

植物生态学报 ›› 2023, Vol. 47 ›› Issue (3): 418-433.DOI: 10.17521/cjpe.2022.0049
白雪, 李玉靖, 景秀清, 赵晓东, 畅莎莎, 荆韬羽, 刘晋汝, 赵鹏宇( )
)
收稿日期:2022-02-02
接受日期:2022-06-22
出版日期:2023-03-20
发布日期:2022-07-15
通讯作者:
赵鹏宇
作者简介:* (394382557@qq.com)基金资助:
BAI Xue, LI Yu-Jing, JING Xiu-Qing, ZHAO Xiao-Dong, CHANG Sha-Sha, JING Tao-Yu, LIU Jin-Ru, ZHAO Peng-Yu( )
)
Received:2022-02-02
Accepted:2022-06-22
Online:2023-03-20
Published:2022-07-15
Contact:
ZHAO Peng-Yu
Supported by:摘要:
重金属铬(Cr)污染对农田中农作物产生毒害作用并破坏土壤微生物群落稳态, 但不同农作物及其根际土壤微生物群落对Cr胁迫的响应机制均有所差异。该研究在时间序列上分析Cr胁迫对谷子(正名: 粱, Setaria italica)长势、谷子差异表达基因(DEGs)的功能途径及土壤微生物群落结构和功能等方面的影响, 阐明谷子及土壤微生物群落的响应机制, 为Cr胁迫下的谷子生长及污染土壤的生态修复治理提供理论依据。基于室内盆栽实验, 以谷子幼苗和种植谷子的土壤为实验材料, 在Cr胁迫前(CK)及胁迫后6 h和6 d (Cr_6h、Cr_6d)的时间序列上分别进行样本采集, 同时测量幼苗生理性状指标及土壤理化指标。通过转录组分析, 研究Cr胁迫时间序列上谷子幼苗基因表达及所富集的功能途径的变化趋势; 通过高通量测序分析, 研究Cr胁迫时间序列上土壤微生物群落结构、物种多样性、群落功能的动态变化过程及与土壤理化性质的相关性。结果发现: 1)转录组分析结果表明Cr胁迫诱导基因表达上调(上调DEGs 54%); Gene Ontology (GO)富集分析表明DEGs在CK和Cr_6h、Cr_6h和Cr_6d样本对中与光合作用相关的基因的表达显著下调; 在Cr_6h和Cr_6d样本对中与防御和损伤调控相关的基因的表达显著上调, 细胞壁及细胞膜和细胞分裂相关基因的表达下调。2)高通量测序结果表明Cr胁迫时间序列上土壤细菌与真菌在门、属水平组成变化显著; 细菌群落α多样性呈现出由应激到稳定的阶段性变化特征(CK、Cr_6h和Cr_6d的Shannon多样性指数分别为6.09、5.93和6.05; Simpson多样性指数分别为0.006 8、0.007 8和0.006 8; Chao多样性指数分别为2 818.49、2 630.73和2 769.38), 而真菌群落α多样性显著下降(Shannon-Wiener多样性指数分别为4.17、3.81和3.23); 细菌与真菌群落的β多样性在Cr胁迫时间序列的分布差异显著。3)土壤理化性质与微生物群落相关性分析表明土壤理化因子与多种真菌群落显著相关, 而与细菌群落的关联关系较弱。结果表明Cr胁迫在时间序列上通过降低叶绿素含量、光系统活性并影响类囊体等结构组分显著抑制了谷子幼苗的光合作用过程, 通过下调细胞壁及微管相关组分基因表达抑制叶片细胞的增殖分化过程, 但同时激活了植物防御系统以降低自身所受的毒害作用。同时土壤细菌与真菌群落通过群落组成结构及多样性的变化来适应Cr胁迫, 二者在胁迫时间序列上的响应程度和策略均有所不同。
白雪, 李玉靖, 景秀清, 赵晓东, 畅莎莎, 荆韬羽, 刘晋汝, 赵鹏宇. 谷子及其根际土壤微生物群落对铬胁迫的响应机制. 植物生态学报, 2023, 47(3): 418-433. DOI: 10.17521/cjpe.2022.0049
BAI Xue, LI Yu-Jing, JING Xiu-Qing, ZHAO Xiao-Dong, CHANG Sha-Sha, JING Tao-Yu, LIU Jin-Ru, ZHAO Peng-Yu. Response mechanisms of millet and its rhizosphere soil microbial communities to chromium stress. Chinese Journal of Plant Ecology, 2023, 47(3): 418-433. DOI: 10.17521/cjpe.2022.0049
| 指标 Index | 对照 Control | Cr胁迫6 h Cr stress for 6 h | Cr胁迫6 d Cr stress for 6 d |
|---|---|---|---|
| 氮含量 Nitrogen content (mg·kg-1) | 38.89 ± 8.27b | 34.28 ± 3.43b | 80.83 ± 6.56a |
| 磷含量 Phosphorus content (mg·kg-1) | 53.83 ± 12.17b | 48.17 ± 5.50b | 111.28 ± 10.45a |
| 钾含量 Potassium content (mg·kg-1) | 117.34 ± 28.51b | 111.33 ± 11.71b | 262.00 ± 25.32a |
| 电导率 Electrical conductivity (us·cm-1) | 743.61 ± 127.88b | 663.89 ± 79.75b | 1628.78 ± 113.72a |
| pH | 6.90 ± 0.08b | 7.04 ± 0.12b | 7.29 ± 0.13a |
表1 铬(Cr)胁迫下土壤理化性质(平均值±标准差)
Table 1 Soil physical and chemical properties under chromium (Cr) stress (mean ± SD)
| 指标 Index | 对照 Control | Cr胁迫6 h Cr stress for 6 h | Cr胁迫6 d Cr stress for 6 d |
|---|---|---|---|
| 氮含量 Nitrogen content (mg·kg-1) | 38.89 ± 8.27b | 34.28 ± 3.43b | 80.83 ± 6.56a |
| 磷含量 Phosphorus content (mg·kg-1) | 53.83 ± 12.17b | 48.17 ± 5.50b | 111.28 ± 10.45a |
| 钾含量 Potassium content (mg·kg-1) | 117.34 ± 28.51b | 111.33 ± 11.71b | 262.00 ± 25.32a |
| 电导率 Electrical conductivity (us·cm-1) | 743.61 ± 127.88b | 663.89 ± 79.75b | 1628.78 ± 113.72a |
| pH | 6.90 ± 0.08b | 7.04 ± 0.12b | 7.29 ± 0.13a |

图1 铬(Cr)胁迫后谷子幼苗形态的变化。CK, 对照; Cr_6d, Cr胁迫6 d; Cr_6h, Cr胁迫6 h。
Fig. 1 Morphology changes of Setaria italica seedlings after chromium (Cr) stress. CK, control; Cr_6d, chromium stress for 6 d; Cr_6h, Cr stress for 6 h.
