

植物生态学报 ›› 2010, Vol. 34 ›› Issue (4): 462-468.DOI: 10.3773/j.issn.1005-264x.2010.04.012
左照江1,3, 张汝民2, 王勇1, 侯平2, 温国胜2, 高岩2,3,*( )
)
收稿日期:2009-09-16
接受日期:2009-11-24
出版日期:2010-09-16
发布日期:2010-04-01
通讯作者:
高岩
作者简介:* E-mail: gaoyan1960@sohu.com
ZUO Zhao-Jiang1,3, ZHANG Ru-Min2, WANG Yong1, HOU Ping2, WEN Guo-Sheng2, GAO Yan2,3,*( )
)
Received:2009-09-16
Accepted:2009-11-24
Online:2010-09-16
Published:2010-04-01
Contact:
GAO Yan
摘要:
冷蒿(Artemisia frigida)挥发性有机化合物(volatile organic compounds, VOCs)具有特殊气味, 在植物受损伤时, 此气味会更加浓烈。该文通过对未损伤与损伤冷蒿VOCs成分分析、地上部分结构观察, 初步揭示了冷蒿VOCs释放与结构之间的关系。结果表明, 未损伤冷蒿VOCs主要含有22种化合物, 其主要成分是莰烯(14.27%)、(E)-乙酸-3-己烯酯(10.85%)、对-伞花烃(9.05%)、桉树脑(39.80%)、α-萜品醇(10.04%)、β-萜品醇(2.48%)、樟脑(5.66%)和(R)-(-)-对薄荷-1-烯-4-醇(3.84%)。损伤较未损伤冷蒿VOCs增加了12种物质, 其中相对含量大于1%的化合物分别为顺-3-己烯醛(1.15%)、2-己烯醛(1.34%)、顺-牻牛儿醇(2.66%)、冰片(4.47%)、(1R,4R)(+)-对-薄荷-2,8-二烯(9.15%)、乙酸冰片酯(1.37%)和4(14), 11-桉叶双烯(1.30%)。冷蒿叶片中栅栏组织发达, 叶柄内具有2-3处栅栏组织, 并且栅栏组织中都具有发达的气室, 同时气室与气孔相连。因此, 损伤较未损伤冷蒿VOCs种类和浓度增多的原因可能为: 冷蒿VOCs合成后大量储存于气室中, 当叶片损伤时, VOCs大量释放出来, 同时合成释放一些新的VOCs, 致使损伤冷蒿VOCs种类和浓度增加。
左照江, 张汝民, 王勇, 侯平, 温国胜, 高岩. 冷蒿挥发性有机化合物主要成分分析及其地上部分结构研究. 植物生态学报, 2010, 34(4): 462-468. DOI: 10.3773/j.issn.1005-264x.2010.04.012
ZUO Zhao-Jiang, ZHANG Ru-Min, WANG Yong, HOU Ping, WEN Guo-Sheng, GAO Yan. Analysis of main volatile organic compounds and study of aboveground structures in Artemisia frigida. Chinese Journal of Plant Ecology, 2010, 34(4): 462-468. DOI: 10.3773/j.issn.1005-264x.2010.04.012
| 保留时间 Retention time (min) | 挥发性有机化合物 Volatile organic compounds | 分子式 Chemical formula | 相对含量 Relative content (%) | |||
|---|---|---|---|---|---|---|
| 未损伤 UDa | 损伤Da | 中文名称 Chinese name | 英文名称 English name | 未损伤 UDa | 损伤 Da | |
| 13.51 | 顺-3-己烯醛 | cis-3-Hexenal | C6H10O | - | 1.15 ± 0.13 | |
| 15.57 | 2-己烯醛 | 2-Hentenal | C6H10O | - | 1.34 ± 0.18 | |
| 15.57 | 15.82 | 3-己烯醇 | 3-Hexenol(c,t) | C6H12O | 0.05 ± 0.01 | 2.81 ± 0.23 |
| 16.14 | 己醇 | 1-Hexanol | C6H14O | - | 0.50 ± 0.04 | |
