

植物生态学报 ›› 2024, Vol. 48 ›› Issue (11): 1524-1535.DOI: 10.17521/cjpe.2023.0386 cstr: 32100.14.cjpe.2023.0386
李民青1,2,3, 周孝明4, 王双龙5, 陈丽丹1,2,3, 李从娟6, 刘冉1,2,*( )
)
收稿日期:2023-12-22
接受日期:2024-05-06
出版日期:2024-11-20
发布日期:2024-07-03
通讯作者:
*刘冉(liuran@ms.xjb.ac.cn)
基金资助:
LI Min-Qing1,2,3, ZHOU Xiao-Ming4, WANG Shuang-Long5, CHEN Li-Dan1,2,3, LI Cong-Juan6, LIU Ran1,2,*( )
)
Received:2023-12-22
Accepted:2024-05-06
Online:2024-11-20
Published:2024-07-03
Contact:
*LIU Ran (liuran@ms.xjb.ac.cn)
Supported by:摘要:
枝干光合作用是维持植物碳平衡不可忽视的重要部分, 探究荒漠木本植物枝干光合在干旱胁迫下的响应机制有助于深入理解荒漠植物抵抗干旱的能力。该研究采用盆栽控制实验, 以两年生乔木状沙拐枣(Calligonum arborescens)幼苗为研究对象, 遮光组用铝箔对幼苗枝干进行遮光处理(无枝干光合), 对照组枝干维持正常光照(有枝干光合), 在干旱初始、干旱15天与30天后测定两组植物枝干和叶片光合特征、水力性状以及非结构性碳水化合物(NSC)含量等参数。结果表明: (1)乔木状沙拐枣的枝干光合平均速率为1.0-2.0 μmol·m-2·s-1, 且未受干旱时间延长的影响, 在干旱初始、干旱15天和干旱30天的枝干光合速率均值分别为1.42、1.28和1.21 μmol·m-2·s-1; 干旱30天后枝干释放CO2速率显著降低, 枝干光合减少枝干释放CO2比例显著提高; (2)随着干旱持续, 幼苗的边材比导率、叶片/枝干含水量、水势以及叶片光合速率均显著降低, 但是存在枝干光合的幼苗的下降趋势更为缓慢; (3)与遮光组相比, 干旱15天后枝干光合显著降低木质部导度损失率, 且显著增加叶片和枝干NSC含量; 干旱30天后枝干光合显著减少了木质部栓塞导管数量与横截面积比例, 分别减少33.8%和22.8%; (4)同时期对照组的幼苗叶片光合速率均显著高于遮光组, 在干旱15天和30天后分别高2.3和3.2 μmol·m-2·s-1。研究结果表明, 枝干光合能够提高乔木状沙拐枣栓塞抵抗力, 同时对NSC储存以及叶片气体交换均有显著影响。该文为深入理解荒漠木本植物干旱适应策略与生存机制提供理论基础。
李民青, 周孝明, 王双龙, 陈丽丹, 李从娟, 刘冉. 干旱胁迫下乔木状沙拐枣枝干光合作用对水力性状与叶片光合作用的影响. 植物生态学报, 2024, 48(11): 1524-1535. DOI: 10.17521/cjpe.2023.0386
LI Min-Qing, ZHOU Xiao-Ming, WANG Shuang-Long, CHEN Li-Dan, LI Cong-Juan, LIU Ran. Effects of stem photosynthesis on hydraulic traits and leaf photosynthesis in Calligonum arborescens under drought stress. Chinese Journal of Plant Ecology, 2024, 48(11): 1524-1535. DOI: 10.17521/cjpe.2023.0386
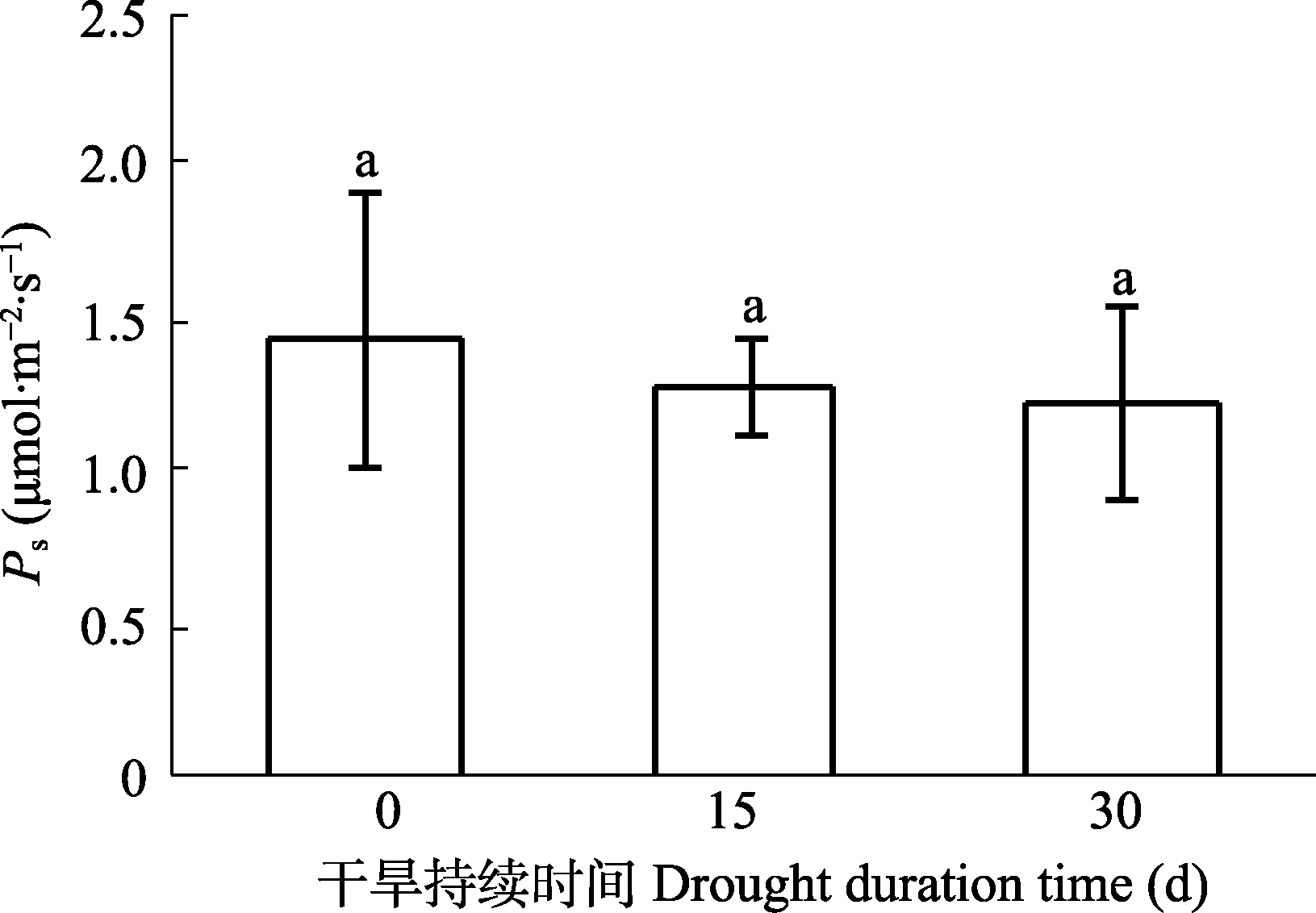
图1 乔木状沙拐枣枝干光合速率(Ps)随干旱持续时间的变化(平均值±标准差, n = 5)。相同小写字母之间表示处理间无显著差异(p > 0.05)。
Fig. 1 Stem photosynthetic rate (Ps) of Calligonum arborescens under drought duration (mean ± SD, n = 5). Same lowercase letters indicates no significant differences between treatments (p > 0.05).