| 指标 Index | 对照 Control | Cr胁迫6 h Cr stress for 6 h | Cr胁迫6 d Cr stress for 6 d |
|---|---|---|---|
| 茎长 Stem length (cm) | 5.86 ± 0.55b | 5.52 ± 0.55b | 7.73 ± 1.12a |
| 根长 Root length (cm) | 4.18 ± 0.81a | 3.64 ± 0.23a | 3.83 ± 0.75a |
| 干质量 Dry mass (g) | 0.003 1 ± 0.000 5b | 0.003 4 ± 0.000 5b | 0.005 0 ± 0.000 6a |
| 鲜质量 Fresh mass (g) | 0.028 3 ± 0.005 3b | 0.029 4 ± 0.002 6b | 0.037 8 ± 0.005 1a |
| 叶绿素含量 Chlorophyll content (SPAD) | 24.68 ± 1.70a | 18.84 ± 1.77b | 16.34 ± 2.00b |
| 氮含量 Nitrogen content (mg·g-1) | 7.49 ± 0.51a | 6.19 ± 0.52b | 5.45 ± 0.58b |
表2 铬(Cr)胁迫时间序列上谷子长势及生物量测定(平均值±标准差)
Table 2 Setaria italica growth and biomass determination in the chromium (Cr) stress time series (mean ± SD)
| 指标 Index | 对照 Control | Cr胁迫6 h Cr stress for 6 h | Cr胁迫6 d Cr stress for 6 d |
|---|---|---|---|
| 茎长 Stem length (cm) | 5.86 ± 0.55b | 5.52 ± 0.55b | 7.73 ± 1.12a |
| 根长 Root length (cm) | 4.18 ± 0.81a | 3.64 ± 0.23a | 3.83 ± 0.75a |
| 干质量 Dry mass (g) | 0.003 1 ± 0.000 5b | 0.003 4 ± 0.000 5b | 0.005 0 ± 0.000 6a |
| 鲜质量 Fresh mass (g) | 0.028 3 ± 0.005 3b | 0.029 4 ± 0.002 6b | 0.037 8 ± 0.005 1a |
| 叶绿素含量 Chlorophyll content (SPAD) | 24.68 ± 1.70a | 18.84 ± 1.77b | 16.34 ± 2.00b |
| 氮含量 Nitrogen content (mg·g-1) | 7.49 ± 0.51a | 6.19 ± 0.52b | 5.45 ± 0.58b |
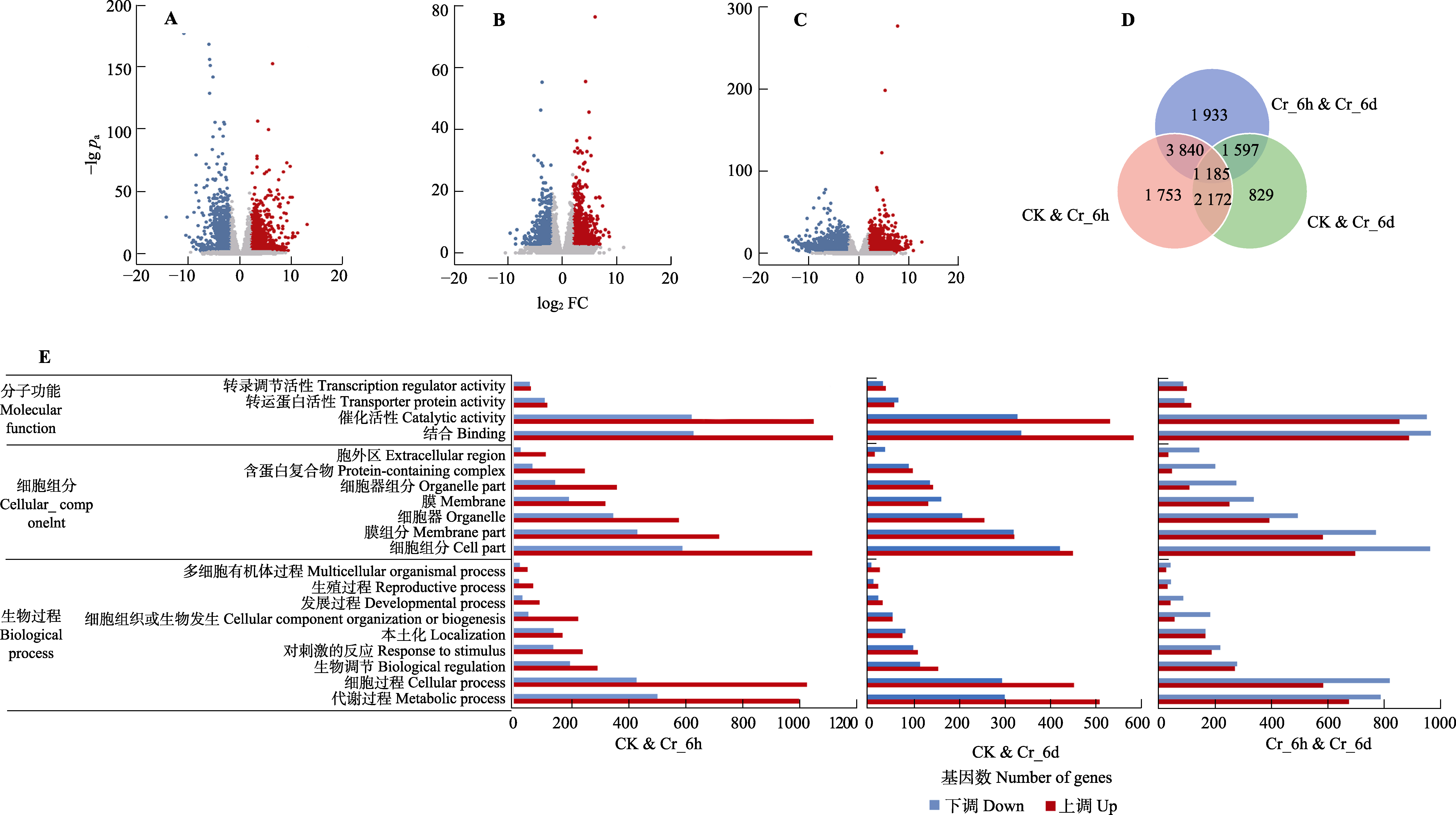
图2 铬(Cr)胁迫时间序列上谷子叶片中的差异表达基因(DEGs)。A-C, CK、Cr_6h、Cr_6d处理中谷子叶片DEGs火山图。蓝点、红点、灰点分别代表下调、上调和无显著变化基因。D, 不同处理谷子叶片中的DEGs Venn图。E, Cr胁迫下谷子叶片中DEGs的GO注释分析。CK, 对照; Cr_6d, Cr胁迫6 d; Cr_6h; Cr胁迫6 h。FC, 差异表达倍数; pa, 矫正的p值。
Fig. 2 Differentially expressed genes (DEGs) in leaves of Setaria italica in the chromium (Cr) stress time series. A-C, Volcano plots of DEGs in leaves of S. italica from three sample pairs. The blue, red and gray dots represent down-regulated, up-regulated and no significant change genes, respectively. D, Venn diagram showing the effect of different samples on DEGs in leaves of S. italica. E, GO annotation analysis of DEGs in leaves of S. italica under Cr treatment. CK, control; Cr_6d, Cr stress for 6 d; Cr_6h, Cr stress for 6 h. FC, differential expression multiple; pa, the adjusted p value.
