± 0.06 | 2.74 ± 0.06 |
| 5 | 山梨醛 Sorbaldehyde | C6H8O | 1.08 ± 0.06 | 2.82 ± 0.08 | 1.90 ± 0.04 | 3.05 ± 0.64 | 1.80 ± 0.08 | - | 2.61 ± 0.11 | 2.33 ± 0.08 |
| 6 | 辛烯醛 2-Ethylhexenal | C8H14O | - | 1.91 ± 0.04 | 4.86 ± 0.81 | - | 3.77 ± 0.12 | 1.37 ± 0.06 | 0.67 ± 0.02 | - |
| 7 | 壬醛 n-Nonanal | C9H18O | - | - | - | - | 0.76 ± 0.02 | 1.21 ± 0.04 | 0.90 ± 0.05 | 0.53 ± 0.02 |
| 8 | 反-3-己烯醇 trans-3-Hexenol | C6H12O | 6.80 ± 0.45 | 8.07 ± 0.62 | 7.85 ± 0.67 | 4.12 ± 0.35 | 9.26 ± 1.61 | 2.50 ± 0.27 | 5.38 ± 1.01 | 3.20 ± 0.32 |
| 9 | (3E)-3-壬烯醇 (3E)-3-Nonen-1-ol | C9H18O | - | - | - | - | - | - | 1.24 ± 0.07 | 1.17 ± 0.11 |
| 10 | 2-乙基己醇 2-Ethylhexan-1-ol | C8H18O | 4.28 ± 0.14 | 1.25 ± 0.08 | - | 1.93 ± 0.07 | - | 8.29 ± 0.61 | 2.08 ± 0.10 | 2.15 ± 0.09 |
| 11 | 反罗勒烯 Ocimene | C10H16 | 1.65 ± 0.06 | 1.53 ± 0.11 | 2.02 ± 0.08 | 1.85 ± 0.05 | 1.55 ± 0.08 | - | 0.80 ± 0.02 | - |
| 12 | 薄荷烯 cis-p-Menthane | C10H20 | 3.38 ± 0.04 | - | 2.03 ± 0.06 | - | 1.50 ± 0.12 | 2.48 ± 0.21 | - | 1.08 ± 0.05 |
| 13 | 柠檬烯 Limonene | C10H16 | 3.83 ± 0.07 | 1.42 ± 0.12 | 1.61 ± 0.12 | 1.74 ± 0.10 | 3.29 ± 0.29 | 1.65 ± 0.15 | 1.67 ± 0.03 | 0.94 ± 0.04 |
| 14 | 罗勒烯Ocimene | C10H16 | 0.18 ± 0.01 | 3.25 ± 0.24 | 2.58 ± 0.22 | 1.44 ± 0.14 | 1.40 ± 0.10 | - | 0.94 ± 0.05 | - |
| 15 | α-蒎烯alpha-Pinene | C10H16 | 0.07 ± 0.01 | 2.68 ± 0.16 | 3.13 ± 0.02 | 2.52 ± 0.09 | 5.24 ± 0.34 | 4.83 ± 0.37 | 3.60 ± 0.34 | 2.69 ± 0.27 |
| 16 | 紫苏烯 Perillen | C10H14O | 0.34 ± 0.04 | 0.53 ± 0.05 | 1.05 ± 0.11 | 1.60 ± 0.06 | 1.03 ± 0.02 | 0.63 ± 0.01 | 0.54 ± 0.02 | - |
| 17 | 长叶烯 Longifolene | C15H24 | 1.77 ± 0.13 | 3.69 ± 0.05 | 2.05 ± 0.23 | 0.71 ± 0.03 | 0.83 ± 0.01 | 1.46 ± 0.04 | 1.03 ± 0.02 | 0.88 ± 0.04 |
| 18 | 丁香烯π Caryophyllen | C15H24 | - | - | - | - | - | 1.07 ± 0.05 | - | - |
| 19 | 顺-3-乙酸叶醇酯 cis-3-Hexenyl acetate | C8H14O2 | 1.55 ± 0.06 | 1.61 ± 0.86 | 2.25 ± 0.61 | 4.73 ± 1.37 | 6.11 ± 1.01 | 2.63 ± 0.31 | 2.20 ± 0.92 | 4.09 ± 0.56 |
| 20 | 丙烯酸正己酯 Hexyl acrylate | C9H16O2 | 1.90 ± 0.09 | - | - | - | - | 2.58 ± 0.18 | 1.55 ± 0.02 | 1.08 ± 0.04 |