| 16.23 | 乙酸异戊酯 | Isopentyl alcohol,acetate | C7H14O2 | - | 0.17 ± 0.05 | |
| 18.01 | 18.02 | α-蒎烯 | α-Pinene | C10H16 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| 18.38 | 18.40 | β-蒎烯 | β-Pinene | C10H16 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| 19.05 | 19.06 | 檀香三烯 | Santolina triene | C10H16 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 19.71 | 19.71 | 水芹烯 | Phellandrene | C10H16 | 0.15 ± 0.02 | 0.39 ± 0.09 |
| 20.07 | 20.09 | 莰烯 | Camphene | C10H16 | 14.27 ± 1.29 | 6.28 ± 0.58 |
| 20.29 | 1-壬烯-3-醇 | 1-Nonen-3-ol | C9H18O | - | 0.58 ± 0.07 | |
| 20.56 | 20.74 | (E)-乙酸-3-己烯酯 | (E)-3-Hexen-1-ol,acetate | C8H14O2 | 10.85 ± 0.83 | 10.96 ± 1.37 |
| 21.54 | 21.79 | 对-伞花烃 | p-Cymene | C10H14 | 9.05 ± 0.79 | 4.11 ± 0.08 |
| 21.95 | 22.02 | 桉树脑 | Eucalyptol | C10H18O | 39.80 ± 2.24 | 22.43 ± 2.49 |
| 22.20 | 2,4-癸二烯-1-醇 | 2,4-Decadien-1-ol | C10H18O | 0.15 ± 0.03 | - | |
| 22.47 | 22.55 | 萜品烯 | Terpinen | C10H16 | 0.93 ± 0.11 | 1.12 ± 0.27 |
| 23.08 | 23.27 | α-萜品醇 | α-Terpineol | C10H18O | 10.04 ± 0.98 | 11.01 ± 0.99 |
| 23.75 | 里纳醇 | Linalol | C10H18O | - | 0.13 ± 0.07 | |
| 23.90 | 23.94 | 黄瓜醇 | Cucumber alcohol | C9H16O | 0.07 ± 0.01 | 0.23 ± 0.04 |
| 24.07 | 24.22 | β-萜品醇 | β-Terpineol | C10H18O | 2.48 ± 0.45 | 3.56 ± 0.26 |
| 25.67 | 25.76 | 樟脑 | Camphor | C10H18O | 5.66 ± 0.97 | 7.85 ± 0.84 |
| 25.87 | 顺-牻牛儿醇 | cis-Geraniol | C10H18O | - | 2.66 ± 0.47 | |
| 26.45 | (R)-(-)-对-薄荷-1-烯-4-醇 | (R)-(-)-p-Menth-1-en-4-ol | C10H18O | 3.84 ± 0.74 | - | |
| 26.58 | 冰片 | Borneol | C10H18O | - | 4.47 ± 0.33 | |
| 26.82 | 26.94 | (S)-(-)-对-薄荷-1-烯-8-醇 | (S)-(-)-p-menth-1-en-8-ol | C10H18O | 0.88 ± 0.12 | 1.80 ± 0.18 |
| 28.64 | 乙酸橙花酯 | Nerol acetate | C12H20O2 | 1.00 ± 0.21 | - | |
| 28.81 | (1R,4R)-(+)-对-薄荷2,8-二烯 | (1R,4R)-(+)-p-Mentha-2,8-diene | C12H20 | - | 9.15 ± 0.86 | |
| 29.27 | 乙酸冰片酯 | Bornyl acetate | C12H20O2 | - | 1.37 ± 0.29 | |
| 31.71 | 31.75 | 古巴烯 | Copaene | C15H24 | 0.25 ± 0.04 | 1.50 ± 0.15 |
| 32.98 | 33.02 | (Z)-法呢烯 | (Z)-Farnesene | C15H24 | 0.02 ± 0.00 | 0.13 ± 0.02 |