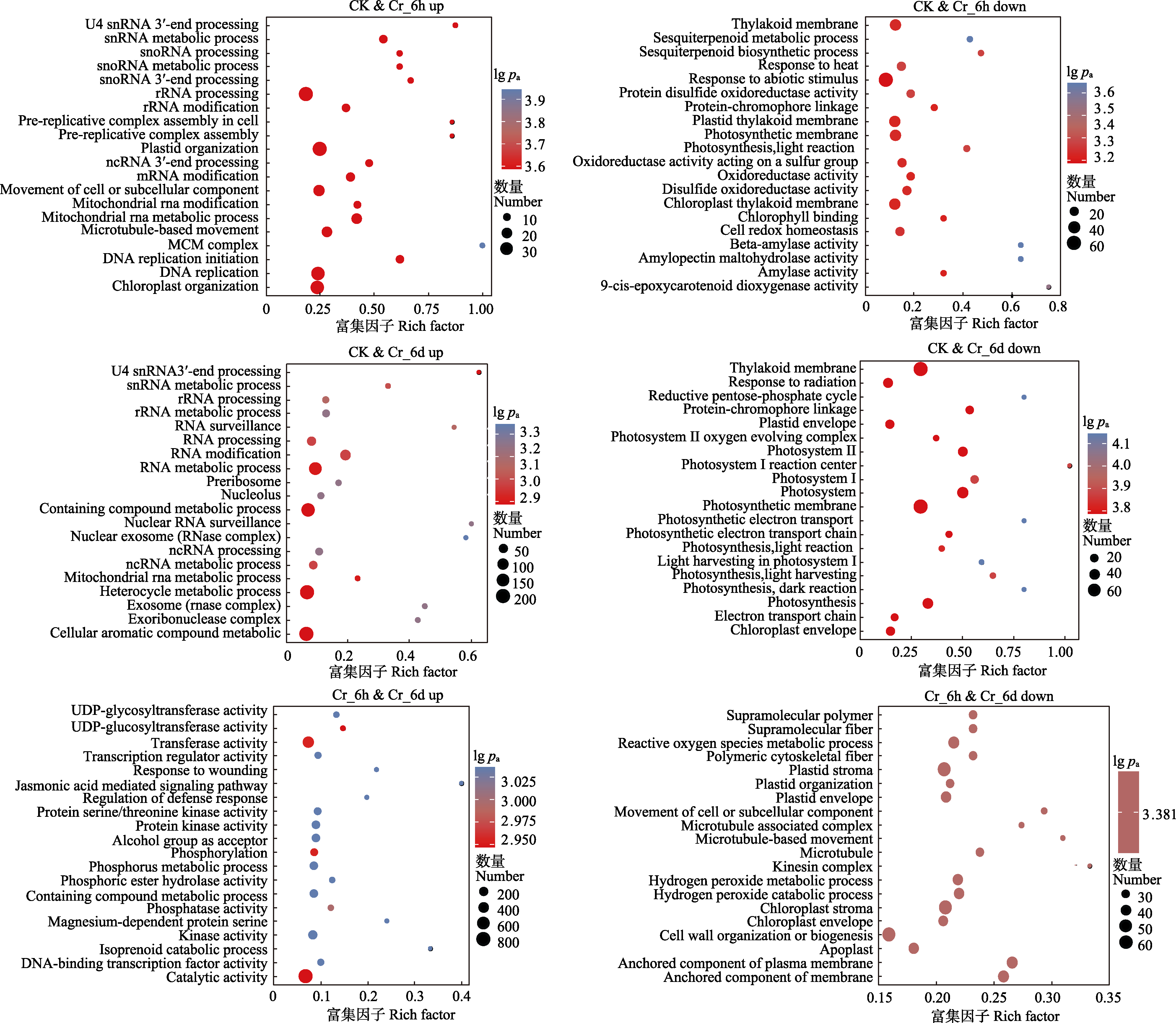
图3 铬(Cr)胁迫下谷子叶片中差异表达基因(DEGs)的Gene Ontology (GO)富集分析。down, 下调; up, 上调。CK, 对照; Cr_6d, Cr胁迫6 d ; Cr_6h, Cr胁迫6 h。9-cis-epoxycarotenoid dioxygenase activity, 9-顺式-环氧类胡萝卜素双加氧酶活性; Alcohol group as acceptor, 醇基作为受体; Amylase activity, 淀粉酶活性; Amylopectin maltohydrolase activity, 支链淀粉麦芽糖水解酶活性; Anchored component of membrance, 膜的锚定成分; Anchored component of plasma membrane, 质膜的锚定成分; Apoplast, 质外体; Beta-amylase activity, β-淀粉酶活性; Catalytic activity, 催化活性; Cell redox homeostasis, 细胞氧化还原稳态; Cell wall organization or biogenesis, 细胞壁组织或生物发生; Cellular aromatic compound metabolic, 细胞芳香族化合物代谢; Chlorophy II binding, 叶绿素II结合; Chloroplast envelope, 叶绿体包膜; Chloroplast thylakoid membrance, 叶绿体类囊体膜; Cloroplast organization, 叶绿体组织; Containing compound metabolic process, 含复合代谢过程; Containing compound metabolic process, 含化合物代谢过程; Disulfide oxidoreductase activity, 二硫键氧化还原酶活性; DNA replication initiation, DNA复制起始; DNA replication, DNA复制; DNA-binding transcription factor activity, DNA结合转录因子活性; Electron transport chain, 电子传递链; Exoribonuclease complex, 外核糖核酸酶复合物; Exosome (RNase complex), 外泌体(核糖核酸酶复合物); Hetercycle metabolic process, 杂环代谢过程; Hydrogen peroxide catabolic process, 过氧化氢分解代谢过程; Hydrogen peroxide metabolic process, 过氧化氢代谢过程; Isoprenoid catabolic process, 类异戊二烯分解代谢过程; Jasmonic acid mediated signaling pathway, 茉莉酸介导的信号通路; Kinase activity, 激酶活性; Kinesin complex, 驱动蛋白复合物; Light harvesting in photosystem I, 光系统I光捕获; Magnesium-dependent protein serine, 镁依赖性蛋白丝氨酸; MCM complex, MCM复合物; Microtubule associated complex, 微管相关复合物; Microtubule, 微管; Microtubule-based movement, 基于微管的运动; Mitochondrial RNA metabolic process, 线粒体RNA代谢过程; Mitochondrial RNA modification, 线粒体RNA修饰; Movement of cell or subcellular component, 细胞或亚细胞成分的运动; mRNA modification, mRNA修饰; ncRNA metabolic processing, ncRNA代谢过程; ncRNA processing, ncRNA加工; Nuclear exosome (RNase complex), 核外泌体(核糖核酸酶复合体); Nucleolus, 核仁; Oxidoreductase activity acting on a sulfur group, 作用于硫的氧化还原酶活性; Oxidoreductase activity, 氧化还原酶活性; Phosphatase activity, 磷酸酶活性; Phosphoric ester hydrolase activity, 磷酸酯水解酶活性; Phosphorus metabolism process, 磷代谢过程; Phosphprylation, 磷酸化; Photosynthesis dark reaction, 光合作用暗反应; Photosynthesis light harvesting, 光合作用光捕获; Photosynthesis light reaction, 光合作用光反应; Photosynthesis, 光合作用; Photosynthetic membrane, 光合膜; Photosystem I, 光系统I; Photosystem II oxygen evolving complex, 光系统II氧演替复合物; Photosystem, 光系统; Photosythetic electron transport chain, 光合电子传递链; Plastic organization, 塑料组织; Plastid envelope, 质体包膜; Plastid processing, 质体加工; Plastid thylakoid membrance, 质体类囊体膜; Polymeric cytoskeletal fiber, 聚合细胞骨架纤维; Pre-replicative complex assembly in cell, 细胞内复制前复合物组装; Pre-replicative complex assembly, 复制前复合物组装; Preribosome, nucleolus, 前核糖体; Protein disulfide oxidoreductase activity, 蛋白质二硫键氧化还原酶活性; Protein kinase activity, 蛋白激酶活性; Protein serine/threonine kinase activity, 蛋白丝氨酸/苏氨酸激酶活性; Protein-chromophore linkage, 蛋白质-发色团键; Protein-chromophore linkage, 蛋白质-发色团连接; Reactive oxygen species metabolic process, 活性氧代谢过程; Reductive pentose-phosphate cycle, 还原性戊糖-磷酸循环; Regulation of defense response, 防御反应的调节; Response to abiotic stimulus, 对非生物刺激的反应; Response to heat, 对热的反应; Response to radiation, 对刺激的反应; Response to wounding, 对创伤的反应; RNA modification, RNA修饰; RNA processing, RNA加工; RNA surveillance, RNA监测; rRNA metabolic processing, rRNA代谢加工; rRNA modification, rRNA修饰; rRNA processing, rRNA加工; Sesquiterpenoid biosynthetic process, 倍半萜类生物合成过程; Sesquiterpenoid metabolic process, 倍半萜类代谢过程; SnoRNA 3?-end processing, snoRNA 3?端加工; snoRNA processing, snoRNA加工; snRNA metabolic process, snRNA代谢过程; Supramolecular fiber, 超分子纤维; Supramolecular polymer, 超分子聚合物; Thylakoid membrane, 类囊体膜; Transcription regulator activity, 转录调节活性; Transferase activity, 转移酶活性; U4 snRNA 3?-end processing, U4 snRNA 3?端加工; UDP-glycosyltransferase activity, UDP-糖基转移酶活性。pa, 矫正的p值。
Fig. 3 Gene Ontology (GO) enrichment analysis of differentially expressed genes (DEGs) in chromium (Cr) stressed Setaria italica leaves. pa, the adjusted p value. CK, control; Cr_6d, Cr stress for 6 d; Cr_6h, Cr stress for 6 h.