| 21 | 异丁酸庚酯 Heptyl isobutyrate | C11H22O2 | - | - | 0.96 ± 0.03 | 5.66 ± 0.63 | 2.45 ± 0.23 | 6.00 ± 0.93 | 3.10 ± 0.43 | 2.47 ± 0.41 |
| 22 | 丁酸庚酯 Heptyl butyrate | C11H22O2 | 1.39 ± 0.05 | 1.31 ± 0.03 | 4.22 ± 0.08 | 2.05 ± 0.39 | 5.02 ± 0.71 | 4.40 ± 1.21 | 2.79 ± 0.94 | 2.18 ± 0.52 |
| 23 | 丁酸丁酯 Butyl butyrate | C8H16O2 | 3.87 ± 0.14 | 3.07 ± 0.54 | 5.93 ± 0.35 | 1.71 ± 0.48 | 1.25 ± 0.08 | - | - | - |
| 合计 | 38.89 | 40.07 | 48.25 | 47.75 | 53.4 | 52.81 | 44.83 | 37.37 | ||
Table 1 The main components of the volatile organic compounds (VOCs) from the leaf of Osmanthus fragrans ‘Boyejingui’ (peak area, A × 105·10 g-1) (mean ± SE)
| 序号 No. | 挥发性有机物 Volatile organic compounds | 分子式 Chemical formula | 28 ℃ | 40 ℃ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 对照 Control | 轻度干旱 Light drought | 中度干旱 Moderate drought | 重度干旱 Severe drought | 对照 Control | 轻度干旱 Light drought | 中度干旱 Moderate drought | 重度干旱 Severe drought | |||
| 1 | 己烯醛 3-Hexenal | C6H10O | 3.33 ± 0.23 | 0.58 ± 0.65 | 2.27 ± 0.12 | - | 3.61 ± 0.26 | - | - | 1.15 ± 0.05 |
| 2 | 2-甲基-4-戊烯醛 2-Methyl-4-pentenal | C6H10O | 0.99 ± 0.11 | 2.36 ± 0.23 | - | 5.97 ± 0.35 | - | 2.88 ± 0.12 | 3.14 ± 0.16 | 1.69 ± 0.13 |
| 3 | 2-已烯醛 (E)-2-Hexenal | C6H10O | 2.48 ± 0.18 | 2.14 ± 0.23 | 2.54 ± 0.21 | 8.67 ± 1.14 | 4.53 ± 0.54 | 4.35 ± 0.08 | 8.01 ± 0.24 | 7.03 ± 0.19 |
| 4 | 苯基丁醛 4-Phenylbutanal | C10H12O | - | 1.85 ± 0.11 | - | - | - | 5.48 ± 0.13 | 2.58 ± 0.06 | 2.74 ± 0.06 |
| 5 | 山梨醛 Sorbaldehyde | C6H8O | 1.08 ± 0.06 | 2.82 ± 0.08 | 1.90 ± 0.04 | 3.05 ± 0.64 | 1.80 ± 0.08 | - | 2.61 ± 0.11 | 2.33 ± 0.08 |
| 6 | 辛烯醛 2-Ethylhexenal | C8H14O | - | 1.91 ± 0.04 | 4.86 ± 0.81 | - | 3.77 ± 0.12 | 1.37 ± 0.06 | 0.67 ± 0.02 | - |
| 7 | 壬醛 n-Nonanal | C9H18O | - | - | - | - | 0.76 ± 0.02 | 1.21 ± 0.04 | 0.90 ± 0.05 | 0.53 ± 0.02 |
| 8 | 反-3-己烯醇 trans-3-Hexenol | C6H12O | 6.80 ± 0.45 | 8.07 ± 0.62 | 7.85 ± 0.67 | 4.12 ± 0.35 | 9.26 ± 1.61 | 2.50 ± 0.27 | 5.38 ± 1.01 | 3.20 ± 0.32 |
| 9 | (3E)-3-壬烯醇 (3E)-3-Nonen-1-ol | C9H18O | - | - | - | - | - | - | 1.24 ± 0.07 | 1.17 ± 0.11 |