| 34.32 | 柏木烯 | Cedrene | C15H24 | - | 0.33 ± 0.03 | |
| 34.42 | 34.50 | 大根叶香烯D | Germacrene D | C15H24 | 0.12 ± 0.01 | 2.48 ± 0.14 |
| 34.70 | 异石竹烯 | Isocaryophillene | C15H24 | 0.21 ± 0.01 | - | |
| 34.76 | 4(14), 11-桉叶双烯 | Eudesma-4(14),11-diene | C15H24 | - | 1.30 ± 0.13 | |
表1 冷蒿挥发性有机化合物(VOCs)主要成分
Table 1 The main components of the volatile organic compounds (VOCs) from Artemisia frigida
| 保留时间 Retention time (min) | 挥发性有机化合物 Volatile organic compounds | 分子式 Chemical formula | 相对含量 Relative content (%) | |||
|---|---|---|---|---|---|---|
| 未损伤 UDa | 损伤Da | 中文名称 Chinese name | 英文名称 English name | 未损伤 UDa | 损伤 Da | |
| 13.51 | 顺-3-己烯醛 | cis-3-Hexenal | C6H10O | - | 1.15 ± 0.13 | |
| 15.57 | 2-己烯醛 | 2-Hentenal | C6H10O | - | 1.34 ± 0.18 | |
| 15.57 | 15.82 | 3-己烯醇 | 3-Hexenol(c,t) | C6H12O | 0.05 ± 0.01 | 2.81 ± 0.23 |
| 16.14 | 己醇 | 1-Hexanol | C6H14O | - | 0.50 ± 0.04 | |
| 16.23 | 乙酸异戊酯 | Isopentyl alcohol,acetate | C7H14O2 | - | 0.17 ± 0.05 | |
| 18.01 | 18.02 | α-蒎烯 | α-Pinene | C10H16 | 0.03 ± 0.01 | 0.04 ± 0.00 |
| 18.38 | 18.40 | β-蒎烯 | β-Pinene | C10H16 | 0.06 ± 0.01 | 0.05 ± 0.01 |
| 19.05 | 19.06 | 檀香三烯 | Santolina triene | C10H16 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 19.71 | 19.71 | 水芹烯 | Phellandrene | C10H16 | 0.15 ± 0.02 | 0.39 ± 0.09 |
| 20.07 | 20.09 | 莰烯 | Camphene | C10H16 | 14.27 ± 1.29 | 6.28 ± 0.58 |
| 20.29 | 1-壬烯-3-醇 | 1-Nonen-3-ol | C9H18O | - | 0.58 ± 0.07 | |
| 20.56 | 20.74 | (E)-乙酸-3-己烯酯 | (E)-3-Hexen-1-ol,acetate | C8H14O2 | 10.85 ± 0.83 | 10.96 ± 1.37 |
| 21.54 | 21.79 | 对-伞花烃 | p-Cymene | C10H14 | 9.05 ± 0.79 | 4.11 ± 0.08 |
| 21.95 | 22.02 | 桉树脑 | Eucalyptol | C10H18O | 39.80 ± 2.24 | 22.43 ± 2.49 |
| 22.20 | 2,4-癸二烯-1-醇 | 2,4-Decadien-1-ol | C10H18O | 0.15 ± 0.03 | - | |
| 22.47 | 22.55 | 萜品烯 | Terpinen | C10H16 | 0.93 ± 0.11 | 1.12 ± 0.27 |
| 23.08 | 23.27 | α-萜品醇 | α-Terpineol | C10H18O | 10.04 ± 0.98 | 11.01 ± 0.99 |
| 23.75 | 里纳醇 | Linalol | C10H18O | - | 0.13 ± 0.07 | |