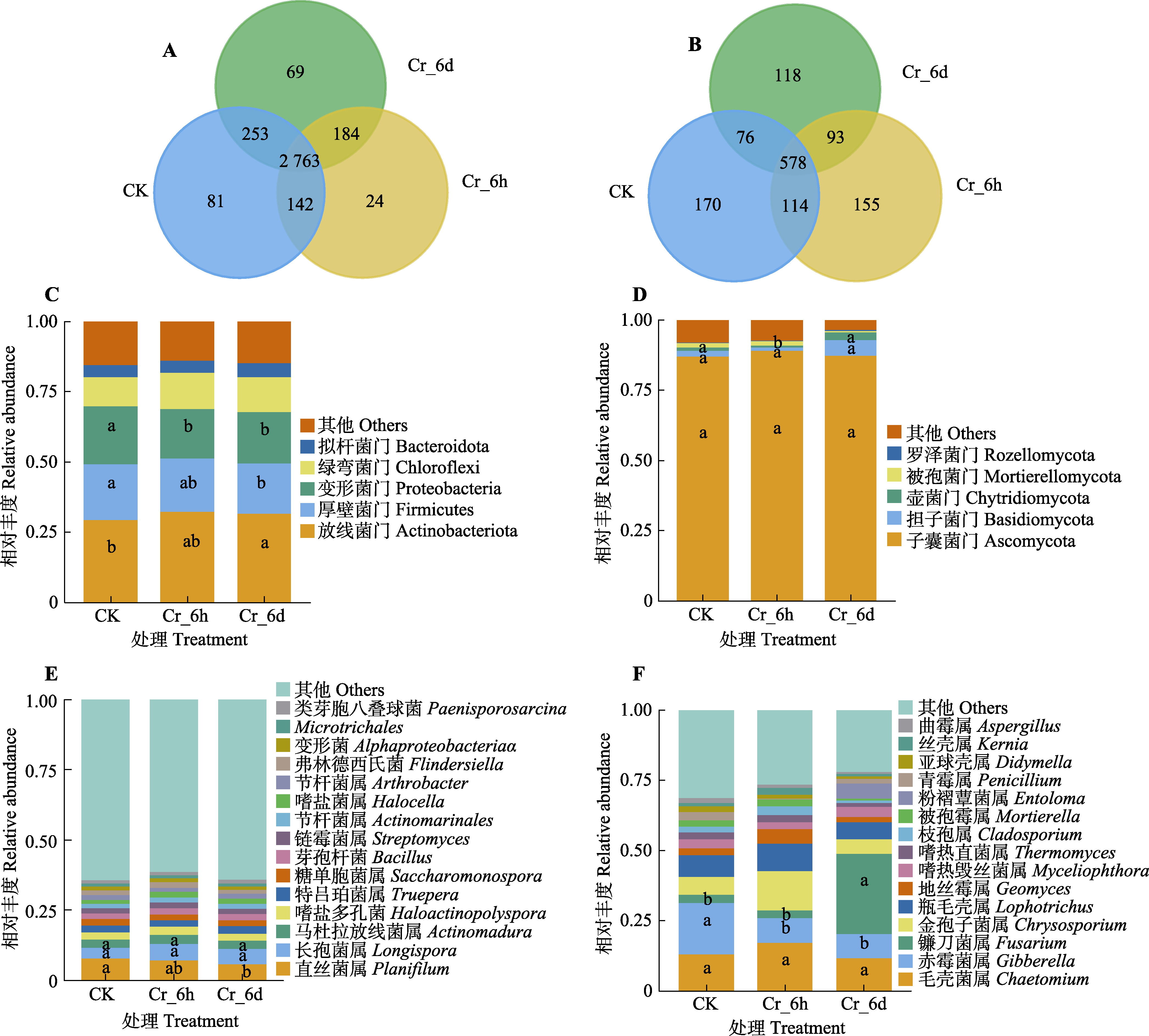
图4 铬(Cr)胁迫时间序列上谷子根际土壤细菌和真菌群落门水平与属水平群落结构及群落间差异。A、B, 细菌和真菌Venn图。图中的数字代表的是不同分组中分类操作单元的个数。C、D, 细菌和真菌门水平群落组成。E、F, 细菌和真菌属水平群落组成。CK, 对照; Cr_6d, Cr胁迫6 d; Cr_6h, Cr胁迫6 h。不同小写字母表示各处理间差异显著(p < 0.05)。
Fig. 4 Community structure and inter-community differences between bacterial and fungal communities in the rhizosphere soil of Setaria italica at phylum level and genus level in the chromium (Cr) stress time series. A, B, Bacterial and fungal Venn diagrams. Numbers in these figures represent the number of operational taxonomic units in different groups. C, D, Bacterial and fungal phylum-level community composition. E, F, Bacterial and fungal genus-level community composition. CK, control; Cr_6d, Cr stress for 6 d ; Cr_6h, Cr stress for 6 h. Different lowercase letters indicate significant differences among treatments (p < 0.05).