| 10 | 2-乙基己醇 2-Ethylhexan-1-ol | C8H18O | 4.28 ± 0.14 | 1.25 ± 0.08 | - | 1.93 ± 0.07 | - | 8.29 ± 0.61 | 2.08 ± 0.10 | 2.15 ± 0.09 |
| 11 | 反罗勒烯 Ocimene | C10H16 | 1.65 ± 0.06 | 1.53 ± 0.11 | 2.02 ± 0.08 | 1.85 ± 0.05 | 1.55 ± 0.08 | - | 0.80 ± 0.02 | - |
| 12 | 薄荷烯 cis-p-Menthane | C10H20 | 3.38 ± 0.04 | - | 2.03 ± 0.06 | - | 1.50 ± 0.12 | 2.48 ± 0.21 | - | 1.08 ± 0.05 |
| 13 | 柠檬烯 Limonene | C10H16 | 3.83 ± 0.07 | 1.42 ± 0.12 | 1.61 ± 0.12 | 1.74 ± 0.10 | 3.29 ± 0.29 | 1.65 ± 0.15 | 1.67 ± 0.03 | 0.94 ± 0.04 |
| 14 | 罗勒烯Ocimene | C10H16 | 0.18 ± 0.01 | 3.25 ± 0.24 | 2.58 ± 0.22 | 1.44 ± 0.14 | 1.40 ± 0.10 | - | 0.94 ± 0.05 | - |
| 15 | α-蒎烯alpha-Pinene | C10H16 | 0.07 ± 0.01 | 2.68 ± 0.16 | 3.13 ± 0.02 | 2.52 ± 0.09 | 5.24 ± 0.34 | 4.83 ± 0.37 | 3.60 ± 0.34 | 2.69 ± 0.27 |
| 16 | 紫苏烯 Perillen | C10H14O | 0.34 ± 0.04 | 0.53 ± 0.05 | 1.05 ± 0.11 | 1.60 ± 0.06 | 1.03 ± 0.02 | 0.63 ± 0.01 | 0.54 ± 0.02 | - |
| 17 | 长叶烯 Longifolene | C15H24 | 1.77 ± 0.13 | 3.69 ± 0.05 | 2.05 ± 0.23 | 0.71 ± 0.03 | 0.83 ± 0.01 | 1.46 ± 0.04 | 1.03 ± 0.02 | 0.88 ± 0.04 |
| 18 | 丁香烯π Caryophyllen | C15H24 | - | - | - | - | - | 1.07 ± 0.05 | - | - |
| 19 | 顺-3-乙酸叶醇酯 cis-3-Hexenyl acetate | C8H14O2 | 1.55 ± 0.06 | 1.61 ± 0.86 | 2.25 ± 0.61 | 4.73 ± 1.37 | 6.11 ± 1.01 | 2.63 ± 0.31 | 2.20 ± 0.92 | 4.09 ± 0.56 |
| 20 | 丙烯酸正己酯 Hexyl acrylate | C9H16O2 | 1.90 ± 0.09 | - | - | - | - | 2.58 ± 0.18 | 1.55 ± 0.02 | 1.08 ± 0.04 |
| 21 | 异丁酸庚酯 Heptyl isobutyrate | C11H22O2 | - | - | 0.96 ± 0.03 | 5.66 ± 0.63 | 2.45 ± 0.23 | 6.00 ± 0.93 | 3.10 ± 0.43 | 2.47 ± 0.41 |
| 22 | 丁酸庚酯 Heptyl butyrate | C11H22O2 | 1.39 ± 0.05 | 1.31 ± 0.03 | 4.22 ± 0.08 | 2.05 ± 0.39 | 5.02 ± 0.71 | 4.40 ± 1.21 | 2.79 ± 0.94 | 2.18 ± 0.52 |
| 23 | 丁酸丁酯 Butyl butyrate | C8H16O2 | 3.87 ± 0.14 | 3.07 ± 0.54 | 5.93 ± 0.35 | 1.71 ± 0.48 | 1.25 ± 0.08 | - | - | - |
| 合计 | 38.89 | 40.07 | 48.25 | 47.75 | 53.4 | 52.81 | 44.83 | 37.37 | ||
| [1] |
Agrawal SB, Singh S, Agrawal M ( 2009). Ultraviolet-B induced changes in gene expression and antioxidants in plants. Advances in Botanical Research, 52, 47-86.
DOI URL |