| 23.90 | 23.94 | 黄瓜醇 | Cucumber alcohol | C9H16O | 0.07 ± 0.01 | 0.23 ± 0.04 |
| 24.07 | 24.22 | β-萜品醇 | β-Terpineol | C10H18O | 2.48 ± 0.45 | 3.56 ± 0.26 |
| 25.67 | 25.76 | 樟脑 | Camphor | C10H18O | 5.66 ± 0.97 | 7.85 ± 0.84 |
| 25.87 | 顺-牻牛儿醇 | cis-Geraniol | C10H18O | - | 2.66 ± 0.47 | |
| 26.45 | (R)-(-)-对-薄荷-1-烯-4-醇 | (R)-(-)-p-Menth-1-en-4-ol | C10H18O | 3.84 ± 0.74 | - | |
| 26.58 | 冰片 | Borneol | C10H18O | - | 4.47 ± 0.33 | |
| 26.82 | 26.94 | (S)-(-)-对-薄荷-1-烯-8-醇 | (S)-(-)-p-menth-1-en-8-ol | C10H18O | 0.88 ± 0.12 | 1.80 ± 0.18 |
| 28.64 | 乙酸橙花酯 | Nerol acetate | C12H20O2 | 1.00 ± 0.21 | - | |
| 28.81 | (1R,4R)-(+)-对-薄荷2,8-二烯 | (1R,4R)-(+)-p-Mentha-2,8-diene | C12H20 | - | 9.15 ± 0.86 | |
| 29.27 | 乙酸冰片酯 | Bornyl acetate | C12H20O2 | - | 1.37 ± 0.29 | |
| 31.71 | 31.75 | 古巴烯 | Copaene | C15H24 | 0.25 ± 0.04 | 1.50 ± 0.15 |
| 32.98 | 33.02 | (Z)-法呢烯 | (Z)-Farnesene | C15H24 | 0.02 ± 0.00 | 0.13 ± 0.02 |
| 34.32 | 柏木烯 | Cedrene | C15H24 | - | 0.33 ± 0.03 | |
| 34.42 | 34.50 | 大根叶香烯D | Germacrene D | C15H24 | 0.12 ± 0.01 | 2.48 ± 0.14 |
| 34.70 | 异石竹烯 | Isocaryophillene | C15H24 | 0.21 ± 0.01 | - | |
| 34.76 | 4(14), 11-桉叶双烯 | Eudesma-4(14),11-diene | C15H24 | - | 1.30 ± 0.13 | |
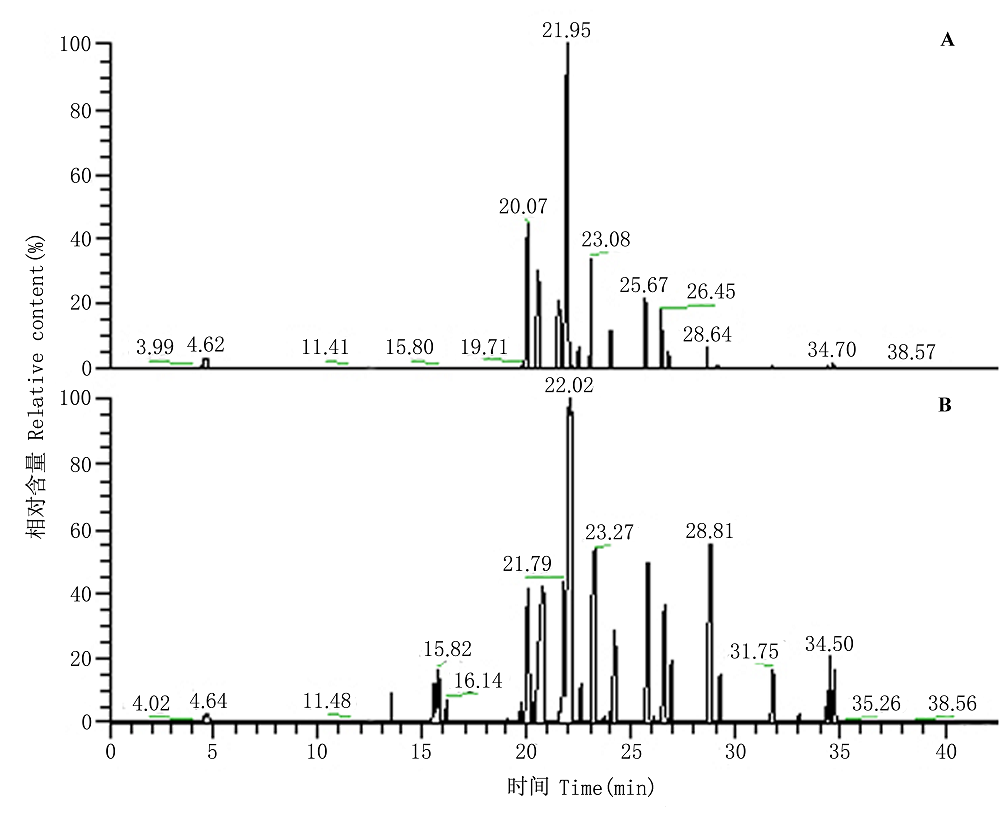
图1 未损伤冷蒿(A)和损伤冷蒿(B)挥发性有机化合物(VOCs)经热脱附/气相色谱/质谱联用仪(TCT/GC/MS)分析的总离子流图(TIC)。
Fig. 1 The total ion current (TIC) of volatile organic compounds (VOCs) from undamaged (A) to damaged (B) Artemisia frigida was analysed by thermal-desorption cold trap/gas chromatography/mass spectrum (TCT/GC/MS).
| [1] | Baldlacchi D, Guenther A, Harley P, Klinger L, Zimmerman P, Lamb B, Westberg H (1995). The fluxes and air chemistry of isoprene above a deciduous hardwood forest. Philosophical Transactions of the Royal Society A, 350, 279-296. |
| [2] |
Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA (2006). Volatile signaling in plant-plant interactions: talking trees in the genomics era. Science, 311, 812-815.