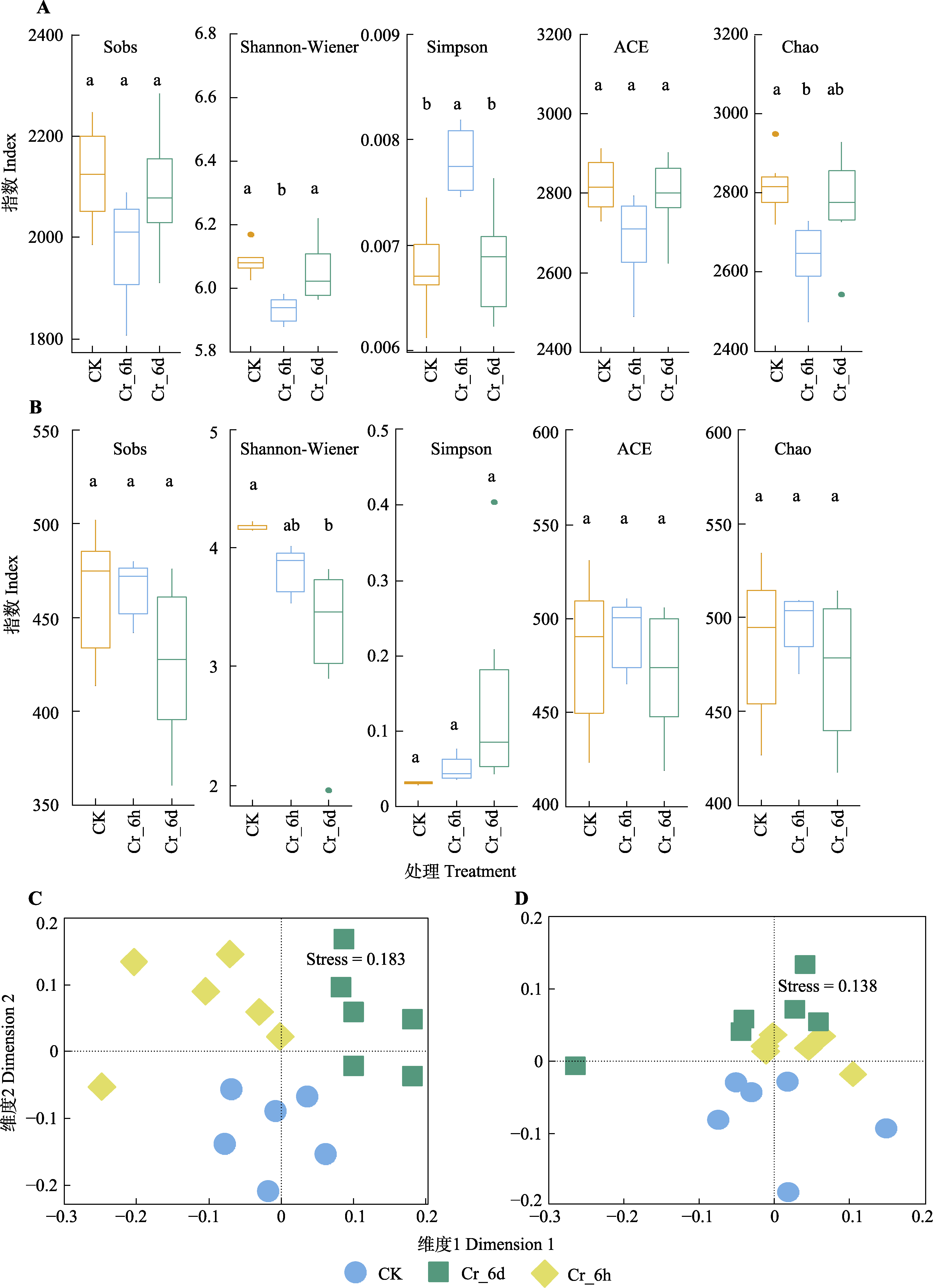
图5 铬(Cr)胁迫时间序列上土壤中细菌(A、C)与真菌(B、D)群落的α多样性及β多样性。A、B, 细菌和真菌群落的α多样性。不同小写字母表示不同处理间差异显著(p < 0.05)。C、D, 细菌和真菌群落的β多样性, 两样本点越接近, 表明两样本物种组成越相似。CK, 对照组; Cr_6d, Cr胁迫6 d ; Cr_6h, Cr胁迫6 h。Stress为反映模型合适程度的指标。
Fig. 5 Analysis of α-diversity and β-diversity of bacterial (A, C) and fungal (B, D) communities in the chromium (Cr) stress time series. A, B, α-diversity of bacterial and fungal communities. Different lowercase letters mean significant differences among treatments (p < 0.05). C, D, β-diversity of bacterial chromium fungal community. The closer the points of two samples, the more similar the species composition of the two samples. CK, control; Cr_6h, Cr stress for 6 h; Cr_6d, Cr stress for 6 d. Stress is a metric reflecting the suitability of the model.
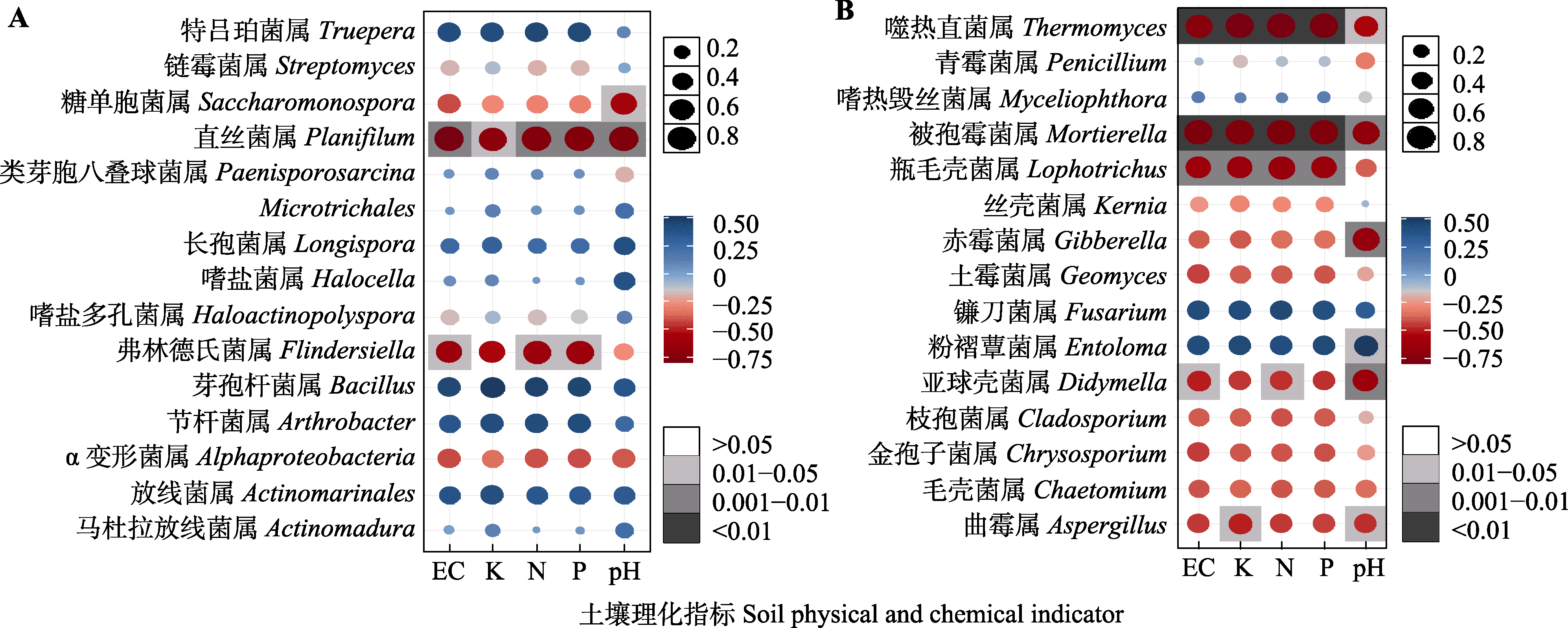
图6 土壤理化因子与细菌(A)和真菌(B)群落的相关性分析。EC, 电导率; K, 钾含量, N, 氮含量; P, 磷含量。圆形点的大小表示相关系数的大小, 颜色表示相关系数的正负, 正方形色块表示相关性检验的p值。
Fig. 6 Correlation analysis of soil physicochemical factors with bacterial (A) and fungal (B) communities。EC, electrical conductivity; K, potassium content, N, nitrogen content; P, phosphorus content. The size of the circular dots indicates the magnitude of the correlation coefficient, the color indicates the positive or negative correlation coefficient, and the square color block indicates the p value of the correlation test.