| [2] | Arab L, Kreuzwieser J, Kruse J, Zimmer I, Ache P, Alfarraj S, Al-Rasheid KAS, Schnitzler JP, Hedrich R, Rennenberg H ( 2016). Acclimation to heat and drought—Lessons to learn from the date palm ( Phoenix dactylifera). Environmental & Experimental Botany, 125, 20-30. |
| [3] | Bourtsoukidis E, Kawaletz H, Radacki D, Schütz S, Hakola H, Hellén H, Noe S, M?lder I, Ammer C, Bonn B ( 2014). Impact of flooding and drought conditions on the emission of volatile organic compounds of Quercus robur and Prunus serotina. Trees, 28, 193-204. |
| [4] |
Bradford MM ( 1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254.
DOI URL |
| [5] | Chen KC ( 2013). The warming trend and the distribution of the seasons in Hangzhou from 1961 to 2012. Chinese Agricultural Science Bulletin, 29, 345-350. |
| [ 陈柯辰 ( 2013). 1961-2012年杭州的升温趋势和四季分配之变化. 中国农学通报, 29, 345-350.] | |
| [6] |
Cheng LL, Chen M, Leng PS, Hu ZH ( 2015). Effect of different temperature levels on the emission of terpenoid volatile compounds in Ocimum basilicum ‘Purple Ruffles’. Journal of Beijing University of Agriculture, 30(2), 78-82.
DOI URL |
|
[ 程璐璐, 陈敏, 冷平生, 胡增辉 ( 2015). 不同温度对紫罗勒萜烯类挥发物释放的影响. 北京农学院学报, 30(2), 78-82.]
DOI URL |
|
| [7] |
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez-Vilalta J, Mayr S, Mencuccini M, Mitchell PJ, Nardini A, Pittermann J, Pratt RB, Sperry JS, Westoby M, Wright IJ, Zanne AE ( 2012). Global convergence in the vulnerability of forests to drought. Nature, 491, 752-755.
DOI URL |
| [8] | Copolovici L, K?nnaste A, Remmel T, Niinemets ü ( 2014). Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environmental & Experimental Botany, 100, 55-63. |
| [9] |
Dicke M, Loreto F ( 2010). Induced plant volatiles: From genes to climate change. Trends in Plant Science, 15, 115-117.
DOI URL PMID |
| [10] |
Dudareva N, Klempien A, Muhlemann JK, Kaplan I ( 2013). Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytologist, 198, 16-32.
DOI URL |
| [11] |
Gao Y, Jin YJ, Li HD, Chen HJ ( 2005). Volatile organic compounds and their roles in bacteriostasis in five conifer species. Journal of Integrative Plant Biology, 47, 499-507.