| 干旱时间 Drought time (d) | Ps (μmol·m-2·s-1) | Rs (μmol·m-2·s-1) | Rd (%) |
|---|---|---|---|
| 0 | 1.45 ± 0.29a | 2.64 ± 0.33a | 55.6 ± 12.9a |
| 15 | 1.28 ± 0.17a | 2.09 ± 0.25a | 62.4 ± 14.1a |
| 30 | 1.21 ± 0.33a | 1.19 ± 0.29b | 100.9 ± 10.4b |
表1 干旱条件下枝干释放CO2速率和枝干光合对枝干释放CO2的固定比例(平均值±标准差, n = 5)
Table 1 Stem respiration rate and stem respiration rate refixation ratio under drought conditions (mean ± SD, n = 5)
| 干旱时间 Drought time (d) | Ps (μmol·m-2·s-1) | Rs (μmol·m-2·s-1) | Rd (%) |
|---|---|---|---|
| 0 | 1.45 ± 0.29a | 2.64 ± 0.33a | 55.6 ± 12.9a |
| 15 | 1.28 ± 0.17a | 2.09 ± 0.25a | 62.4 ± 14.1a |
| 30 | 1.21 ± 0.33a | 1.19 ± 0.29b | 100.9 ± 10.4b |
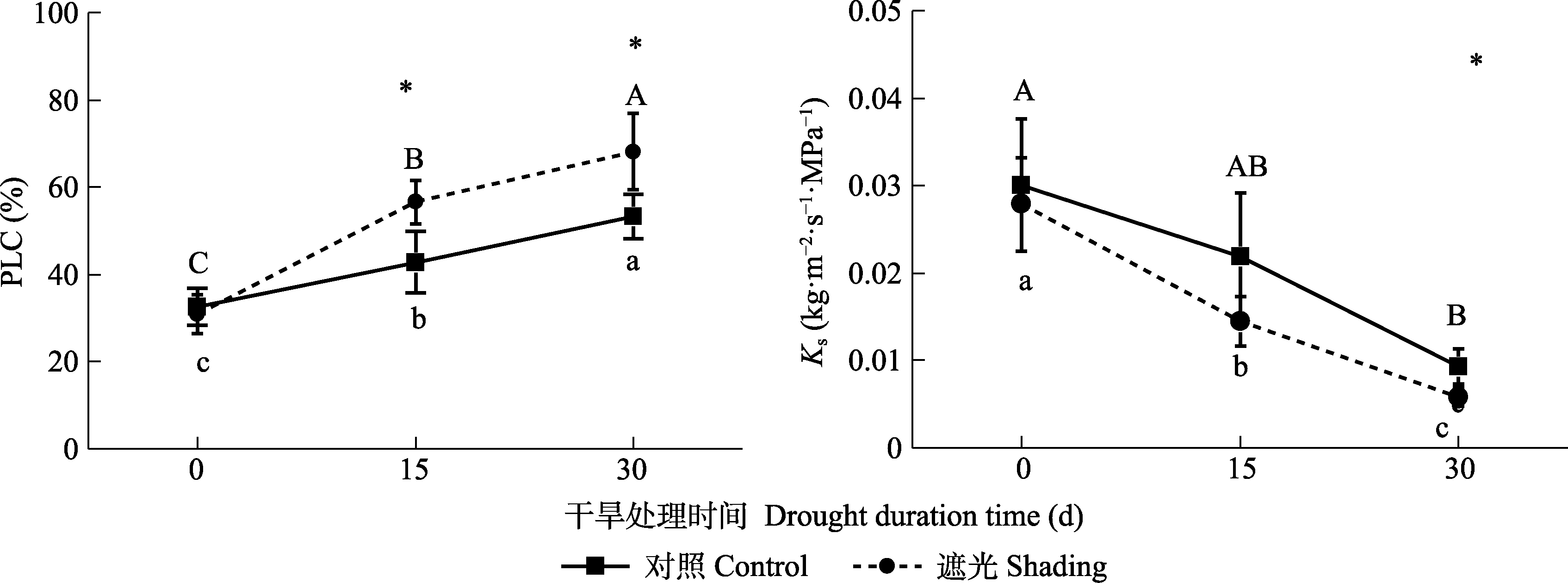
图2 干旱条件下导度损失率(PLC)和边材比导率变化(Ks) (平均值±标准差, n = 5)。不同大小写字母表示同一处理不同干旱时间存在显著差异。*表示在p < 0.05水平下相同干旱时间对照组和遮光组之间的差异显著。
Fig. 2 Percentage loss of conductivity (PLC) and specific hydraulic conductivity (Ks) under drought conditions (mean ± SD, n = 5). Different uppercase or lowercase letters represent the significant difference between the drought time of control or shading. * indicates the significant difference between the control and shading for the same drought time at the p < 0.05 level.