DOI URL PMID |
| [3] |
Dixon RA (2001). Natural products and plant disease resistance. Nature, 411, 843-847.
URL PMID |
| [4] | Du JW (杜家纬) (2001). Plant-insect chemical communication and its behavior control. Acta Photophysiologica Sinica (植物生理学报), 27, 193-200. (in Chinese with English abstract) |
| [5] |
Dudareva N, Pichersky E (2000). Biochemistry and molecular aspects of floral scent. Plant Physiology, 122, 627-634.
URL PMID |
| [6] |
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004). Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781-1785.
DOI URL PMID |
| [7] |
Fall R, Karl T, Hansel A, Jordan A, Lindinger W (1999). Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. Journal of Geophysical Research, 104, 15963-15974.
DOI URL |
| [8] |
Gao Y, Jin YJ, Li HD, Chen HJ (2005). Volatile organic compounds and their roles in bacteriostasis in five conifer species. Journal of Integrative Plant Biology, 47, 499-507.
DOI URL |
| [9] |
Guenther AB, Monson RK, Fall R (1991). Isoprene and monoterpene emission rate variability: observations with Eucalyptus and emission rate algorithm development. Journal of Geophysical Research, 96, 10799-10808.
DOI URL |
| [10] |
Heil M (2007). Indirect defence via tritrophic interactions. New Phytologist, 178, 41-61.
DOI URL |
| [11] | Heil M, Bueno JCS (2007). Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proceedings of the National Academy of Sciences of the United States of America, 140, 5467-5472. |
| [12] |
Lerdau M, Dilts SB, Westberg H, Lamb BK, Aiiwine EJ (1994). Monoterpene emission from Ponderosa pine. Journal of Geophysical Research, 99, 16609-16615.
DOI URL |
| [13] | Li XG (李新岗), Liu HX (刘惠霞), Liu LP (刘拉平), Ma YM (马养民) (2006). Study on host-plant volatiles affecting the host selection of Dioryctria pryeri. Scientia Silvae Sinicae (林业科学), 42, 71-78. (in Chinese with English abstract) |
| [14] |
Loreto F, Pinelli P, Manes F, Kollist H (2004). Impact of ozone on monoterpene emissions and evidence for an isoprene- like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiology, 24, 361-367.
DOI URL PMID |
| [15] | Ma RJ (马瑞君), Wang ML (王明理), Zhu XT (朱学泰), Lu XW (鲁先文), Sun K (孙坤) (2005). Allelopathy and chemical constituents of Ligularia virgaurea volatile. Chinese Journal of Applied Ecology (应用生态学报), 16, 1826-1829. (in Chinese with English abstract) |
| [16] |
Pàre PW, Tumlinson JH (1997). Denovo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiology, 114, 1161-1167.
DOI URL PMID |
| [17] |
Peñuelas J, Llusià J (2003). BVOCs: plant defense against climate warming. Trends in Plant Science, 8, 105-109.
URL PMID |
| [18] |
Rosenstiel TN, Ebbets AL, Khatri WC, Fall R, Monson RK (2004). Induction of poplar leaf nitrate reductase: a test of extrachloroplastic control of isoprene emission rate. Plant Biology, 6, 12-21.
DOI URL PMID |
| [19] |
Sharkey TD, Chen X, Yeh S (2001). Isoprene increase thermotolerance of fosmidomycin fed leaves. Plant Physiology, 125, 2001-2006.
URL PMID |
| [20] |
Singsaas L (2000). Terpenes and the thermotolerance of photosynthesis. New Phytologist, 146, 1-16.
DOI URL |
| [21] |
Steinberg S, Dicke M, Vet LEM (1993). Relative importance of info chemicals from first and second trophic level in long range host location by the larval parasitoid Cotesia (= Apanteles) glomerata. Journal of Chemical Ecology, 19, 47-59.
DOI URL PMID |
| [22] |
van den Boom CE, van Beek TA, Posthumus MA, de Groot A, Dicke M (2004). Qualitative and quantitative variation among volatile profiles induced by Tetranychus urticae feeding on plants from various families. Journal of Chemical Ecology, 30, 69-89.
DOI URL PMID |
| [23] |
Vuorinen T, Nerg AM, Ibrahim MA, Reddy GVP, Holopainen JK (2004). Emission of Plutella xylostella―induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiology, 135, 1984-1992.
DOI URL PMID |
| [24] | Yin J (尹姣), Cao YZ (曹雅忠), Luo LZ (罗礼智), Hu Y (胡毅) (2005). Oviposition preference of the meadow moth, Loxostege sticticalis L., on different host plants and its chemical mechanism. Acta Ecologica Sinica (生态学报), 25, 1844-1852. (in Chinese with English abstract) |
| [25] | Zuo ZJ (左照江), Zhang RM (张汝民), Gao Y (高岩) (2009a). Research advances in volatile signals among plants. Chinese Bulletin of Botany (植物学报), 44, 245-252. (in Chinese with English abstract) |
| [26] | Zuo ZJ (左照江), Zhang RM (张汝民), Zhu JH (朱金胡), Wen GS (温国胜), Hou P (侯平), Gao Y (高岩) (2009b). Effects of volatile organic compounds (VOCs) from Artemisia frigida on germination and growth of four plant types. Journal of Zhejiang Forestry College (浙江林学院学报), 26, 76-82. (in Chinese with English abstract) |
| [1] | 程思祺, 姜峰, 金光泽. 温带森林阔叶植物幼苗叶经济谱及其与防御性状的关系[J]. 植物生态学报, 2022, 46(6): 678-686. |
| [2] | 孙文泰, 马明. 黄土高原长期覆膜苹果园土壤物理退化与细根生长响应[J]. 植物生态学报, 2021, 45(9): 972-986. |
| [3] | 董琳琳, 普晓妍, 张璐璐, 宋亮, 鲁志云, 李苏. 亚热带森林附生地衣压力-体积曲线分析及其适用性[J]. 植物生态学报, 2021, 45(3): 274-285. |
| [4] | 董正武, 赵英, 雷加强, 喜银巧. 塔克拉玛干沙漠不同区域柽柳沙包土壤盐分分布特征及其影响因素[J]. 植物生态学报, 2018, 42(8): 873-884. |
| [5] | 汪俊宇, 王小东, 马元丹, 傅卢成, 周欢欢, 王彬, 张汝民, 高岩. ‘波叶金桂’对干旱和高温胁迫的生理生态响应[J]. 植物生态学报, 2018, 42(6): 681-691. |
| [6] | 刘盟盟, 贾丽, 程路芸, 张洪芹, 臧晓琳, 宝音陶格涛, 张汝民, 高岩. 冷蒿酚酸及其抗氧化防御酶活性对机械损伤的响应[J]. 植物生态学报, 2017, 41(2): 219-230. |
| [7] | 徐世琴, 吉喜斌, 金博文. 典型固沙植物梭梭生长季蒸腾变化及其对环境因子的响应[J]. 植物生态学报, 2015, 39(9): 890-900. |
| [8] | 刘娜娜,田秋英,张文浩. 内蒙古典型草原优势种冷蒿和克氏针茅对土壤低磷环境适应策略的比较[J]. 植物生态学报, 2014, 38(9): 905-915. |
| [9] | 刘芳,左照江,许改平,吴兴波,郑洁,高荣孚,张汝民,高岩. 迷迭香对干旱胁迫的生理响应及其诱导挥发性有机化合物的释放[J]. 植物生态学报, 2013, 37(5): 454-463. |
| [10] | 陈天翌, 刘增辉, 娄安如. 刺萼龙葵种群在中国不同分布地区的表型变异[J]. 植物生态学报, 2013, 37(4): 344-353. |
| [11] | 慈敦伟,戴良香,宋文武,张智猛. 花生萌发至苗期耐盐胁迫的基因型差异[J]. 植物生态学报, 2013, 37(11): 1018-1027. |
| [12] | 李贺, 张维康, 王国宏. 中国云杉林的地理分布与气候因子间的关系[J]. 植物生态学报, 2012, 36(5): 372-381. |
| [13] | 张苏芳, 张真, 王鸿斌, 孔祥波. 植物防御的新发现: 植物-植物相互交流[J]. 植物生态学报, 2012, 36(10): 1120-1124. |
| [14] | 魏丽萍, 王孝安, 王世雄, 朱志红, 郭华, 孙嘉男, 郝江勃. 黄土高原马栏林区基于不同植被组织尺度的群落物种多样性[J]. 植物生态学报, 2011, 35(1): 17-26. |
| [15] | 杨娟, 葛剑平, 刘丽娟, 丁易, 谭迎春. 卧龙自然保护区针阔混交林林隙更新规律[J]. 植物生态学报, 2007, 31(3): 425-430. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2026 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()