DOI URL |
| [12] | Hartikainen K, Nerg AM, Kivim?enp?? M, Kontunen-Soppela S, M?enp?? M, Oksanen E, Rousi M, Holopainen T ( 2009). Emissions of volatile organic compounds and leaf structural characteristics of European aspen ( Populus tremula) grown under elevated ozone and temperature. Tree Physiology, 29, 1163-1173. |
| [13] |
Hartmann H, Ziegler W, Trumbore S ( 2013). Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Functional Ecology, 27, 413-427.
DOI URL |
| [14] |
Hassan MN, Zainal Z, Ismail I ( 2015). Green leaf volatiles: biosynthesis, Biological functions and their applications in biotechnology. Plant Biotechnology Journal, 13, 727-739.
DOI URL PMID |
| [15] |
Hasunuma T, Takeno S, Hayashi S, Sendai M, Bamba T, Yoshimura S, Tomizawa K, Fukusaki E, Miyake C ( 2008). Overexpression of 1-Deoxy-D-xylulose-5-phosphate reductoisomerase gene in chloroplast contributes to increment of isoprenoid production. Journal of Bioscience and Bioengineering, 105, 518-526.
DOI URL |
| [16] |
Hatanaka A ( 1993). The biogeneration of green odour by green leaves. Phytochemistry, 34, 1201-1218.
DOI URL |
| [17] | Hijioka Y, Lin E, Pereira J, Corlett R, Cui X, Insarov G, Lasco R, Lindgren E, Surjan A ( 2014). Asia. In: Christopher B, Vicente R eds. Climate Change 2014: Impacts, Adaptation, and Vulnerability, Part B: Regional Aspects, Contribution of Working Group II, Fifth Assessment Report of the Intergovernmental Panel on Climate Change . Cambridge University Press, Cambridge,UK . 1327-1370. |
| [18] |
Hoch G, Richter A, K?rner C ( 2003). Non-structural carbon compounds in temperate forest trees. Plant, Cell & Environment, 26, 1067-1081.
DOI URL |
| [19] | Joó é, Dewulf J, Amelynck C, Schoon N, Pokorska O, ?impraga M, Steppe K, Aubinet M, van Langenhove H ( 2011). Constitutive versus heat and biotic stress induced BVOC emissions in Pseudotsuga menziesii. Atmospheric Environment, 45, 3655-3662. |
| [20] | Jud W, Vanzo E, Li Z, Ghirardo A, Zimmer I, Sharkey TD, Hansel1 A, Schnitzler JP ( 2016). Effects of heat and drought stress on post-illumination bursts of volatile organic compounds in isoprene-emitting and non-emitting poplar. Plant, Cell & Environment, 39, 1204-1215. |
| [21] |
Klein T, Hoch G, Dan Y, K?rner C ( 2014). Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiology, 34, 981-992.
DOI URL |
| [22] |
Li L, Sheen J ( 2016). Dynamic and diverse sugar signaling. Current Opinion in Plant Biology, 33, 116-125.