| 功能分组 Guild | 对照 Control | Cr胁迫6 h Cr stress for 6 h | Cr胁迫6 d Cr stress for 6 d | |
|---|---|---|---|---|
| 细菌 Bacterial | 全局和概述 Global and overview maps | 34 369 841.00 ± 4 720 050.73a | 34 518 331.33 ± 3 830 827.80a | 35 436 018.33 ± 2 851 467.46a |
| 碳水化合物代谢 Carbohydrate metabolism | 7 915 005.25 ± 1 088 978.09a | 7 977 677.58 ± 893 493.00a | 8 182 307.00 ± 66 8193.19a | |
| 氨基酸代谢 Amino acid metabolism | 7 063 478.92 ± 966 823.00a | 7 092 711.17 ± 792 150.10a | 7 279 350.08 ± 586 179.57a | |
| 能量代谢 Energy metabolism | 3 723 520.25 ± 510 073.28a | 3 742 113.67 ± 409 484.99a | 3 833 272.92 ± 303 226.45a | |
| 辅助因子和维生素代谢 Metabolism of cofactors and vitamins | 3 596 104.96 ± 488 259.88a | 3 580 709.88 ± 393 862.62a | 3 674 852.42 ± 301 407.93a | |
| 真菌 Fungal | 未定义的腐生菌 Undefined saprotroph | 11 919.33 ± 1 237.78a | 9 633.50 ± 2 636.32a | 18 537.33 ± 10 095.58a |
| 动物病原体-内生菌-植物病原体-未定义腐生菌 Animal pathogen-endophyte-plant pathogen- undefined saprotroph | 10 675.17 ± 1 585.46ab | 12 060.50 ± 1 946.59a | 7 946.33 ± 3 433.26b | |
| 动物病原体-粪便腐生菌-内生菌-附生植物- 植物腐生菌-木材腐生菌 Animal pathogen-dungsaprotroph-endophyte- epiphytelant saprotroph-wood saprotroph | 7 631.33 ± 1 345.52a | 9 334.50 ± 2 107.57a | 6 545.83 ± 4 562.01a | |
| 植物病原体 Plant pathogen | 10 021.17 ± 2 090.68a | 5 757.83 ± 1763.85b | 4 860.67 ± 2 198.03b | |
| 动物病原体-内生菌-地衣寄生虫-植物病原体- 土壤腐生菌-木材腐生菌 Animal pathogen-endophyte-lichen parasite-plant pathogen-soil saprotroph-wood saprotroph | 1 255.17 ± 416.48a | 1 119.50 ± 217.63a | 11 740.17 ± 17 952.63a | |
表3 铬(Cr)胁迫时间序列上土壤微生物群落的功能预测分析(平均值±标准差)
Table 3 Functional prediction analysis of soil microbial community in the chromium (Cr) stress time series (mean ± SD)
| 功能分组 Guild | 对照 Control | Cr胁迫6 h Cr stress for 6 h | Cr胁迫6 d Cr stress for 6 d | |
|---|---|---|---|---|
| 细菌 Bacterial | 全局和概述 Global and overview maps | 34 369 841.00 ± 4 720 050.73a | 34 518 331.33 ± 3 830 827.80a | 35 436 018.33 ± 2 851 467.46a |
| 碳水化合物代谢 Carbohydrate metabolism | 7 915 005.25 ± 1 088 978.09a | 7 977 677.58 ± 893 493.00a | 8 182 307.00 ± 66 8193.19a | |
| 氨基酸代谢 Amino acid metabolism | 7 063 478.92 ± 966 823.00a | 7 092 711.17 ± 792 150.10a | 7 279 350.08 ± 586 179.57a | |
| 能量代谢 Energy metabolism | 3 723 520.25 ± 510 073.28a | 3 742 113.67 ± 409 484.99a | 3 833 272.92 ± 303 226.45a | |
| 辅助因子和维生素代谢 Metabolism of cofactors and vitamins | 3 596 104.96 ± 488 259.88a | 3 580 709.88 ± 393 862.62a | 3 674 852.42 ± 301 407.93a | |
| 真菌 Fungal | 未定义的腐生菌 Undefined saprotroph | 11 919.33 ± 1 237.78a | 9 633.50 ± 2 636.32a | 18 537.33 ± 10 095.58a |
| 动物病原体-内生菌-植物病原体-未定义腐生菌 Animal pathogen-endophyte-plant pathogen- undefined saprotroph | 10 675.17 ± 1 585.46ab | 12 060.50 ± 1 946.59a | 7 946.33 ± 3 433.26b | |
| 动物病原体-粪便腐生菌-内生菌-附生植物- 植物腐生菌-木材腐生菌 Animal pathogen-dungsaprotroph-endophyte- epiphytelant saprotroph-wood saprotroph | 7 631.33 ± 1 345.52a | 9 334.50 ± 2 107.57a | 6 545.83 ± 4 562.01a | |
| 植物病原体 Plant pathogen | 10 021.17 ± 2 090.68a | 5 757.83 ± 1763.85b | 4 860.67 ± 2 198.03b | |
| 动物病原体-内生菌-地衣寄生虫-植物病原体- 土壤腐生菌-木材腐生菌 Animal pathogen-endophyte-lichen parasite-plant pathogen-soil saprotroph-wood saprotroph | 1 255.17 ± 416.48a | 1 119.50 ± 217.63a | 11 740.17 ± 17 952.63a | |
| [1] |
Abdu N, Abdullahi AA, Abdulkadir A (2017). Heavy metals and soil microbes. Environmental Chemistry Letters, 15, 65-84.
DOI |
| [2] |
Ali B, Huang CR, Qi ZY, Ali S, Daud MK, Geng XX, Liu HB, Zhou WJ (2013). 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environmental Science and Pollution Research, 20, 7256-7267.
DOI URL |
| [3] |
Artursson V, Finlay RD, Jansson JK (2006). Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environmental Microbiology, 8, 1-10.
DOI PMID |
| [4] | Bai X, Zhao XY, Jing XQ, Zhao XD, Yan PM, Zhao PY (2022). Response mechanism of soil fungal community in farmland during a period of chromium stress. Chinese Journal of Eco-Agriculture, 30, 105-115. |
| [白雪, 赵鑫宇, 景秀清, 赵晓东, 燕平梅, 赵鹏宇 (2022). 农田土壤中真菌群落在时间序列对铬胁迫的响应机制. 中国生态农业学报, 30, 105-115.] | |
| [5] |
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17, 478-486.
DOI PMID |
| [6] |
Cervantes C, Campos-Garcı́a J, Devars S, Gutiérrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R (2001). Interactions of chromium with microorganisms and plants. FEMS Microbiology Reviews, 25, 335-347.