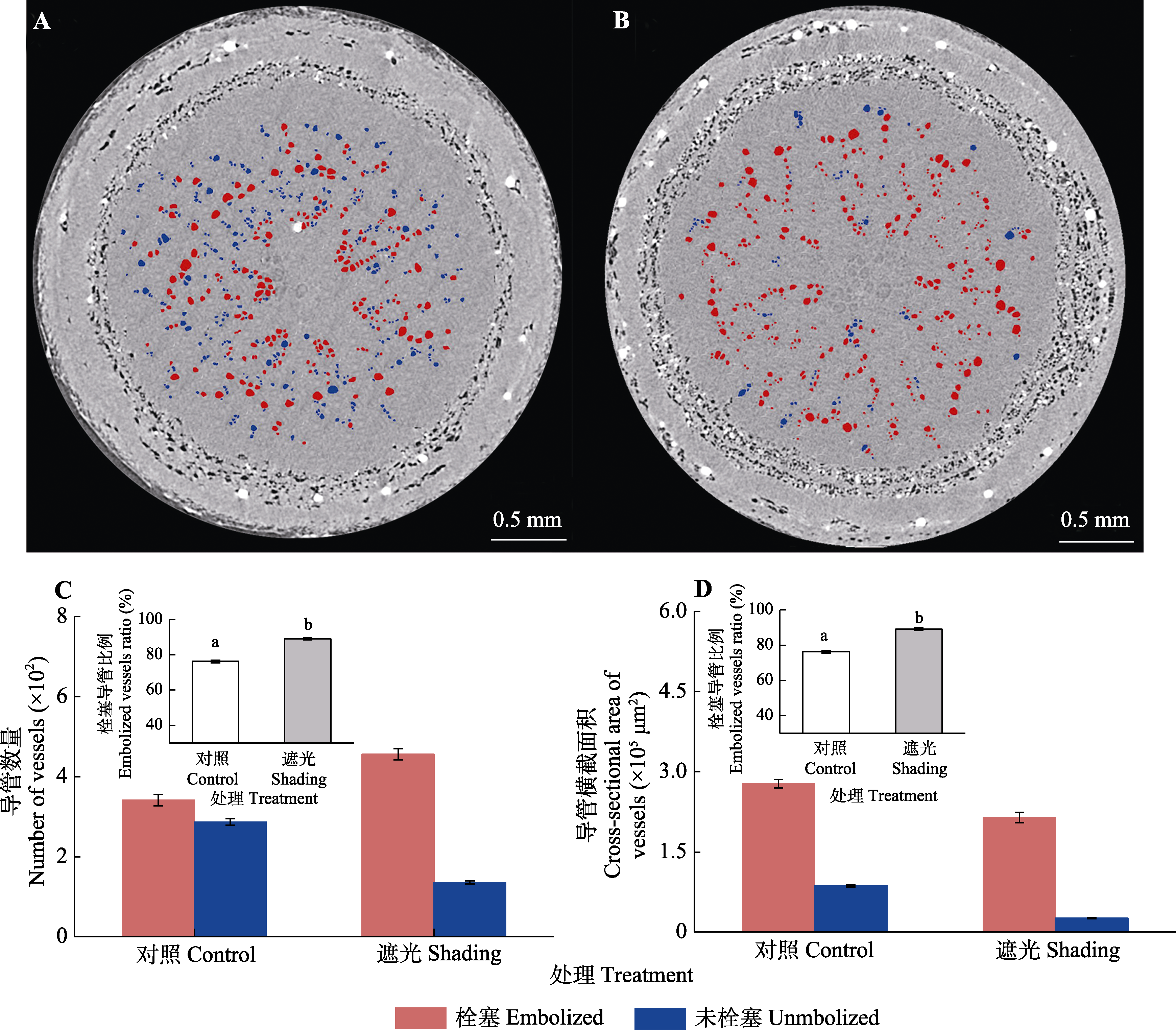
图3 Micro-CT扫描干旱30天后导管栓塞状态。A, 对照组幼茎横截面。B, 遮光组幼茎横截面。C, 导管数量统计及栓塞导管数量比例(平均值±标准差)。D, 导管横截面积统计及栓塞导管横截面积比例(平均值±标准差)。C和D中不同小写字母代表对照组和遮光组差异显著(p < 0.05)。
Fig. 3 Micro-CT scan of the vessels after 30 d of drought treatment. A, 2D cross-section of young stem treated of control. B, 2D cross-section of young stem treated of shading. C, Number of vessels statistics and embolized vessels number ratio (mean ± SD). D, Cross-sectional area statistics of vessels and embolized vessels cross-sectional area ratio (mean ± SD). Different lowercase letters represent the significant difference of control and shading in C and D (p < 0.05).
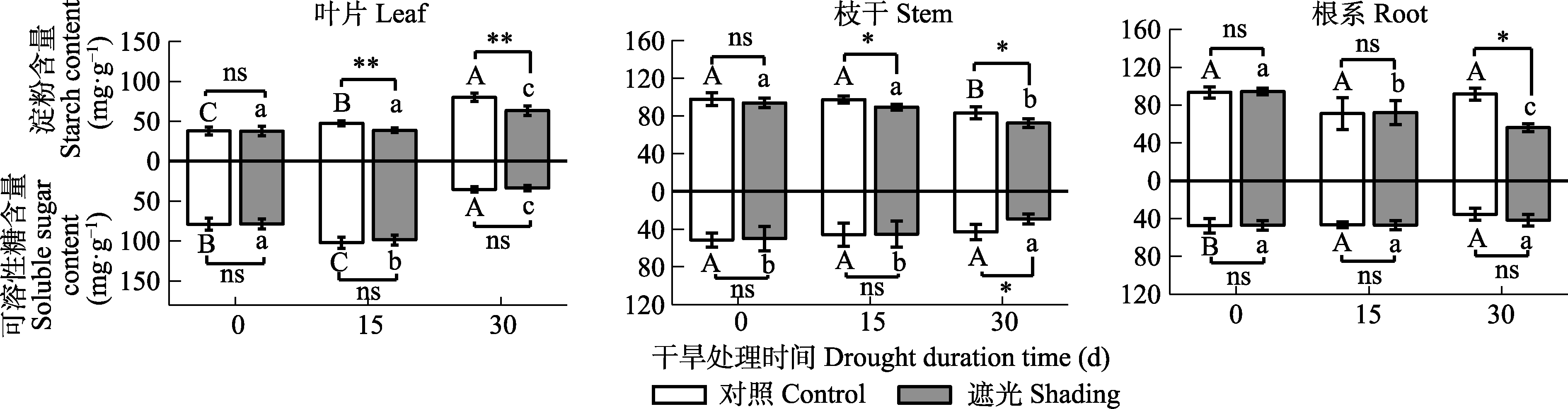
图4 干旱条件下根、枝干和叶淀粉含量和可溶性糖含量(平均值±标准差, n = 5)。不同大/小写字母表示同一处理不同干旱时间存在显著差异(p < 0.05)。*和**分别表示在p < 0.05和p < 0.01水平下相同干旱时间对照组和遮光组之间的差异显著。
Fig. 4 Starch and soluble sugar contents in root, stem and leaf under drought conditions (mean ± SD, n = 5). Different uppercase or lower case letters represent the significant difference between the drought time of control or shading. * and ** indicate the significant difference between the control and shading for the same drought time at the p < 0.05 and p < 0.01 level.
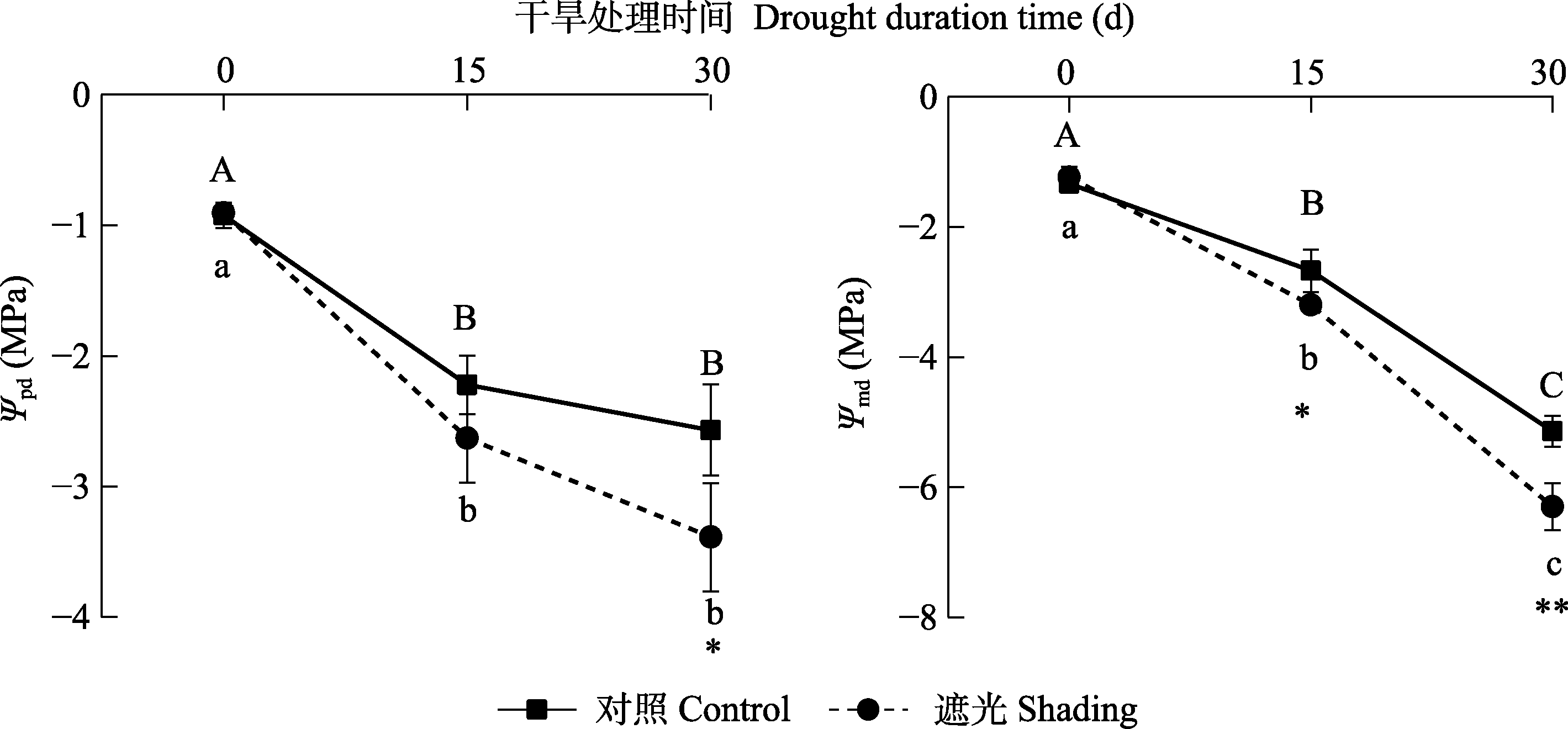
图5 干旱条件下叶黎明前水势(Ψpd)和正午水势变化(Ψmd) (平均值±标准差, n = 5)。不同大/小写字母表示同一处理不同干旱时间差异显著(p < 0.05)。*和**分别表示在p < 0.05和p < 0.01水平下相同干旱时间对照组和遮光组之间差异显著。
Fig. 5 Predawn water potential (Ψpd) and midday water potential (Ψmd) under drought conditions (mean ± SD, n = 5). Different uppercase or lowercase letters represent significant difference between the drought time of control or shading (p < 0.05). * and ** indicate the significant difference between the control and shading for the same drought time at the p < 0.05 and p < 0.01 level.
| 干旱时间 Drought time (d) | 对照 Treatment | AWCl | AWCs | RWCl | RWCs |
|---|---|---|---|---|---|
| 0 | 对照 Control | 3.14 ± 0.42a | 0.81 ± 0.06a | 0.93 ± 0.04a | 0.71 ± 0.01a |
| 遮光 Shading | 3.03 ± 0.41a | 0.80 ± 0.05a | 0.90 ± 0.05a | 0.72 ± 0.04a | |
| 15 | 对照 Control | 2.70 ± 0.34a | 0.81 ±0.04a | 0.78 ± 0.05a | 0.68 ± 0.07a |
| 遮光 Shading | 2.64 ± 0.24a | 0.72 ± 0.05b | 0.76 ± 0.05a | 0.61 ± 0.04a | |
| 30 | 对照 Control | 1.88 ± 0.27a | 0.61 ± 0.03a | 0.73 ± 0.05a | 0.63 ± 0.03a |
| 遮光 Shading | 1.22 ± 0.36b | 0.54 ± 0.03b | 0.55 ± 0.03b | 0.45 ± 0.07b |
表2 干旱条件下枝干和叶片绝对含水量和相对含水量(平均值±标准差)
Table 2 Absolute and relative water content of stem and leaf under drought conditions (mean ± SD)
| 干旱时间 Drought time (d) | 对照 Treatment | AWCl | AWCs | RWCl | RWCs |
|---|---|---|---|---|---|
| 0 | 对照 Control | 3.14 ± 0.42a | 0.81 ± 0.06a | 0.93 ± 0.04a | 0.71 ± 0.01a |
| 遮光 Shading | 3.03 ± 0.41a | 0.80 ± 0.05a | 0.90 ± 0.05a | 0.72 ± 0.04a | |
| 15 | 对照 Control | 2.70 ± 0.34a | 0.81 ±0.04a | 0.78 ± 0.05a | 0.68 ± 0.07a |
| 遮光 Shading | 2.64 ± 0.24a | 0.72 ± 0.05b | 0.76 ± 0.05a | 0.61 ± 0.04a | |
| 30 | 对照 Control | 1.88 ± 0.27a | 0.61 ± 0.03a | 0.73 ± 0.05a | 0.63 ± 0.03a |
| 遮光 Shading | 1.22 ± 0.36b | 0.54 ± 0.03b | 0.55 ± 0.03b | 0.45 ± 0.07b |
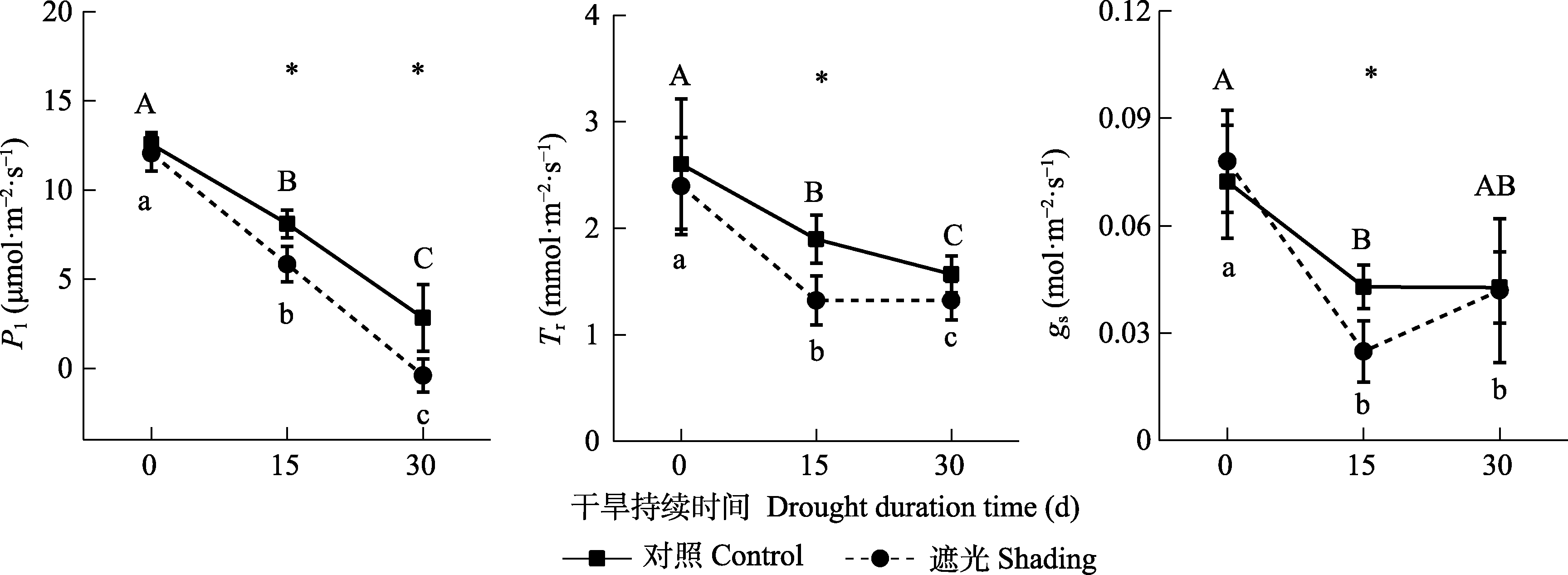
图6 干旱条件下的叶片光合速率(Pl)、蒸腾速率(Tr)和叶片气孔导度(gs)变化(平均值±标准差, n = 5)。ns, p > 0.05; *, p < 0.05; **, p < 0.01。不同大/小写字母表示同一处理不同干旱时间差异显著(p < 0.05)。*和**分别表示在p < 0.05和p < 0.01水平下相同干旱时间对照组和遮光组之间差异显著。
Fig. 6 Leaf photosynthetic rate (Pl), transpiration rate (Tr) and leaf stomatal conductance (gs) under drought conditions (mean ± SD, n = 5). Different uppercase or lowercase letters represent the significant difference between the drought time of control or shading (p < 0.05). * and ** indicate the significant difference between the control and shading for the same drought time at the p < 0.05 and p < 0.01 level.
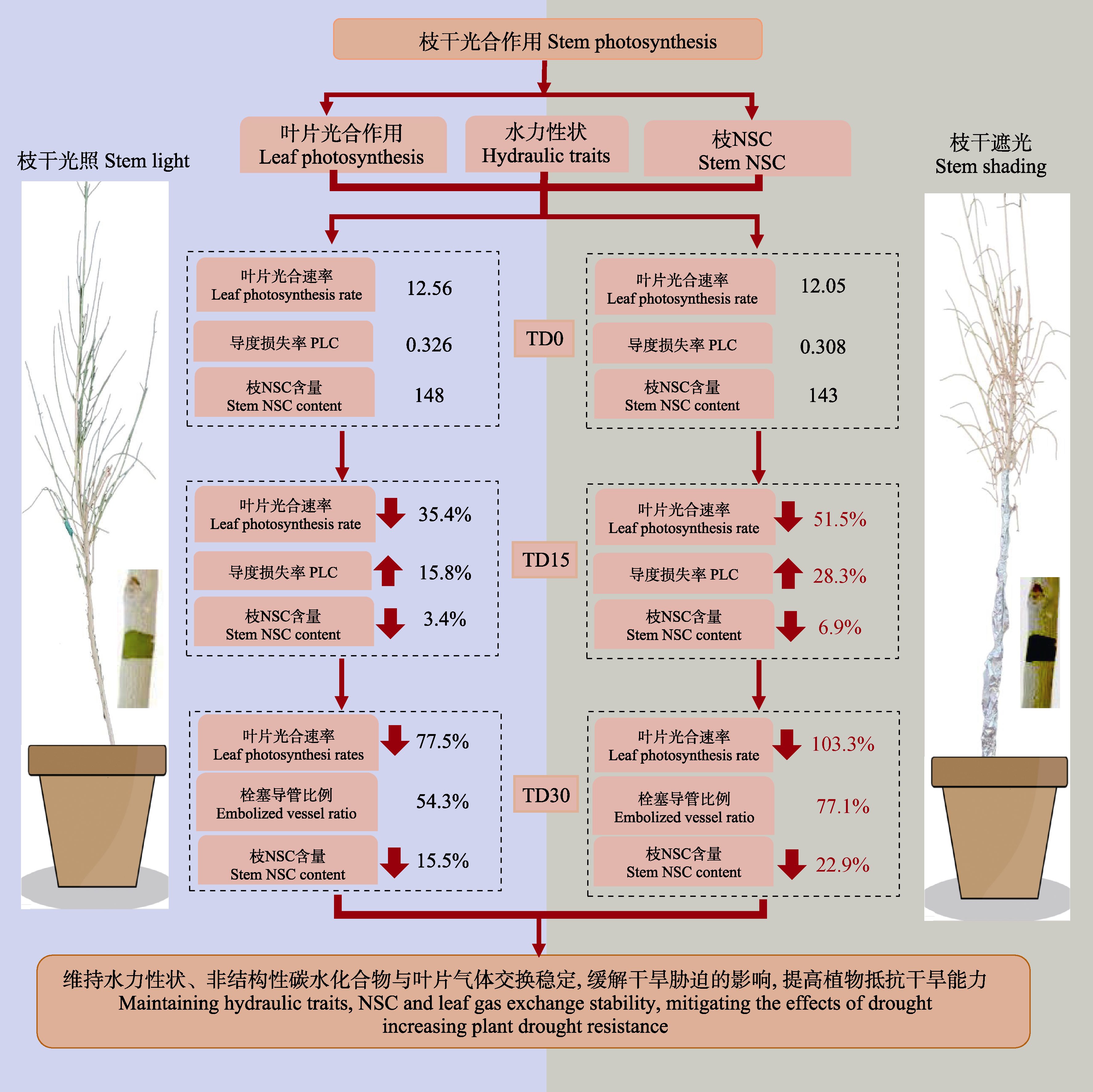
图7 乔木状沙拐枣枝干光合抵抗干旱的概念示意图。叶片光合速率单位μmol·m-2·s-1, 枝干非结构性碳水化合物(NSC)含量单位mg·g-1。TD0, 干旱初始; TD15, 干旱15天后; TD30, 干旱30天后。15天和30天下降比例均相对于干旱初始。PLC, 导度损失率; NSC, 非结构性碳水化合物。
Fig. 7 Conceptual diagram illustrating stem photosynthesis of Calligonum arborescens resistance drought stress. The unit of leaf photosynthesis is μmol·m-2·s-1, and the unit of non-structural carbohydrate content is mg·g-1. TD0, initial drought; TD15, after 15 d of drought; TD30, after 30 d of drought. The proportion of TD15 and TD30 decline is relative to TD0. PLC, percentage loss of conductivity; NSC, non-structural carbohydrates.
| 干旱天数 Drought time (d) | 处理 Treatment | NSCl | NSCs | NSCr |
|---|---|---|---|---|
| 0 | 对照组Control | 119.3 ± 9.6a | 90.8 ± 6.8a | 108.6 ± 8.9a |
| 遮光组Shading | 111.0 ± 5.9a | 87.0 ± 7.5a | 113.2 ± 15.2a | |
| 15 | 对照组Control | 94.3 ± 10.5a | 106.9 ± 7.0a | 103.1 ± 12.9a |
| 遮光组Shading | 76.5 ± 2.5b | 73.7 ± 4.0b | 107.6 ± 13.6a | |
| 30 | 对照组Control | 83.1 ± 6.0a | 117.3 ± 9.7a | 96.0 ± 5.5a |
| 遮光组Shading | 63.9 ± 6.1b | 88.6 ± 8.0b | 79.1 ± 7.1b |
附录III 干旱条件下乔木状沙拐枣叶片、枝干和根系非结构性碳水化合物(NSCl、NSCs、NSCr)含量(平均值±标准差, n = 5)。
Supplement III Non-structure carbohydrate content in leaf, stem and root (NSCl, NSCs, NSCr) under drought conditions of Calligonum arborescens (mean ± SD, n = 5).
| 干旱天数 Drought time (d) | 处理 Treatment | NSCl | NSCs | NSCr |
|---|---|---|---|---|
| 0 | 对照组Control | 119.3 ± 9.6a | 90.8 ± 6.8a | 108.6 ± 8.9a |
| 遮光组Shading | 111.0 ± 5.9a | 87.0 ± 7.5a | 113.2 ± 15.2a | |
| 15 | 对照组Control | 94.3 ± 10.5a | 106.9 ± 7.0a | 103.1 ± 12.9a |
| 遮光组Shading | 76.5 ± 2.5b | 73.7 ± 4.0b | 107.6 ± 13.6a | |
| 30 | 对照组Control | 83.1 ± 6.0a | 117.3 ± 9.7a | 96.0 ± 5.5a |
| 遮光组Shading | 63.9 ± 6.1b | 88.6 ± 8.0b | 79.1 ± 7.1b |
| [1] | Adams HD, Zeppel MJB, Anderegg WRL, Hartmann H, Landhäusser SM, Tissue DT, Huxman TE, Hudson PJ, Franz TE, Allen CD, Anderegg LDL, Barron-Gafford GA, Beerling DJ, Breshears DD, Brodribb TJ, et al. (2017). A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nature Ecology & Evolution, 1, 1285-1291. |
| [2] | Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, 259, 660-684. |
| [3] |
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012). The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences of the United States of America, 109, 233-237.
DOI PMID |
| [4] | Anderegg WRL, Konings AG, Trugman AT, Yu KL, Bowling DR, Gabbitas R, Karp DS, Pacala S, Sperry JS, Sulman BN, Zenes N (2018). Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature, 561, 538-541. |
| [5] | Ávila E, Herrera A, Tezara W (2014). Contribution of stem CO2 fixation to whole-plant carbon balance in nonsucculent species. Photosynthetica, 52, 3-15. |
| [6] |
Ávila-Lovera E, Zerpa AJ, Santiago LS (2017). Stem photosynthesis and hydraulics are coordinated in desert plant species. New Phytologist, 216, 1119-1129.
DOI PMID |
| [7] | Bloemen J, McGuire MA, Aubrey DP, Teskey RO, Steppe K (2013a). Transport of root-respired CO2 via the transpiration stream affects aboveground carbon assimilation and CO2 efflux in trees. New Phytologist, 197, 555-565. |
| [8] | Bloemen J, Overlaet-Michiels L, Steppe K (2013b). Understanding plant responses to drought: How important is woody tissue photosynthesis? Acta Horticulturae, 991, 149-155. |
| [9] | Cai XA, Zeng XP, Chen YQ (2015). Stem corticular photosynthesis: ecophysiological functions and their measurement. Acta Ecologica Sinica, 35, 6909-6922. |
| [蔡锡安, 曾小平, 陈远其 (2015). 树干皮层光合作用——生理生态功能和测定方法. 生态学报, 35, 6909-6922.] | |
| [10] |
Cernusak LA, Cheesman AW (2015). The benefits of recycling: How photosynthetic bark can increase drought tolerance. New Phytologist, 208, 995-997.
DOI PMID |
| [11] | Cernusak LA, Marshall JD (2000). Photosynthetic refixation in branches of western white pine. Functional Ecology, 14, 300-311. |
| [12] |
Chastain DR, Snider JL, Collins GD, Perry CD, Whitaker J, Byrd SA (2014). Water deficit in field-grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. Journal of Plant Physiology, 171, 1576-1585.
DOI PMID |
| [13] | Chen QC, Hu T, Li XH, Song CP, Zhu JK, Chen LQ, Zhao Y (2022). Phosphorylation of SWEET sucrose transporters regulates plant root:shoot ratio under drought. Nature Plants, 8, 68-77. |
| [14] | Chen X, Zhao P, Zhao X, Wang Q, Ouyang L, Larjavaara M, Zhu L, Ni G (2021). Involvement of stem corticular photosynthesis in hydraulic maintenance of Eucalyptus trees and its effect on leaf gas exchange. Environmental and Experimental Botany, 186, 104451. DOI: 10.1016/j.envexpbot.2021.104451. |
| [15] | Choat B, Brodribb TJ, Brodersen CR, Duursma RA, López R, Medlyn BE (2018). Triggers of tree mortality under drought. Nature, 558, 531-539. |
| [16] | Cramer MD, Hoffmann V, Verboom GA (2008). Nutrient availability moderates transpiration in Ehrharta calycina. New Phytologist, 179, 1048-1057. |
| [17] | Dai AG (2011). Drought under global warming: a review. WIREs Climate Change, 2, 45-65. |
| [18] |
de Baerdemaeker NJF, Salomón RL, de Roo L, Steppe K (2017). Sugars from woody tissue photosynthesis reduce xylem vulnerability to cavitation. New Phytologist, 216, 720-727.
DOI PMID |
| [19] | de Roo L, Salomón RL, Oleksyn J, Steppe K (2020a). Woody tissue photosynthesis delays drought stress in Populus tremula trees and maintains starch reserves in branch xylem tissues. New Phytologist, 228, 70-81. |
| [20] | de Roo L, Salomón RL, Steppe K (2020b). Woody tissue photosynthesis reduces stem CO2 efflux by half and remains unaffected by drought stress in young Populus tremula trees. Plant, Cell & Environment, 43, 981-991. |
| [21] |
Fang YJ, Xiong LZ (2015). General mechanisms of drought response and their application in drought resistance improvement in plants. Cellular and Molecular Life Sciences, 72, 673-689.
DOI PMID |
| [22] | Feng XL, Huang XH, Li MQ, Ma J, Liu R (2022a). Changes of stem photosynthetic characteristics before and after germination in seven woody species. Chinese Journal of Ecology, 41, 654-660. |
| [冯晓龙, 黄新焕, 李民青, 马杰, 刘冉 (2022a). 7种木本植物萌芽前后枝干光合特征变化. 生态学杂志, 41, 654-660.] | |
| [23] | Feng XL, Liu R, Li CJ, Wang YG, Kong L, Wang ZR (2022b). Stem photosynthesis and its main influencing factors of Haloxylon ammodendron and Tamarix ramosissima. Chinese Journal of Applied Ecology, 33, 344-352. |
| [冯晓龙, 刘冉, 李从娟, 王玉刚, 孔璐, 王增如 (2022b). 梭梭和多枝柽柳的枝干光合及其主要影响因子. 应用生态学报, 33, 344-352.] | |
| [24] | Feng X, Liu R, Li C, Zhang H, Slot M (2023). Contrasting responses of two C4 desert shrubs to drought but consistent decoupling of photosynthesis and stomatal conductance at high temperature. Environmental and Experimental Botany, 209, 105295. DOI: 10.1016/j.envexpbot.2023.105295. |
| [25] |
Gupta A, Rico-Medina A, Caño-Delgado AI (2020). The physiology of plant responses to drought. Science, 368, 266-269.
DOI PMID |
| [26] | Hartmann H (2015). Carbon starvation during drought-induced tree mortality—Are we chasing a myth? Journal of Plant Hydraulics, 2, e005. DOI: 10.20870/jph.2015.e005. |
| [27] |
Huang JP, Yu HP, Guan XD, Wang GY, Guo RX (2016). Accelerated dryland expansion under climate change. Nature Climate Change, 6, 166-171.
DOI |
| [28] |
Li R, Jiang ZM, Zhang SX, Cai J (2015). A review of new research progress on the vulnerability of xylem embolism of woody plants. Chinese Journal of Plant Ecology, 39, 838-848.
DOI |
|
[李荣, 姜在民, 张硕新, 蔡靖 (2015). 木本植物木质部栓塞脆弱性研究新进展. 植物生态学报, 39, 838-848.]
DOI |
|
| [29] | Li Y, Zheng XJ, Wang YG, Xu GQ, Liu R (2021). Experiment and simulation platform for oasis-desert symbiotic relationship (ODP). Bulletin of Chinese Academy of Sciences, 36, 1506-1514. |
| [李彦, 郑新军, 王玉刚, 徐贵青, 刘冉 (2021). 绿洲-荒漠共生关系实验模拟平台(绿洲-荒漠平台). 中国科学院院刊, 36, 1506-1514.] | |
| [30] | Li ZM, Wang CK, Luo DD (2017). Variations and interrelationships of foliar hydraulic and photosynthetic traits for Larix gmelinii. Chinese Journal of Plant Ecology, 41, 1140-1148. |
|
[李志民, 王传宽, 罗丹丹 (2017). 兴安落叶松叶水力与光合性状的变异性和相关性. 植物生态学报, 41, 1140-1148.]
DOI |
|
| [31] | Liu JX, Gu L, Yu YC, Huang P, Wu ZG, Zhang Q, Qian YQ, Wan XC, Sun ZY (2019). Corticular photosynthesis drives bark water uptake to refill embolized vessels in dehydrated branches of Salix matsudana. Plant, Cell & Environment, 42, 2584-2596. |
| [32] |
Marchin RM, Medlyn BE, Tjoelker MG, Ellsworth DS (2023). Decoupling between stomatal conductance and photosynthesis occurs under extreme heat in broadleaf tree species regardless of water access. Global Change Biology, 29, 6319-6335.
DOI PMID |
| [33] |
Maurel C, Nacry P (2020). Root architecture and hydraulics converge for acclimation to changing water availability. Nature Plants, 6, 744-749.
DOI PMID |
| [34] | McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011). The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends in Ecology & Evolution, 26, 523-532. |
| [35] | Mu Q, Dong MQ, Xu J, Cao YX, Ding YB, Sun SK, Cai HJ (2022). Photosynthesis of winter wheat effectively reflected multiple physiological responses under short-term drought-rewatering conditions. Journal of the Science of Food and Agriculture, 102, 2472-2483. |
| [36] |
Nardini A, Lo Gullo MA, Salleo S (2011). Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant Science, 180, 604-611.
DOI PMID |
| [37] | Pang J, Zhao H, Bansal R, Bohuon E, Lambers H, Ryan MH, Siddique KHM (2018). Leaf transpiration plays a role in phosphorus acquisition among a large set of chickpea genotypes. Plant, Cell & Environment, 41, 2069-2079. |
| [38] |
Poorter L, Kitajima K (2007). Carbohydrate storage and light requirements of tropical moist and dry forest tree species. Ecology, 88, 1000-1011.
PMID |
| [39] | Qi J, Fan Z, Fu P, Zhang Y, Sterck F (2021). Differential determinants of growth rates in subtropical evergreen and deciduous juvenile trees: carbon gain, hydraulics and nutrient-use efficiencies. Tree Physiology, 41, 12-23. |
| [40] |
Sala A, Woodruff DR, Meinzer FC (2012). Carbon dynamics in trees: feast or famine? Tree Physiology, 32, 764-775.
DOI PMID |
| [41] | Saveyn A, Steppe K, Ubierna N, Dawson TE (2010). Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant, Cell & Environment, 33, 1949-1958. |
| [42] |
Schmitz N, Egerton JJG, Lovelock CE, Ball MC (2012). Light-dependent maintenance of hydraulic function in mangrove branches: Do xylary chloroplasts play a role in embolism repair? New Phytologist, 195, 40-46.
DOI PMID |
| [43] | Stephenson NL, Das AJ (2020). Height-related changes in forest composition explain increasing tree mortality with height during an extreme drought. Nature Communications, 11, 3402. DOI: 10.1038/s41467-019-12380-6. |
| [44] | Teskey RO, Saveyn A, Steppe K, McGuire MA (2008). Origin, fate and significance of CO2 in tree stems. New Phytologist, 177, 17-32. |
| [45] | Trenberth KE, Dai AG, van der Schrier G, Jones PD, Barichivich J, Briffa KR, Sheffield J (2014). Global warming and changes in drought. Nature Climate Change, 4, 17-22. |
| [46] |
Vandegehuchte MW, Bloemen J, Vergeynst LL, Steppe K (2015). Woody tissue photosynthesis in trees: salve on the wounds of drought? New Phytologist, 208, 998-1002.
DOI PMID |
| [47] | Wittmann C, Pfanz H (2018). More than just CO2-recycling: corticular photosynthesis as a mechanism to reduce the risk of an energy crisis induced by low oxygen. New Phytologist, 219, 551-564. |
| [48] | Wu QX, Wu FZ, Hu Y, Kang ZJ, Zhang YY, Yang J, Yue K, Ni XY, Yang YS (2021). Difference in non-structural carbohydrates between fresh and senescent leaves of 11 tree species in a subtropical common-garden. Chinese Journal of Plant Ecology, 45, 771-779. |
|
[吴秋霞, 吴福忠, 胡仪, 康自佳, 张耀艺, 杨静, 岳楷, 倪祥银, 杨玉盛 (2021). 亚热带同质园11个树种新老叶非结构性碳水化合物含量比较. 植物生态学报, 45, 771-779.]
DOI |
|
| [49] | Yang B, Liu ZZ, Peng FR, Cao F, Chen T, Deng QJ, Chen WJ (2017). Growth and photosynthetic characteristics for pecan cultivars during drought stress and recovery. Journal of Zhejiang A&F University, 34, 991-998. |
| [杨标, 刘壮壮, 彭方仁, 曹凡, 陈涛, 邓秋菊, 陈文静 (2017). 干旱胁迫和复水下不同薄壳山核桃品种的生长和光合特性. 浙江农林大学学报, 34, 991-998.] | |
| [50] | Yin J, Qiu GY, He F, He KN, Tian JH, Zhang WQ, Xiong YJ, Zhao SH, Liu JX (2008). Leaf area characteristics of plantation stands in semi-arid loess hill-gully region of China. Chinese Journal of Plant Ecology, 32, 440-447. |
|
[尹婧, 邱国玉, 何凡, 贺康宁, 田晶会, 张卫强, 熊育久, 赵少华, 刘建新 (2008). 半干旱黄土丘陵区人工林叶面积特征. 植物生态学报, 32, 440-447.]
DOI |
|
| [51] |
Zhang ZH, Cao BL, Gao S, Xu K (2019). Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma, 256, 1013-1024.
DOI PMID |
| [1] | 彭仲韬, 金光泽, 刘志理. 小兴安岭三种槭树叶性状随植株大小和林冠条件的变异[J]. 植物生态学报, 2024, 48(6): 730-743. |
| [2] | 苏炜, 陈平, 吴婷, 刘岳, 宋雨婷, 刘旭军, 刘菊秀. 氮添加与干季延长对降香黄檀幼苗非结构性碳水化合物、养分与生物量的影响[J]. 植物生态学报, 2023, 47(8): 1094-1104. |
| [3] | 余海霞, 曲鲁平, 汤行昊, 刘南, 张子雷, 王浩, 王艺璇, 邵长亮, 董刚, 胡亚林. 闽楠和木荷非结构性碳水化合物对不同模式热浪的差异性响应[J]. 植物生态学报, 2023, 47(2): 249-261. |
| [4] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [5] | 李变变, 张凤华, 赵亚光, 孙秉楠. 不同刈割程度对油莎豆非结构性碳水化合物代谢及生物量的影响[J]. 植物生态学报, 2023, 47(1): 101-113. |
| [6] | 伍敏, 田雨, 樊大勇, 张祥雪. 干旱胁迫下毛白杨和元宝槭的水力学调控[J]. 植物生态学报, 2022, 46(9): 1086-1097. |
| [7] | 董涵君, 王兴昌, 苑丹阳, 柳荻, 刘玉龙, 桑英, 王晓春. 温带不同材性树种树干非结构性碳水化合物的径向分配差异[J]. 植物生态学报, 2022, 46(6): 722-734. |
| [8] | 李思源, 张照鑫, 饶良懿. 桑苗非结构性碳水化合物和生长激素对水淹胁迫的响应[J]. 植物生态学报, 2022, 46(3): 311-320. |
| [9] | 秦慧君, 焦亮, 周怡, 薛儒鸿, 柒常亮, 杜达石. 祁连山优势树木碳水化合物资源分配的海拔和树种效应[J]. 植物生态学报, 2022, 46(2): 208-219. |
| [10] | 林夏珍, 刘林, 董婷婷, 方琦博, 郭庆学. 非结构性碳水化合物与氮分配对美洲黑杨和青杨耐盐能力的影响[J]. 植物生态学报, 2021, 45(9): 961-971. |
| [11] | 吴秋霞, 吴福忠, 胡仪, 康自佳, 张耀艺, 杨静, 岳楷, 倪祥银, 杨玉盛. 亚热带同质园11个树种新老叶非结构性碳水化合物含量比较[J]. 植物生态学报, 2021, 45(7): 771-779. |
| [12] | 宋琳, 雒文涛, 马望, 何鹏, 梁潇洒, 王正文. 极端干旱对草甸草原优势植物非结构性碳水化合物的影响[J]. 植物生态学报, 2020, 44(6): 669-676. |
| [13] | 章异平, 海旭莹, 徐军亮, 吴文霞, 曹鹏鹤, 安文静. 秦岭东段栓皮栎枝条非结构性碳水化合物含量的季节动态[J]. 植物生态学报, 2019, 43(6): 521-531. |
| [14] | 周慧敏, 李品, 冯兆忠, 张殷波. 地表臭氧浓度升高与干旱交互作用对杨树非结构性碳水化合物积累和叶根分配的短期影响[J]. 植物生态学报, 2019, 43(4): 296-304. |
| [15] | 王兆国, 王传宽. 碳供给与碳利用对树木生长的限制机制[J]. 植物生态学报, 2019, 43(12): 1036-1047. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19