DOI URL PMID |
| [23] | Li P, Sun YF, Wang SG, Wang XD, Cai X, Zhu WZ, Paolo C, Li MH ( 2008). Altitudinal changes in leaf mass per unit area and tissue non-structural carbohydrates content of Abies fabri on Gongga Mountain of southwest China. Chinese Journal of Applied Ecology, 19, 8-12. |
| [ 李蟠, 孙玉芳, 王三根, 王小丹, 蔡小虎, 朱万泽, Cherybini Paolo, 李迈和 ( 2008). 贡嘎山地区不同海拔冷杉比叶质量和非结构性碳水化合物含量变化. 应用生态学报, 19, 8-12.] | |
| [24] | Lin SK, Lin J, Liu QL, Ai YF, Ke YQ, Chen C, Zhang ZY, He H ( 2014). Time-course of photosynthesis and non-?structural carbon compounds in the leaves of tea plants ( Camellia sinensis, L.) in response to deficit irrigation. Agricultural Water Management, 144, 98-106. |
| [25] | Liu F, Zuo ZJ, Xu GP, Wu XB, Zheng J, Gao RF, Zhang RM, Gao Y ( 2013). Physiological responses to drought stress and the emission of induced volatile organic compounds in Rosmarinus officinalis. Chinese Journal of Plant Ecology, 37, 454-463. |
| [ 刘芳, 左照江, 许改平, 吴兴波, 郑洁, 高荣孚, 张汝民, 高岩 ( 2013). 迷迭香对干旱胁迫的生理响应及其诱导挥发性有机化合物的释放. 植物生态学报, 37, 454-463.] | |
| [26] |
Loreto F, Schnitzler JP ( 2010). Abiotic stresses and induced BVOCs. Trends in Plant Science, 15, 154-166.
DOI URL PMID |
| [27] | Ma C, Feng YL, Zhang J, Wang HZ, Yuan JL, Li YJ ( 2017). Effects of exogenous methyl jasmonate on endogenous hormones and yield formation in wheat after anthesis under drought stress. Plant Physiology Journal, 53, 1051-1058. |
| [ 马超, 冯雅岚, 张均, 王贺正, 原佳乐, 李友军 ( 2017). 外源茉莉酸甲酯对干旱胁迫下小麦花后内源激素含量及产量形成的影响. 植物生理学报, 53, 1051-1058.] | |
| [28] |
Maguire AJ, Kobe RK ( 2015). Drought and shade deplete non-structural carbohydrate reserves in seedlings of five temperate tree species. Ecology and Evolution, 5, 5711-5721.
DOI URL |
| [29] | Marias DE, Meinzer FC, Woodruff DR, McCulloh KA ( 2017). Thermotolerance and heat stress responses of Douglas-fir and ponderosa pine seedling populations from contrasting climates. Tree Physiology, 37, 301-315. |
| [30] |
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA ( 2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178, 719-739.
DOI URL |
| [31] | Morshedloo MR, Craker LE, Salami A, Nazeri V, Sang H, Maggi F ( 2017). Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono- and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiology & Biochemistry, 111, 119-128. |
| [32] | Moses T, Pollier J, Thevelein JM, Goossens A ( 2013). Bioengineering of plant (tri) terpenoids: From metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytologist, 200, 27-43. |
| [33] |
Mu ZZ ( 2015). Research on the Impacts of High Temperature and Drought on Korla Fragrant Pear Photosynthetic Characteristics. Master degree dissertation, Xinjiang Agricultural University, ürümqi.
DOI URL |
|
[ 穆蓁蓁 ( 2015). 高温干旱对库尔勒香梨光合特性的影响研究. 新疆农业大学, 乌鲁木齐.]
DOI URL |
|
| [34] | Murcia G, Pontin M, Reinoso H, Baraldi R, Bertazza G, Gómez-Talquenca S, Bottini R, Piccoli PN ( 2015). ABA and GA3 increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-?structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters. Physiologia Plantarum, 156, 323-337. |
| [35] | Nahar K, Hasanuzzaman M, Alam MM, Fujita M ( 2015). Exogenous glutathione confers high temperature stress tolerance in mung bean ( Vigna radiate L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environmental & Experimental Botany, 112, 44-54. |
| [36] |
Pan QM, Han XG, Bai YF, Yang JC ( 2002). Advances in physiology and ecology studies on stored non-structure carbohydrates in plants. Chinese Bulletin of Botany, 19, 30-38.
DOI URL |
|
[ 潘庆民, 韩兴国, 白永飞, 杨景成 ( 2002). 植物非结构性贮藏碳水化合物的生理生态学研究进展. 植物学报, 19, 30-38.]