PMID |
| [7] | Chen WX, Li Q, Wang Z, Sun ZJ (2020). Spatial distribution characteristics and pollution evaluation of heavy metals in arable land soil of China. Environmental Science, 41, 2822-2833. |
| [陈文轩, 李茜, 王珍, 孙兆军 (2020). 中国农田土壤重金属空间分布特征及污染评价. 环境科学, 41, 2822-2833.] | |
| [8] |
Chodak M, Gołębiewski M, Morawska-Płoskonka J, Kuduk K, Niklińska M (2013). Diversity of microorganisms from forest soils differently polluted with heavy metals. Applied Soil Ecology, 64, 7-14.
DOI URL |
| [9] |
Coreño-Alonso A, Acevedo-Aguilar FJ, Reyna-López GE, Tomasini A, Fernández FJ, Wrobel K, Wrobel K, Gutiérrez- Corona JF (2009). Cr(VI) reduction by an Aspergillus tubingensis strain: role of carboxylic acids and implications for natural attenuation and biotreatment of Cr(VI) contamination. Chemosphere, 76, 43-47.
DOI PMID |
| [10] |
de Oliveira LM, Gress J, De J, Rathinasabapathi B, Marchi G, Chen YS, Ma LQ (2016). Sulfate and chromate increased each other’s uptake and translocation in As-hyperaccumulator Pteris vittata. Chemosphere, 147, 36-43.
DOI PMID |
| [11] |
Deng LJ, Zeng GM, Fan CZ, Lu LH, Chen XF, Chen M, Wu HP, He XX, He Y (2015). Response of rhizosphere microbial community structure and diversity to heavy metal co-pollution in arable soil. Applied Microbiology and Biotechnology, 99, 8259-8269.
DOI PMID |
| [12] |
Diwan HM, Khan I, Ahmad A, Iqbal M (2010). Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regulation, 61, 97-107.
DOI URL |
| [13] |
Fan WJ, Feng YX, Li YH, Lin YJ, Yu XZ (2020). Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Science of the Total Environment, 744, 140951. DOI: 10.1016/j.scitotenv.2020.140951.
DOI |
| [14] | Fang HH, Pei YX, Tian BH, Zhang LP, Qiao ZJ, Liu ZQ (2014). Ca2+ participates in H2S induced Cr6+ tolerance in Setaria italica. Chinese Journal of Cell Biology, 36, 758-765. |
| [方慧慧, 裴雁曦, 田保华, 张丽萍, 乔增杰, 刘志强 (2014). H2S与Ca2+协同增强谷子对Cr6+胁迫的耐受. 中国细胞生物学学报, 36, 758-765.] | |
| [15] |
Glassman SI, Wang IJ, Bruns TD (2017). Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Molecular Ecology, 26, 6960-6973.
DOI PMID |
| [16] |
Guo XP, Yang Y, Niu ZS, Lu DP, Zhu CH, Feng JN, Wu JY, Chen YR, Tou FY, Liu M, Hou LJ (2019). Characteristics of microbial community indicate anthropogenic impact on the sediments along the Yangtze Estuary and its coastal area, China. Science of the Total Environment, 648, 306-314.
DOI URL |
| [17] |
Guo ZH, Zeng P, Xiao XY, Peng C (2021). Physiological, anatomical, and transcriptional responses of mulberry (Morus alba L.) to Cd stress in contaminated soil. Environmental Pollution, 284, 117387. DOI: 10.1016/j.envpol.2021.117387
DOI |
| [18] |
Hossain MA, Piyatida P, da Silva JAT, Fujita M, Polle A (2012). Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. Journal of Botany, 2012, 872875. DOI: 10.1155/2012/872875.
DOI |
| [19] |
Ipsilantis I, Coyne MS (2007). Soil microbial community response to hexavalent chromium in planted and unplanted soil. Journal of Environmental Quality, 36, 638-645.
PMID |
| [20] |
Jiang B, Adebayo A, Jia JL, Xing Y, Deng SQ, Guo LM, Liang YT, Zhang DY (2019). Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. Journal of Hazardous Materials, 362, 187-195.
DOI PMID |
| [21] |
Kadiiska MB, Xiang QH, Mason RP (1994). In vivo free radical generation by chromium (VI): an electron spin resonance spin-trapping investigation. Chemical Research in Toxicology, 7, 800-805.
PMID |
| [22] |
Kasemodel MC, Sakamoto IK, Varesche MBA, Rodrigues VGS (2019). Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Science of the Total Environment, 675, 367-379.
DOI |
| [23] |
Leng Y, Li Y, Wen Y, Zhao H, Wang Q, Li SW (2020). Transcriptome analysis provides molecular evidences for growth and adaptation of plant roots in cadimium-contaminated environments. Ecotoxicology and Environmental Safety, 204, 111098. DOI: 10.1016/j.ecoenv.2020.111098.
DOI |
| [24] |
Lin YB, Ye YM, Hu YM, Shi HK (2019). The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicology and Environmental Safety, 180, 557-564.
DOI PMID |
| [25] | Lin YB, Ye YM, Wu CF, Hu YM, Shi HK (2020). Response analysis of soil bacterial community to different heavy metal pollution levels in paddy fields: a case study of A county. Acta Scientiae Circumstantiae, 40, 224-233. |
| [林耀奔, 叶艳妹, 吴次芳, 胡一鸣, 施昊坤 (2020). 水田土壤细菌群落对不同重金属污染水平的响应分析——以A县为例. 环境科学学报, 40, 224-233.] | |
| [26] |
Mathur S, Kalaji HM, Jajoo A (2016). Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica, 54, 185-192.
DOI URL |
| [27] |
Mohanty M, Patra HK (2011). Attenuation of chromium toxicity by bioremediation technology. Reviews of Environmental Contamination and Toxicology, 210, 1-34.
DOI PMID |
| [28] |
Montes-Holguin MO, Peralta-Videa JR, Meitzner G, Martinez- Martinez A, de la Rosa G, Castillo-Michel HA, Gardea-Torresdey JL 2006). Biochemical and spectroscopic studies of the response of Convolvulus arvensis L. to chromium(III) and chromium(VI) stress. Environmental Toxicology and Chemistry, 25, 220-226.
PMID |
| [29] |
Morales-Barrera L, Cristiani-Urbina E (2008). Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water, Air, and Soil Pollution, 187, 327-336.
DOI URL |
| [30] |
Oladipo OG, Awotoye OO, Olayinka A, Bezuidenhout CC, Maboeta MS (2018). Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Brazilian Journal of Microbiology, 49, 29-37.
DOI PMID |
| [31] |
Ong GH, Ho XH, Shamkeeva S, Manasha Savithri Fernando AS, Wong LS (2017). Biosorption study of potential fungi for copper remediation from Peninsular Malaysia. Remediation Journal, 27, 59-63.
DOI URL |
| [32] |
Orwin KH, Wardle DA (2004). New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biology & Biochemistry, 36, 1907-1912.
DOI URL |
| [33] |
Pasqualetti M, Mulas B, Canzonetti G, Benedetti A, Tempesta S (2012). Effects of long-term heavy metal contamination on soil fungi in the Mediterranean area. Cryptogamie, Mycologie, 33, 43-57.
DOI URL |
| [34] |
Sanderson P, Naidu R, Bolan N, Bowman M, McLure S (2012). Effect of soil type on distribution and bioaccessibility of metal contaminants in shooting range soils. Science of the Total Environment, 438, 452-462.
DOI URL |
| [35] |
Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI (2017). Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere, 178, 513-533.
DOI PMID |
| [36] |
Sharma DC, Sharma CP, Tripathi RD (2003). Phytotoxic lesions of chromium in maize. Chemosphere, 51, 63-68.
PMID |
| [37] | Sharma S, Adholeya A (2011). Detoxification and accumulation of chromium from tannery effluent and spent chrome effluent by Paecilomyces lilacinus fungi. International Biodeterioration & Biodegradation, 65, 309-317. |
| [38] |
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013). Chromium toxicity and tolerance in plants. Environmental Chemistry Letters, 11, 229-254.
DOI URL |
| [39] |
Srivastava D, Tiwari M, Dutta P, Singh P, Chawda K, Kumari M, Chakrabarty D (2021). Chromium stress in plants: toxicity, tolerance and phytoremediation. Sustainability, 13, 4629.
DOI URL |
| [40] | Su SH, Zhang YX, Zhu LF, Li XL, Gao WB (2011). Analysis of bibliography on heavy-metal tolerant bacteria research in China in recent ten years. Journal of Library and Information Sciences in Agriculture, 23(5), 63-67. |
| [苏少华, 张玉秀, 朱凌峰, 李祥雷, 高武斌 (2011). 近十年我国耐重金属细菌研究文献分析. 农业图书情报学刊, 23(5), 63-67.] | |
| [41] |
Subrahmanyam D (2008). Effects of chromium toxicity on leaf photosynthetic characteristics and oxidative changes in wheat (Triticum aestivum L.). Photosynthetica, 46, 339. DOI: 10.1007/s11099-008-0062-4.
DOI |
| [42] |
Vajpayee P, Rai UN, Ali MB, Tripathi RD, Yadav V, Sinha S, Singh SN (2001). Chromium-induced physiologic changes in Vallisneria spiralis L. Bulletin of Environmental Contamination and Toxicology, 67, 246-256.
PMID |
| [43] |
Volpicella M, Leoni C, Fanizza I, Distaso M, Leoni G, Farioli L, Naumann T, Pastorello E, Ceci LR (2017). Characterization of maize chitinase-A, a tough allergenic molecule. Allergy, 72, 1423-1429.
DOI PMID |
| [44] | Wang YY, Xia YQ, Ge GF (2021). Effect of lead stress on microbial flora and functional diversity in yellow- cinnamon soil. Chinese Journal of Soil Science, 52, 1114-1120. |
| [王彦雨, 夏远巧, 葛高飞 (2021). 铅胁迫对黄褐土微生物区系和功能多样性的影响. 土壤通报, 52, 1114-1120.] | |
| [45] | Wu WC, Dong CX, Wu JH, Liu XW, Wu YX, Chen XB, Yu SX (2017). Ecological effects of soil properties and metal concentrations on the composition and diversity of microbial communities associated with land use patterns in an electronic waste recycling region. Science of the Total Environment, 601- 602, 57-65. |
| [46] |
Zaccheo P, Genevini PL, Cocucci SM (1982). Chromium ions toxicity on the membrane transport mechanism in segments of maize seedling roots. Journal of Plant Nutrition, 5, 1217-1227.
DOI URL |
| [47] | Zhang C, Nie S, Liang J, Zeng GM, Wu HP, Hua SS, Liu JY, Yuan YJ, Xiao HB, Deng LJ, Xiang HY (2016). Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Science of the Total Environment, 557- 558, 785-790. |
| [48] | Zhao PY, Yan PM, Zhao XD, Bai X (2021). Reconstruction and functional recovery of soil microbial community after fumigation of metam-sodium. Plant Protection, 47, 44-53. |
| [赵鹏宇, 燕平梅, 赵晓东, 白雪 (2021). 威百亩熏蒸后土壤微生物群落重建及功能恢复. 植物保护, 47, 44-53.] | |
| [49] |
Zhou Y, Duan J, Fujibe T, Yamamoto KT, Tian CG (2012). AtIQM1, a novel calmodulin-binding protein, is involved in stomatal movement in Arabidopsis. Plant Molecular Biology, 79, 333-346.
DOI URL |
| [1] | 刘瑶 钟全林 徐朝斌 程栋梁 郑跃芳 邹宇星 张雪 郑新杰 周云若. 不同大小刨花楠细根功能性状与根际微环境关系[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 张仲富, 王四海, 杨卫, 陈剑. 蒜头果根际细菌群落结构与功能特征对其健康状态的响应[J]. 植物生态学报, 2023, 47(7): 1020-1031. |
| [3] | 林春惠, 顾惠怡, 叶钦良, 张志坚, 钟智明, 易绮斐. 珍稀濒危植物大苞山茶种群结构与动态特征[J]. 植物生态学报, 2023, 47(12): 1684-1692. |
| [4] | 冯继广, 张秋芳, 袁霞, 朱彪. 氮磷添加对土壤有机碳的影响: 进展与展望[J]. 植物生态学报, 2022, 46(8): 855-870. |
| [5] | 聂秀青, 王冬, 周国英, 熊丰, 杜岩功. 三江源地区高寒湿地土壤微生物生物量碳氮磷及其化学计量特征[J]. 植物生态学报, 2021, 45(9): 996-1005. |
| [6] | 裴广廷, 孙建飞, 贺同鑫, 胡宝清. 长期人为干扰对桂西北喀斯特草地土壤微生物多样性及群落结构的影响[J]. 植物生态学报, 2021, 45(1): 74-84. |
| [7] | 罗林, 黄艳, 梁进, 汪恩涛, 胡君, 贺合亮, 赵春章. 西南亚高山针叶林主要树种互作及增温对根区土壤微生物群落的影响[J]. 植物生态学报, 2020, 44(8): 875-884. |
| [8] | 蔚亮, 李均力, 包安明, 白洁, 黄粤, 刘铁, 沈占锋. 塔里木河下游湿地面积时序变化及对生态输水的响应[J]. 植物生态学报, 2020, 44(6): 616-627. |
| [9] | 肖文宏, 周青松, 朱朝东, 吴东辉, 肖治术. 野生动物监测技术和方法应用进展与展望[J]. 植物生态学报, 2020, 44(4): 409-417. |
| [10] | 高贵锋, 褚海燕. 微生物组学的技术和方法及其应用[J]. 植物生态学报, 2020, 44(4): 395-408. |
| [11] | 蒋玉玲, 陈旭辉, 苗青, 曲波. 辽宁省9种兰科植物根内与根际土壤中真菌群落结构的差异[J]. 植物生态学报, 2019, 43(12): 1079-1090. |
| [12] | 石国玺, 王文颖, 蒋胜竞, 成岗, 姚步青, 冯虎元, 周华坤. 黄帚橐吾种群扩张对土壤理化特性与微生物功能多样性的影响[J]. 植物生态学报, 2018, 42(1): 126-132. |
| [13] | 王军, 王冠钦, 李飞, 彭云峰, 杨贵彪, 郁建春, 周国英, 杨元合. 短期增温对紫花针茅草原土壤微生物群落的影响[J]. 植物生态学报, 2018, 42(1): 116-125. |
| [14] | 陈雅涵, 谢宗强. 样品保存条件对土壤与植物全碳全氮含量的影响[J]. 植物生态学报, 2017, 41(6): 632-638. |
| [15] | 张江红, 彭福田, 蒋晓梅, 李民吉, 王中堂. 桃树枝条还田对土壤自毒物质,微生物及植株生长的影响[J]. 植物生态学报, 2016, 40(2): 140-150. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19