DOI URL |
|
| [37] |
Pe?uelas J, Staudt M ( 2010). BVOCs and global change. Trends in Plant Science, 15, 133-144.
DOI URL |
| [38] | Ramak P, Osaloo SK, Ebrahimzadeh H, Sharifi M, Behmanesh M ( 2013). Inhibition of the mevalonate pathway enhances carvacrol biosynthesis and DXR gene expression in shoot cultures of Satureja khuzistanica Jamzad. Journal of Plant Physiology, 170, 1187-1193. |
| [39] | Santino A, Bonsegna S, Domenico SD, Poltronieri P ( 2010). Plant oxylipins and their contribution to plant defense. Current Topics in Plant Biology, 11, 103-111. |
| [40] | Silva EN, Ferreirasilva SL, Fontenele ADV, Ribeiro RV, Viégas RA, Silveira JAG ( 2010). Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. Journal of Plant Physiology, 167, 1157-1164. |
| [41] |
Staudt M, Morin X, Chuine I ( 2017). Contrasting direct and indirect effects of warming and drought on isoprenoid emissions from Mediterranean oaks. Regional Environmental Change, 17, 2121-2133.
DOI URL |
| [42] | Toroser D, Huber SC ( 1998). 3-Hydroxy-3-methylglutaryl-coenzyme A reductase kinase and sucrose-phosphate synthase kinase activities in cauliflower florets: Ca 2+ dependence and substrate specificities . Archives Biochemistry & Biophysics, 355, 291-300. |
| [43] | Trifilò P, Casolo V, Raimondo F, Petrussa E, Boscutti F, Gullo MAL, Nardini A ( 2017). Effects of prolonged drought on stem non-structural carbohydrates content and post-drought hydraulic recovery in Laurus nobilis L.: The possible link between carbon starvation and hydraulic failure. Plant Physiology and Biochemistry, 120, 232-241. |
| [44] |
Velikova V, Varkonyi Z, Szabo M, Maslenkova L, Nogues I, Kovacs L, Peeva V, Busheva M, Garab G, Sharkey TD, Loreto F ( 2011). Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiology, 157, 905-916.
DOI URL |
| [45] |
Vickers CE, Possell M, Cojocariu CI, Velikova VB, Laothawornkitkul J, Ryan A, Mullineaux PM, Nicholas Hewitt C ( 2009). Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant, Cell & Environment, 32, 520-531.
DOI URL PMID |
| [46] | Vitale M, Salvatori E, Loreto F, Fares S, Manes F ( 2008). Physiological responses of Quercus ilex leaves to water stress and acute ozone exposure under controlled conditions. Water, Air & Soil Pollution, 189, 113-125. |
| [47] | Zhang T, Cao Y, Chen Y, Liu G ( 2015). Non-structural carbohydrate dynamics in Robinia pseudoacacia, saplings under three levels of continuous drought stress. Trees, 29, 1837-1849. |
| [48] | Zhao C, Wang HY, Liu MZ ( 2017). Metabolic regulation of soluble sugar, starch and related enzymes in cassava stems under drought stress. Plant Physiology Journal, 53, 795-806. |
| [ 赵超, 王海燕, 刘美珍, 王文泉 ( 2017). 干旱胁迫下木薯茎杆可溶性糖、淀粉及相关酶的代谢规律. 植物生理学报, 53, 795-806.] | |
| [49] | Zhou S, Lin FP, Wang YK, Shen YB, Zhang RM, Gao RF, Gao Y ( 2012). Effects of mechanical damage of leaves on volatile organic compounds and chlorophyll fluorescence parameters in seedlings of Cinnamomum camphora. Chinese Journal of Plant Ecology, 36, 671-680. |
| [ 周帅, 林富平, 王玉魁, 沈应柏, 张汝民, 高荣孚, 高岩 ( 2012). 樟树幼苗机械损伤叶片对挥发性有机化合物及叶绿素荧光参数的影响. 植物生态学报, 36, 671-680.] |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn