

植物生态学报 ›› 2023, Vol. 47 ›› Issue (2): 249-261.DOI: 10.17521/cjpe.2022.0167
余海霞1, 曲鲁平1,*( ), 汤行昊2, 刘南1, 张子雷1, 王浩1, 王艺璇3, 邵长亮3, 董刚4, 胡亚林1
), 汤行昊2, 刘南1, 张子雷1, 王浩1, 王艺璇3, 邵长亮3, 董刚4, 胡亚林1
收稿日期:2022-04-26
接受日期:2022-09-05
出版日期:2023-02-20
发布日期:2023-02-28
通讯作者:
*(基金资助:
YU Hai-Xia1, QU Lu-Ping1,*( ), TANG Xing-Hao2, LIU Nan1, ZHANG Zi-Lei1, WANG Hao1, WANG Yi-Xuan3, SHAO Chang-Liang3, DONG Gang4, HU Ya-Lin1
), TANG Xing-Hao2, LIU Nan1, ZHANG Zi-Lei1, WANG Hao1, WANG Yi-Xuan3, SHAO Chang-Liang3, DONG Gang4, HU Ya-Lin1
Received:2022-04-26
Accepted:2022-09-05
Online:2023-02-20
Published:2023-02-28
Contact:
*(Supported by:摘要:
植物体内非结构性碳水化合物(NSC)存储和调节机制反映了植物生长和代谢过程对环境变化的响应程度。全球气候变暖背景下, 极端气候事件热浪的发生频率增加, 势必会影响植物的碳收支和分配过程。然而目前热浪频率及间隔时间差异形成的复杂模式对植物不同器官之间的NSC分配及调节机制的影响尚不清楚。为了更好地阐明极端气候事件下植物器官水平的碳收支平衡机制, 该研究以亚热带主要阔叶树种闽楠(Phoebe bournei)和木荷(Schima superba)苗木为研究对象, 设置对照和不同频率及不同间隔时间的5个热浪处理, 分别为无热浪(CK)、单次热浪(HW)、短间隔反复热浪(两次热浪间隔7天, 2HW7)、中间隔反复热浪(两次热浪间隔30天, 2HW30)、长间隔反复热浪(两次热浪间隔45天, 2HW45)。测定分析不同树种各器官(茎、叶、根) NSC含量和生物量变化及其分配对不同热浪模式的响应, 揭示复杂极端气候事件对植物生长的影响及不同植物的响应差异。结果表明反复热浪2HW7显著增加了闽楠茎可溶性糖含量, 但对其根、叶可溶性糖及NSC含量无显著影响; 然而2HW7显著增加了木荷茎、根淀粉含量, 对可溶性糖和NSC含量无显著影响, 表明了热浪胁迫下不同阔叶树种NSC分配和调节存在物种和器官差异性。2HW30和2HW45处理下闽楠茎NSC含量显著低于2HW7, 2HW30和2HW45处理下木荷茎、根淀粉含量也显著低于2HW7, 与CK无显著差异, 表明了反复热浪存在累加效应, 且累加效应与反复热浪间隔时间相关。闽楠各器官生物量在2HW7处理组显著增加, 但木荷茎、根生物量在不同热浪模式处理下无显著差异, 表明闽楠增加NSC存储供给植物各器官生长发育以抵御热浪胁迫, 而木荷可能将光合产物以淀粉的形式储存在叶中缓解热浪对叶片光合系统的影响。研究结果表明不同间隔时间及频率的热浪对植物产生累加效应, 而植物NSC对热浪胁迫的调节能力与累加效应有关。
余海霞, 曲鲁平, 汤行昊, 刘南, 张子雷, 王浩, 王艺璇, 邵长亮, 董刚, 胡亚林. 闽楠和木荷非结构性碳水化合物对不同模式热浪的差异性响应. 植物生态学报, 2023, 47(2): 249-261. DOI: 10.17521/cjpe.2022.0167
YU Hai-Xia, QU Lu-Ping, TANG Xing-Hao, LIU Nan, ZHANG Zi-Lei, WANG Hao, WANG Yi-Xuan, SHAO Chang-Liang, DONG Gang, HU Ya-Lin. Divergent responses of non-structural carbohydrates in Phoebe bournei and Schima superba to different heat wave patterns. Chinese Journal of Plant Ecology, 2023, 47(2): 249-261. DOI: 10.17521/cjpe.2022.0167
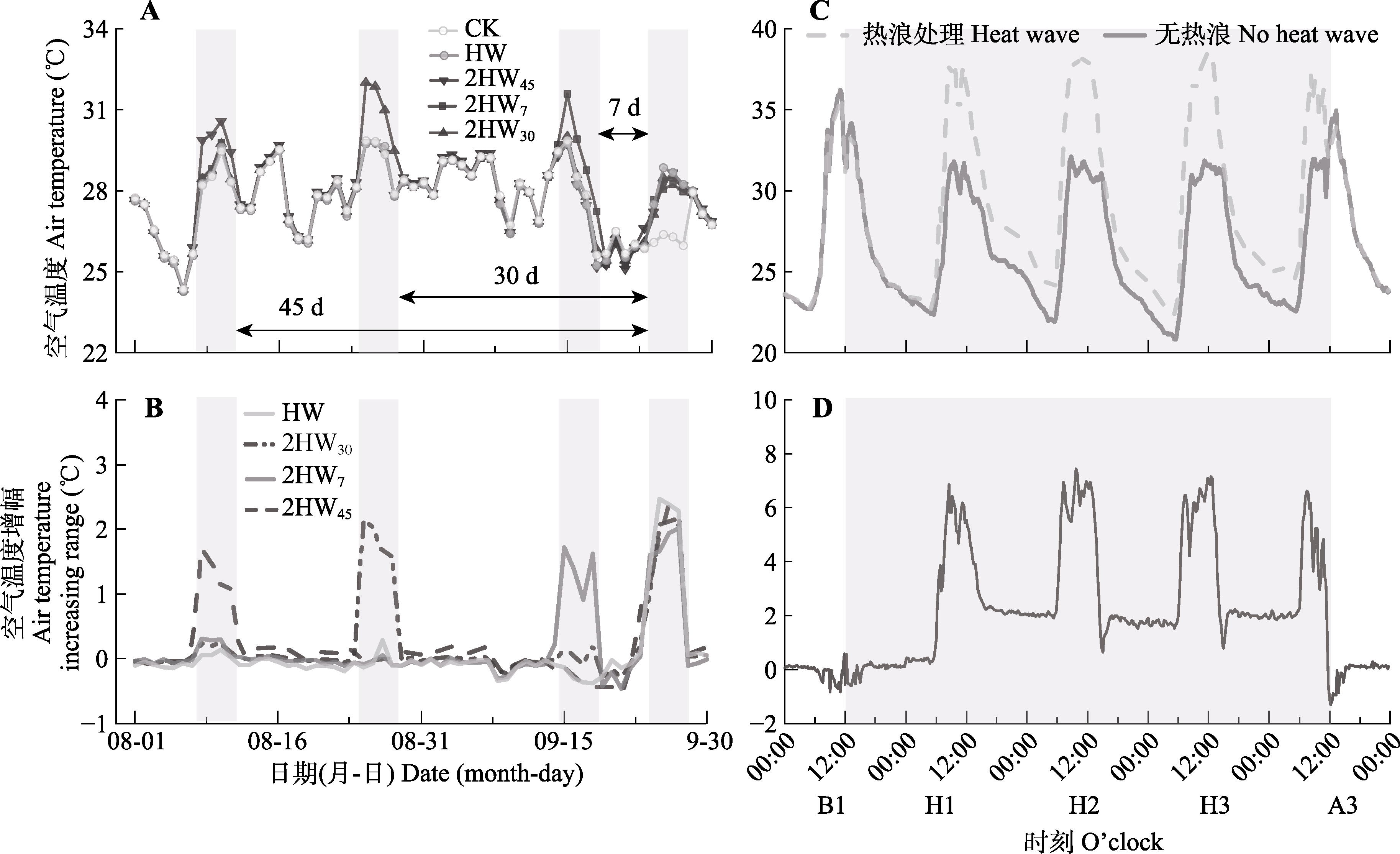
图1 实验阶段不同模式热浪处理日平均气温变化及增温幅度(A、B)及单次热浪模拟阶段空气温度日间变化及增温幅度(C、D)。2HW7, 两次热浪间隔7天; 2HW30, 两次热浪间隔30天; 2HW45, 两次热浪间隔45天; CK, 无热浪; HW, 单次热浪。A3, 热浪后1日; B1, 热浪前1日; H1, 热浪第1日, H2, 热浪第2日; H3, 热浪第3日。
Fig. 1 Daily mean air temperature variation and increasing range in different heat wave treatment during the experiment (A, B) and the illustration of air temperature diurnal variation and increasing range during a heat wave simulation period (C, D). 2HW7, two heat wave interval of 7 days; 2HW30, two heat wave interval of 30 days; 2HW45, two heat wave interval of 45 days; CK, no heat wave; HW, one heat wave. A3, the day after heat wave; B1, the day before heat wave; H1, the first day of heat wave; H2, the second day of heat wave; H3, the third day of heat wave.
| 指标 Index | df | 可溶性糖含量 Soluble sugar content (mg·g-1) | 淀粉含量 Starch content (mg·g-1) | NSC含量 NSC content (mg·g-1) |
|---|---|---|---|---|
| 热浪处理 Heat wave (a) | 4 | 0.173 | <0.001 | 0.015 |
| 树种 Species (b) | 1 | <0.001 | 0.534 | <0.001 |
| 器官 Organs (c) | 2 | <0.001 | <0.001 | <0.001 |
| 热浪处理×树种 a × b | 4 | 0.682 | 0.009 | 0.350 |
| 热浪处理×器官 a × c | 8 | 0.554 | 0.305 | 0.569 |
| 树种×器官 b × c | 2 | <0.001 | <0.001 | <0.001 |
| 热浪处理×树种×器官 a × b × c | 8 | 0.765 | 0.321 | 0.832 |
表1 不同热浪处理、树种和器官对植物非结构性碳水化合物(NSC)含量及分配的三因素方差分析
Table 1 Results of three-way ANOVA of plant non-structural carbohydrates (NSC) content and their allocation under different heatwave treatments, tree species and organs
| 指标 Index | df | 可溶性糖含量 Soluble sugar content (mg·g-1) | 淀粉含量 Starch content (mg·g-1) | NSC含量 NSC content (mg·g-1) |
|---|---|---|---|---|
| 热浪处理 Heat wave (a) | 4 | 0.173 | <0.001 | 0.015 |
| 树种 Species (b) | 1 | <0.001 | 0.534 | <0.001 |
| 器官 Organs (c) | 2 | <0.001 | <0.001 | <0.001 |
| 热浪处理×树种 a × b | 4 | 0.682 | 0.009 | 0.350 |
| 热浪处理×器官 a × c | 8 | 0.554 | 0.305 | 0.569 |
| 树种×器官 b × c | 2 | <0.001 | <0.001 | <0.001 |
| 热浪处理×树种×器官 a × b × c | 8 | 0.765 | 0.321 | 0.832 |
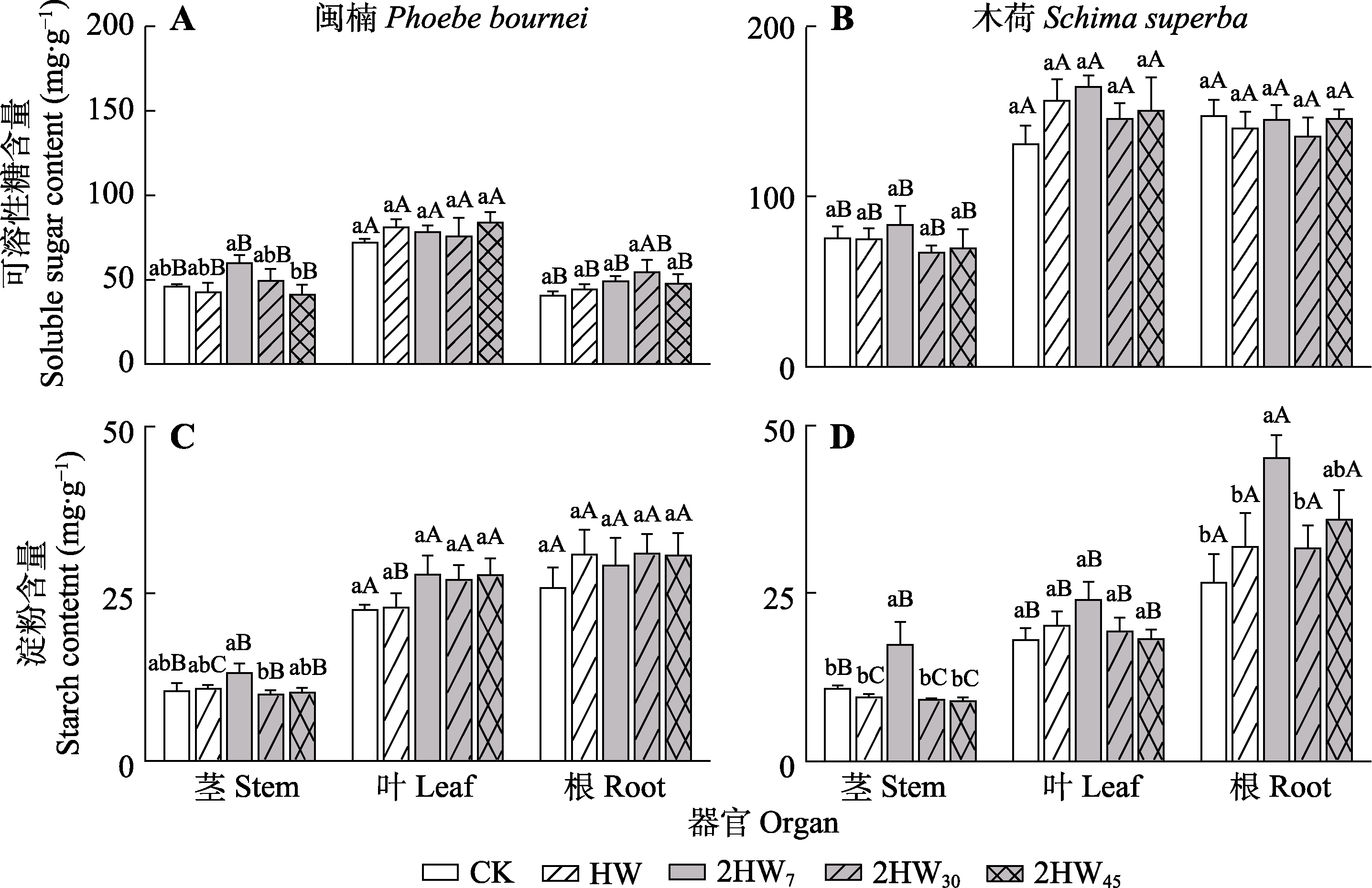
图2 不同模式热浪对闽楠和木荷各器官可溶性糖和淀粉含量的影响(平均值±标准误)。2HW7, 两次热浪间隔7天; 2HW30, 两次热浪间隔30天; 2HW45, 两次热浪间隔45天; CK, 无热浪; HW, 单次热浪。不同小写字母表示同一器官不同热浪频率处理间差异显著(p < 0.05), 不同大写字母表示同一树种相同处理不同器官间差异显著(p < 0.05)。
Fig. 2 Effects of different heat wave patterns on soluble sugar and starch contents in different organs of Phoebe bournei and Schima superba (mean ± SE). 2HW7, two heat wave interval of 7 days; 2HW30, two heat wave interval of 30 days; 2HW45, two heat wave interval of 45 days; CK, no heat wave; HW, one heat wave. Different lowercase letters indicate significant differences among different treatments in the same organs (p < 0.05), and different uppercase letters indicate significant differences among different organs in the same species (p < 0.05).
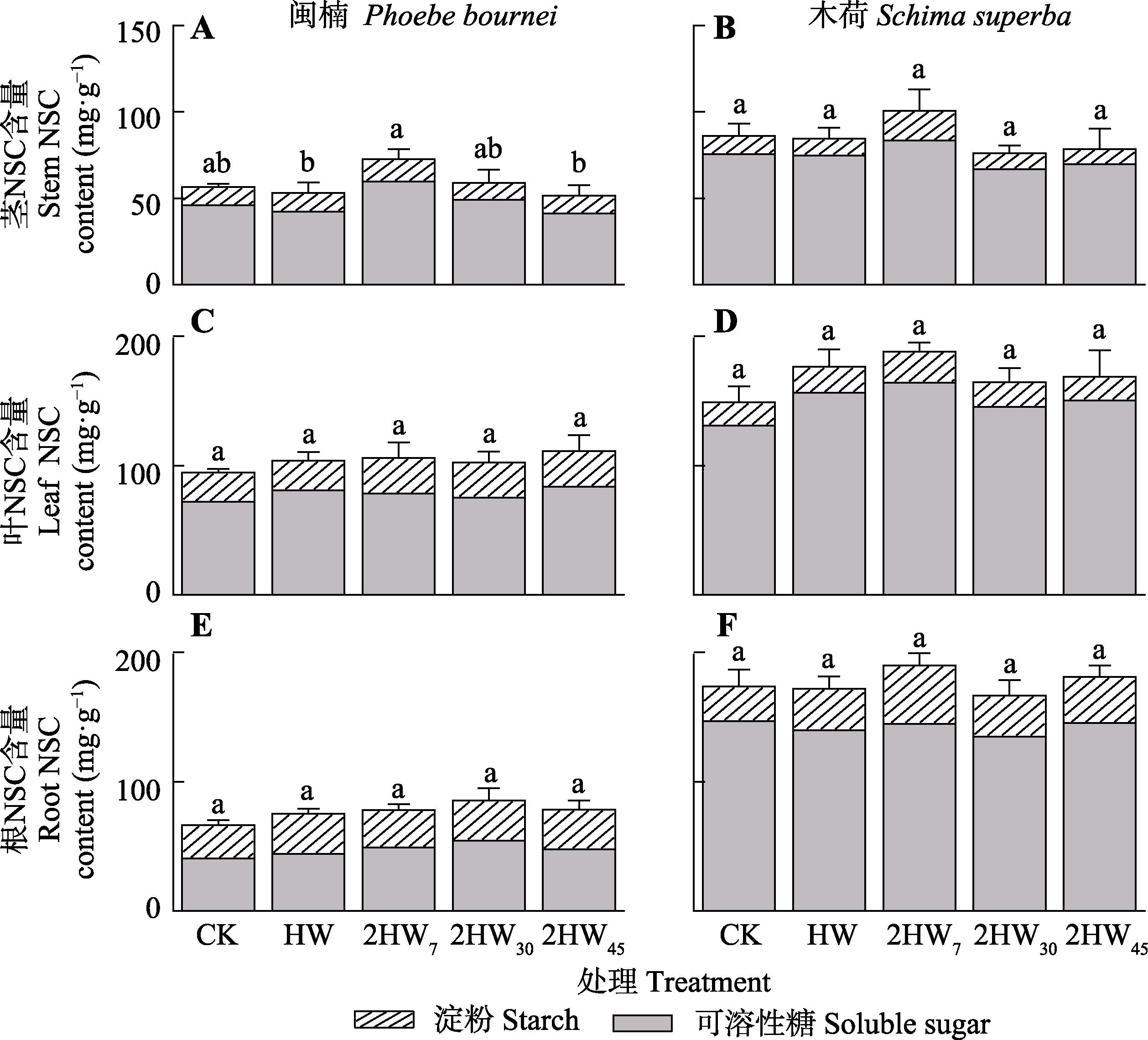
图3 不同模式热浪对闽楠和木荷各器官非结构性碳水化合物(NSC)含量的影响(平均值±标准误)。2HW7, 两次热浪间隔7天; 2HW30, 两次热浪间隔30天; 2HW45, 两次热浪间隔45天; CK, 无热浪; HW, 单次热浪。不同小写字母表示不同处理间存在显著差异(p < 0.05)。
Fig. 3 Effects of different heat wave patterns on non-structural carbohydrates (NSC) content in different organs of Phoebe bournei and Schima superba (mean ± SE). 2HW7, two heat wave interval of 7 days; 2HW30, two heat wave interval of 30 days; 2HW45, two heat wave interval of 45 days; CK, no heat wave; HW, one heat wave. Different lowercase letters indicate significant differences among different treatments (p < 0.05).
| 指标 Index | df | p | ||||
|---|---|---|---|---|---|---|
| 茎生物量 Stem biomass (g) | 叶生物量 Leaf biomass (g) | 根生物量 Root biomass (g) | 总生物量 Total biomass (g) | 根冠比 Root:shoot | ||
| 热浪处理 Heat wave | 4 | 0.034 | 0.107 | 0.004 | 0.015 | 0.172 |
| 树种 Species | 1 | <0.001 | <0.001 | 0.005 | <0.001 | 0.159 |
| 热浪处理×树种 Heat wave × species | 4 | 0.144 | 0.298 | 0.687 | 0.244 | 0.975 |
表2 不同热浪处理及树种对植物生物量影响的双因素分析
Table 2 Results of two-way ANOVA of different plant biomass influenced by heat wave treatments and tree species
| 指标 Index | df | p | ||||
|---|---|---|---|---|---|---|
| 茎生物量 Stem biomass (g) | 叶生物量 Leaf biomass (g) | 根生物量 Root biomass (g) | 总生物量 Total biomass (g) | 根冠比 Root:shoot | ||
| 热浪处理 Heat wave | 4 | 0.034 | 0.107 | 0.004 | 0.015 | 0.172 |
| 树种 Species | 1 | <0.001 | <0.001 | 0.005 | <0.001 | 0.159 |
| 热浪处理×树种 Heat wave × species | 4 | 0.144 | 0.298 | 0.687 | 0.244 | 0.975 |
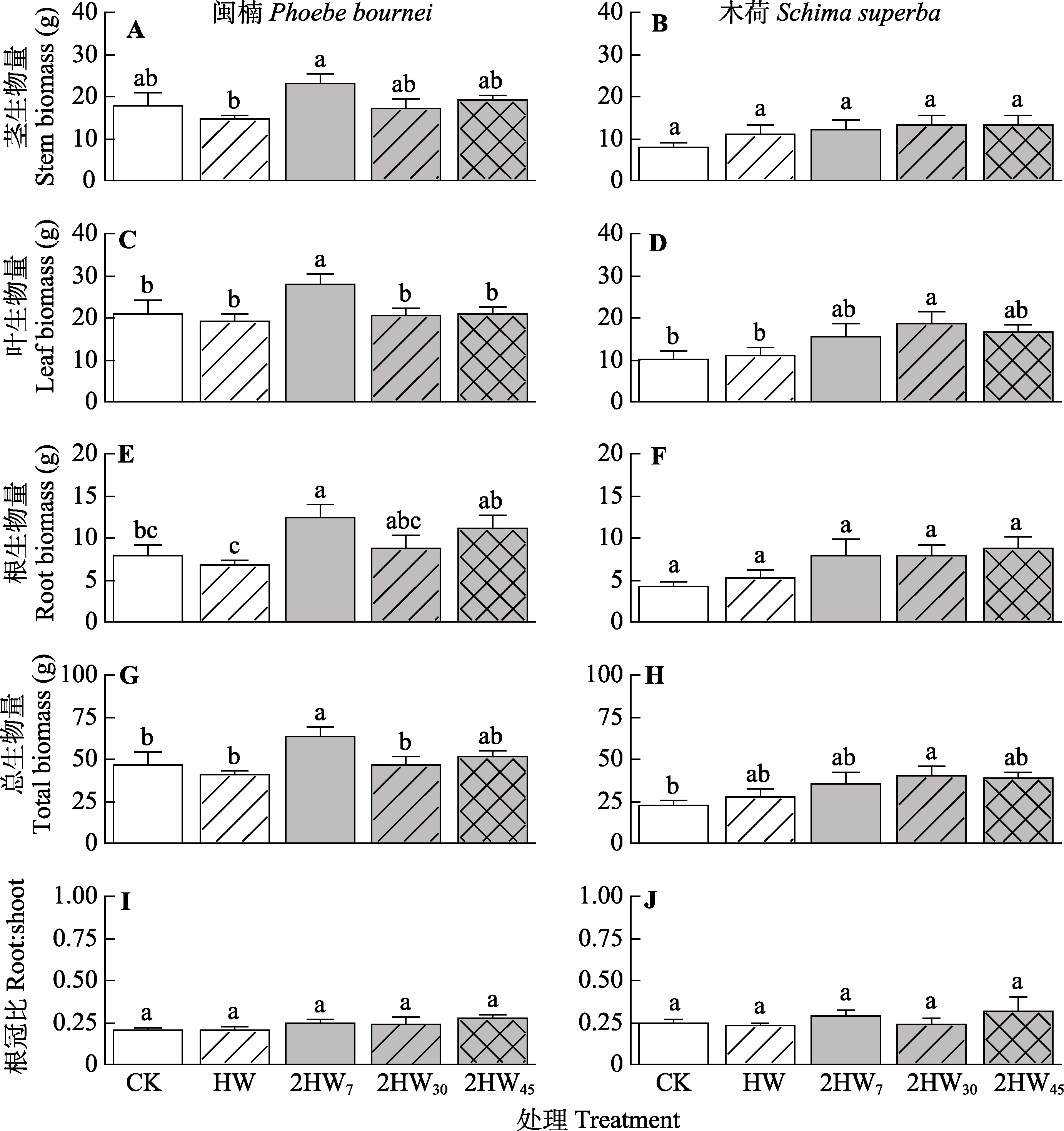
图4 不同模式热浪对闽楠和木荷茎、叶、根、总生物量及根冠比的影响(平均值±标准误)。2HW7, 两次热浪间隔7天; 2HW30, 两次热浪间隔30天; 2HW45, 两次热浪间隔45天; CK, 无热浪; HW, 单次热浪。不同小写字母表示不同处理之间生物量存在显著差异(p < 0.05)。
Fig. 4 Effects of different heat wave patterns on different organ biomass and root:shoot of Phoebe bournei and Schima superba (mean ± SE). 2HW7, two heat wave interval of 7 days; 2HW30, two heat wave interval of 30 days; 2HW45, two heat wave interval of 45 days; CK, no heat wave; HW, one heat wave. Different lowercase letters indicate significant differences among different treatments (p < 0.05).
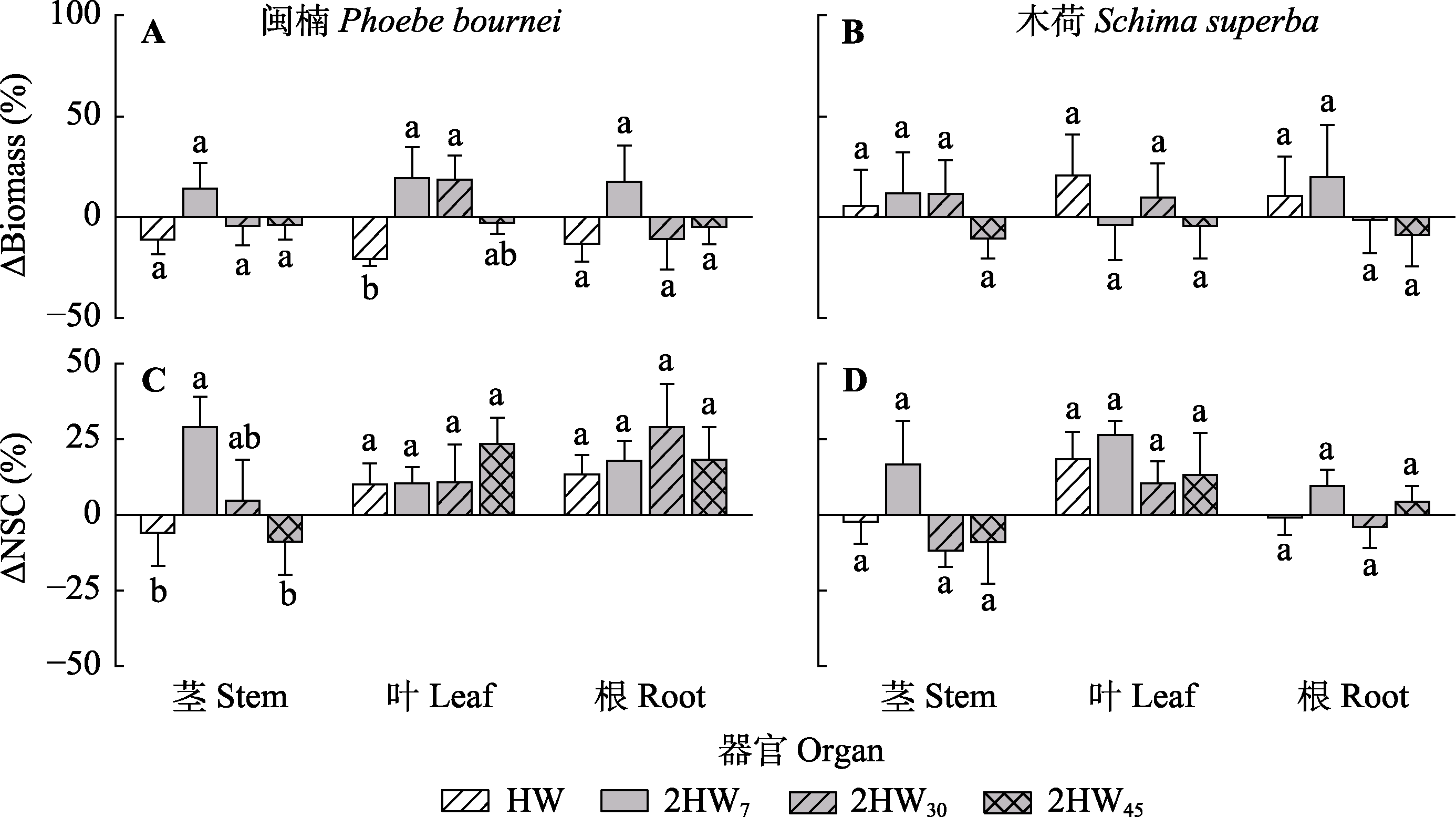
图5 不同模式热浪对闽楠和木荷各器官生物量和非结构性碳水化合物含量的影响百分比变化(平均值±标准误)。变化率由各热浪处理组与无热浪组的差值计算所得; ΔBiomass, 植物各器官生物量在不同模式热浪下的变化百分比; ΔNSC, 非结构性碳水化合物含量对不同频率热浪响应比。2HW7, 两次热浪间隔7天; 2HW30, 两次热浪间隔30天; 2HW45, 两次热浪间隔45天; HW, 单次热浪。不同小写字母表示不同处理间差异显著(p < 0.05)。
Fig. 5 Effects of different heat wave patterns on the percentage changes of biomass and non-structural carbohydrates (NSC) content in different organs of Phoebe bournei and Schima superba (mean ± SE). Percentage changes were calculated by the difference between treatment and control group; ΔBiomass, the biomass percentage changes response to different heat wave patterns; ΔNSC, the NSC content percentage changes response to different heat wave patterns. 2HW7, two heat wave interval of 7 days; 2HW30, two heat wave interval of 30 days; 2HW45, two heat wave interval of 45 days; HW, one heat wave. Different lowercase letters indicate significant differences among different treatments (p < 0.05).
| [1] |
Abeli T, Rossi G, Gentili R, Gandini M, Mondoni A, Cristofanelli P (2012). Effect of the extreme summer heat waves on isolated populations of two orophitic plants in the north Apennines (Italy). Nordic Journal of Botany, 30, 109-115.
DOI URL |
| [2] |
Ahrens CW, Challis A, Byrne M, Leigh A, Nicotra AB, Tissue D, Rymer P (2021). Repeated extreme heatwaves result in higher leaf thermal tolerances and greater safety margins. New Phytologist, 232, 1212-1225.
DOI URL |
| [3] |
Bardgett RD, Mommer L, de Vries FT (2014). Going underground: root traits as drivers of ecosystem processes. Trends in Ecology & Evolution, 29, 692-699.
DOI URL |
| [4] |
Birami B, Gattmann M, Heyer AG, Grote R, Arneth A, Ruehr NK (2018). Heat waves alter carbon allocation and increase mortality of Aleppo pine under dry conditions. Frontiers in Forests and Global Change, 1, 8. DOI: 10.3389/ffgc.2018.00008.
DOI |
| [5] |
Canham CD, Kobe RK, Latty EF, Chazdon RL (1999). Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia, 121, 1-11.
DOI PMID |
| [6] | Chen SR (2010). Effect of different site conditions on the growth of Phoebe bournei. Journal of Fujian College of Forestry, 30, 157-160. |
| [陈淑容 (2010). 不同立地因子对楠木生长的影响. 福建林学院学报, 30, 157-160.] | |
| [7] |
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014). Nonstructural carbon in woody plants. Annual Review of Plant Biology, 65, 667-687.
DOI PMID |
| [8] |
Drake JE, Tjoelker MG, Vårhammar A, Medlyn BE, Reich PB, Leigh A, Pfautsch S, Blackman CJ, López R, Aspinwall MJ, Crous KY, Duursma RA, Kumarathunge D, de Kauwe MG, Jiang MK, et al. (2018). Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biology, 24, 2390-2402.
DOI PMID |
| [9] | Du Y, Han Y, Wang CK (2014). The influence of drought on non-structural carbohydrates in the needles and twigs of Larix gmelinii. Acta Ecologica Sinica, 34, 6090-6100. |
| [杜尧, 韩轶, 王传宽 (2014). 干旱对兴安落叶松枝叶非结构性碳水化合物的影响. 生态学报, 34, 6090-6100.] | |
| [10] |
Duan HL, Wu JP, Huang GM, Zhou SX, Liu WF, Liao YC, Yang X, Xiao ZF, Fan HB (2017). Individual and interactive effects of drought and heat on leaf physiology of seedlings in an economically important crop. AoB Plants, 9, plw090. DOI: 10.1093/aobpla/plw090.
DOI |
| [11] |
Duarte AG, Katata G, Hoshika Y, Hossain M, Kreuzwieser J, Arneth A, Ruehr NK (2016). Immediate and potential long-term effects of consecutive heat waves on the photosynthetic performance and water balance in Douglas-fir. Journal of Plant Physiology, 205, 57-66.
DOI PMID |
| [12] | Galiano L, Timofeeva G, Saurer M, Siegwolf R, Martínez- Vilalta J, Hommel R, Gessler A (2017). The fate of recently fixed carbon after drought release: towards unravelling C storage regulation in Tilia platyphyllos and Pinus sylvestris. Plant, Cell & Environment, 40, 1711-1724. |
| [13] |
Gazol A, Camarero JJ, Sangüesa-Barreda G, Serra-Maluquer X, Sánchez-Salguero R, Coll L, Casals P (2020). Tree species are differently impacted by cumulative drought stress and present higher growth synchrony in dry places. Frontiers in Forests and Global Change, 3, 573346. DOI: 10.3389/ffgc.2020.573346.
DOI |
| [14] |
Hagedorn F, Joseph J, Peter M, Luster J, Pritsch K, Geppert U, Kerner R, Molinier V, Egli S, Schaub M, Liu JF, Li MH, Sever K, Weiler M, Siegwolf RTW, et al. (2016). Recovery of trees from drought depends on belowground sink control. Nature Plants, 2, 16111. DOI: 10.1038/nplants.2016.111.
DOI |
| [15] |
Hartmann H, Link RM, Schuldt B (2021). A whole-plant perspective of isohydry: stem-level support for leaf-level plant water regulation. Tree Physiology, 41, 901-905.
DOI PMID |
| [16] |
Hommel R, Siegwolf R, Zavadlav S, Arend M, Schaub M, Galiano L, Haeni M, Kayler ZE, Gessler A (2016). Impact of interspecific competition and drought on the allocation of new assimilates in trees. Plant Biology, 18, 785-796.
DOI PMID |
| [17] |
Huang JB, Hammerbacher A, Weinhold A, Reichelt M, Gleixner G, Behrendt T, van Dam NM, Sala A, Gershenzon J, Trumbore S, Hartmann H (2019). Eyes on the future- evidence for trade-offs between growth, storage and defense in Norway spruce. New Phytologist, 222, 144-158.
DOI URL |
| [18] |
Ingrisch J, Bahn M (2018). Towards a comparable quantification of resilience. Trends in Ecology & Evolution, 33, 251-259.
DOI URL |
| [19] |
Kannenberg SA, Novick KA, Phillips RP (2018). Coarse roots prevent declines in whole-tree non-structural carbohydrate pools during drought in an isohydric and an anisohydric species. Tree Physiology, 38, 582-590.
DOI PMID |
| [20] |
Kucharik CJ, Foley JA, Delire C, Fisher VA, Coe MT, Lenters JD, Young-Molling C, Ramankutty N, Norman JM, Gower ST (2000). Testing the performance of a dynamic global ecosystem model: water balance, carbon balance, and vegetation structure. Global Biogeochemical Cycles, 14, 795-825.
DOI URL |
| [21] |
Lei Y, Song WY, Xia ZC, Korpelainen H, Niinemets Ü, Li CY (2019). Elevated temperature differently affects growth, photosynthetic capacity, nutrient absorption and leaf ultrastructure of Abies faxoniana and Picea purpurea under intraand interspecific competition. Tree Physiology, 39, 1342-1357.
DOI PMID |
| [22] | Li KJ, Bai QS, Yao J, Wang YL, Lian HM, Zhang Q, He BX, Cai YL (2021). Research progress on cultivation and utilization of Schima superba in China. Forestry and Environmental Science, 37(6), 188-195. |
| [李可见, 白青松, 尧俊, 汪迎利, 连辉明, 张谦, 何波祥, 蔡燕灵 (2021). 我国木荷培育和利用研究进展. 林业与环境科学, 37(6), 188-195.] | |
| [23] |
Li MH, Xiao WF, Wang SG, Cheng GW, Cherubini P, Cai XH, Liu XL, Wang XD, Zhu WZ (2008). Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiology, 28, 1287-1296.
DOI URL |
| [24] | Li N, Sun T, Mao ZJ (2014). Effects of long-term high- temperature stress on the biomass and non-structure carbohydrates of Pinus sylvestris var. mongolica seedlings. Bulletin of Botanical Research, 34, 212-218. |
|
[李娜, 孙涛, 毛子军 (2014). 长期极端高温胁迫对樟子松幼苗生物量及非结构性碳水化合物的影响. 植物研究, 34, 212-218.]
DOI |
|
| [25] | Li X, Tan ND, Wu T, Cheng Y, Liu SZ, Fu SL, Li YY, Liu JX (2021). Plant growth and C:N:P stoichiometry characteristics in response to experimental warming in four co-occurring subtropical forest tree seedlings. Acta Ecologica Sinica, 41, 6146-6158. |
| [李旭, 谭钠丹, 吴婷, 程严, 刘世忠, 傅松玲, 李义勇, 刘菊秀 (2021). 增温对南亚热带常绿阔叶林4种幼树生长和碳氮磷化学计量特征的影响. 生态学报, 41, 6146-6158.] | |
| [26] | Lin LB, Li TH, Wen SZ, Yang L (2019). Study on growth regularity and biomass distribution feature of Phoebe bournei-Schima superba young mixed forest. Journal of Central South University of Forestry & Technology, 39(4), 79-84. |
| [林立彬, 李铁华, 文仕知, 杨柳 (2019). 闽楠木荷混交幼林生长规律及生物量分布特征研究. 中南林业科技大学学报, 39(4), 79-84.] | |
| [27] |
Lu RY, Chen RD (2016). A review of recent studies on extreme heat in China. Atmospheric and Oceanic Science Letters, 9, 114-121.
DOI URL |
| [28] |
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178, 719-739.
DOI PMID |
| [29] |
McDowell NG (2011). Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology, 155, 1051-1059.
DOI PMID |
| [30] |
Miralles DG, Teuling AJ, van Heerwaarden CC, de Arellano JVG (2014). Mega-heatwave temperatures due to combined soil desiccation and atmospheric heat accumulation. Nature Geoscience, 7, 345-349.
DOI |
| [31] |
Mitchell PJ, OʼGrady AP, Tissue DT, White DA, Ottenschlaeger ML, Pinkard EA (2013). Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist, 197, 862-872.
DOI PMID |
| [32] |
Paula S, Pausas JG (2011). Root traits explain different foraging strategies between resprouting life histories. Oecologia, 165, 321-331.
DOI PMID |
| [33] |
Pérez-de-Lis G, García-González I, Rozas V, Olano JM (2016). Feedbacks between earlywood anatomy and non-structural carbohydrates affect spring phenology and wood production in ring-porous oaks. Biogeosciences, 13, 5499-5510.
DOI URL |
| [34] |
Piper FI, Fajardo A, Hoch G (2017). Single-provenance mature conifers show higher non-structural carbohydrate storage and reduced growth in a drier location. Tree Physiology, 37, 1001-1010.
DOI PMID |
| [35] |
Pretzsch H, Grams T, Häberle KH, Pritsch K, Bauerle T, Rötzer T (2020). Growth and mortality of Norway spruce and European beech in monospecific and mixed-species stands under natural episodic and experimentally extended drought. Results of the KROOF throughfall exclusion experiment. Trees, 34, 957-970.
DOI |
| [36] |
Puri E, Hoch G, Körner C (2014). Defoliation reduces growth but not carbon reserves in Mediterranean Pinus pinaster trees. Trees, 29, 1187-1196.
DOI URL |
| [37] |
Qu LP, Chen JQ, Dong G, Jiang SC, Li LH, Guo JX, Shao CL (2016). Heat waves reduce ecosystem carbon sink strength in a Eurasian meadow steppe. Environmental Research, 144, 39-48.
DOI PMID |
| [38] |
Qu LP, Chen JQ, Dong G, Shao CL (2018). Heavy mowing enhances the effects of heat waves on grassland carbon and water fluxes. Science of the Total Environment, 627, 561-570.
DOI URL |
| [39] |
Qu LP, de Boeck HJ, Fan HH, Dong G, Chen JQ, Xu WB, Ge ZQ, Huang ZJ, Shao CL, Hu YL (2020). Diverging responses of two subtropical tree species (Schima superba and Cunninghamia lanceolata) to heat waves. Forests, 11, 513. DOI: 10.3390/f11050513.
DOI |
| [40] |
Reich PB, Sendall KM, Stefanski A, Wei XR, Rich RL, Montgomery RA (2016). Boreal and temperate trees show strong acclimation of respiration to warming. Nature, 531, 633-636.
DOI |
| [41] |
Richardson AD, Carbone MS, Keenan TF, Czimczik CI, Hollinger DY, Murakami P, Schaberg PG, Xu XM (2013). Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytologist, 197, 850-861.
DOI PMID |
| [42] |
Ruehr NK, Grote R, Mayr S, Arneth A (2019). Beyond the extreme: recovery of carbon and water relations in woody plants following heat and drought stress. Tree Physiology, 39, 1285-1299.
DOI PMID |
| [43] |
Ruehr NK, Offermann CA, Gessler A, Winkler JB, Ferrio JP, Buchmann N, Barnard RL (2009). Drought effects on allocation of recent carbon: from beech leaves to soil CO2 efflux. New Phytologist, 184, 950-961.
DOI URL |
| [44] |
Salleo S, Trifilò P, Esposito S, Nardini A, Lo Gullo MA (2009). Starch-to-sugar conversion in wood parenchyma of field-growing Laurus nobilis plants: a component of the signal pathway for embolism repair? Functional Plant Biology, 36, 815-825.
DOI URL |
| [45] |
Secchi F, Zwieniecki MA (2012). Analysis of xylem sap from functional (nonembolized) and nonfunctional (embolized) vessels of Populus nigra: chemistry of refilling. Plant Physiology, 160, 955-964.
DOI URL |
| [46] |
Sehgal A, Sita K, Kumar J, Kumar S, Singh S, Siddique KHM, Nayyar H (2017). Effects of drought, heat and their Interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Frontiers in Plant Science, 8, 1776. DOI: 10.3389/fpls.2017.01776.
DOI |
| [47] |
Siebers MH, Yendrek CR, Drag D, Locke AM, Rios Acosta L, Leakey ADB, Ainsworth EA, Bernacchi CJ, Ort DR (2015). Heat waves imposed during early pod development in soybean (Glycine max) cause significant yield loss despite a rapid recovery from oxidative stress. Global Change Biology, 21, 3114-3125.
DOI PMID |
| [48] |
Signori-Müller C, Oliveira RS, Barros FdV, Tavares JV, Gilpin M, Diniz FC, Zevallos MJM, Yupayccana CAS, Acosta M, Bacca J, Chino RSC, Cuellar GMA, Cumapa ERM, Martinez F, Mullisaca FMP, et al. (2021). Non-structural carbohydrates mediate seasonal water stress across Amazon forests. Nature Communications, 12, 2310-2319.
DOI PMID |
| [49] |
Song L, Luo WT, Ma W, He P, Liang XS, Wang ZW (2020). Extreme drought effects on nonstructural carbohydrates of dominant plant species in a meadow grassland. Chinese Journal of Plant Ecology, 44, 669-676.
DOI |
|
[宋琳, 雒文涛, 马望, 何鹏, 梁潇洒, 王正文 (2020). 极端干旱对草甸草原优势植物非结构性碳水化合物的影响. 植物生态学报, 44, 669-676.]
DOI |
|
| [50] |
Thalmann M, Santelia D (2017). Starch as a determinant of plant fitness under abiotic stress. New Phytologist, 214, 943-951.
DOI PMID |
| [51] |
van der Putten WH, Vet LEM, Harvey JA, Wäckers FL (2001). Linking above- and belowground multitrophic interactions of plants, herbivores, pathogens, and their antagonists. Trends in Ecology & Evolution, 16, 547-554.
DOI URL |
| [52] |
Vlam M, Baker PJ, Bunyavejchewin S, Zuidema PA (2014). Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia, 174, 1449-1461.
DOI PMID |
| [53] | Wang K, Zhao CJ, Lin TT, Yu GQ, Sun J (2019). Effects of different water treatments on non-structural carbohydrates in different organs of Ulmus pumila seedlings in the Horqin sandy land. Arid Zone Research, 36, 113-121. |
| [王凯, 赵成姣, 林婷婷, 于国庆, 孙菊 (2019). 水分处理对榆树幼苗不同器官非结构性碳水化合物的影响. 干旱区研究, 36, 113-121.] | |
| [54] |
Wang PY, Tang JP, Wang SY, Dong XN, Fang J (2018). Regional heatwaves in China: a cluster analysis. Climate Dynamics, 50, 1901-1917.
DOI URL |
| [55] | Wang YL, Xu ZZ, Zhou GS (2004). Changes in biomass allocation and gas exchange characteristics of Leymus chinensis in response to soil water stress. Acta Phytoecologica Sinica, 28, 803-809. |
|
[王云龙, 许振柱, 周广胜 (2004). 水分胁迫对羊草光合产物分配及其气体交换特征的影响. 植物生态学报, 28, 803-809.]
DOI |
|
| [56] |
Woodruff DR, Meinzer FC, Marias DE, Sevanto S, Jenkins MW, McDowell NG (2015). Linking nonstructural carbohydrate dynamics to gas exchange and leaf hydraulic behavior in Pinus edulis and Juniperus monosperma. New Phytologist, 206, 411-421.
DOI PMID |
| [57] | Wu C, Xiong DC, Zhang L, Shi YG, Wu F, Liu XF, Yang ZJ (2021). Effects of warming on leaf non-structural carbohydrates, carbon and nitrogen isotopes of Cunninghamia lanceolata saplings in different seasons. Chinese Journal of Applied and Environmental Biology, 28, 1564-1570. |
| [吴晨, 熊德成, 张丽, 时应贵, 吴帆, 刘小飞, 杨智杰 (2021). 增温对杉木幼树不同季节叶片非结构性碳水化合物及碳氮同位素的影响. 应用与环境生物学报, 28, 1564-1570.] | |
| [58] |
Xu H, Xiao JF, Zhang ZQ (2020). Heatwave effects on gross primary production of northern mid-latitude ecosystems. Environmental Research Letters, 15, 074027. DOI: 10.1088/ 1748-9326/ab8760.
DOI |
| [59] |
Yuan WP, Cai WW, Chen Y, Liu SG, Dong WJ, Zhang HC, Yu GR, Chen ZQ, He HL, Guo WD, Liu D, Liu SM, Xiang WH, Xie ZH, Zhao ZH, Zhou GM (2016). Severe summer heatwave and drought strongly reduced carbon uptake in southern China. Scientific Reports, 6, 18813. DOI: 10.1038/srep18813.
DOI |
| [60] | Zaragoza-Castells J, Sánchez-Gómez D, Valladares F, Hurry V, Atkin OK (2007). Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long-lived leaves. Plant, Cell & Environment, 30, 820-833. |
| [61] | Zhang T, Cao Y, Chen YM, Liu GB (2016). Effects of drought stress on non-structural carbohydrates of Robinia pseudoacacia saplings at the end of the growing season. Journal of Soil and Water Conservation, 30, 297-304. |
| [张婷, 曹扬, 陈云明, 刘国彬 (2016). 生长季末期干旱胁迫对刺槐幼苗非结构性碳水化合物的影响. 水土保持学报, 30, 297-304.] |
| [1] | 俞庆水 倪晓凤 吉成均 朱江玲 唐志尧 方精云. 10年氮磷添加对海南尖峰岭两种热带雨林优势植物叶片非结构性碳水化合物的影响[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 苏炜, 陈平, 吴婷, 刘岳, 宋雨婷, 刘旭军, 刘菊秀. 氮添加与干季延长对降香黄檀幼苗非结构性碳水化合物、养分与生物量的影响[J]. 植物生态学报, 2023, 47(8): 1094-1104. |
| [3] | 白雨鑫, 苑丹阳, 王兴昌, 刘玉龙, 王晓春. 东北地区3种桦木木质部导管特征对气候变化响应的趋同与差异[J]. 植物生态学报, 2023, 47(8): 1144-1158. |
| [4] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [5] | 李变变, 张凤华, 赵亚光, 孙秉楠. 不同刈割程度对油莎豆非结构性碳水化合物代谢及生物量的影响[J]. 植物生态学报, 2023, 47(1): 101-113. |
| [6] | 伍敏, 田雨, 樊大勇, 张祥雪. 干旱胁迫下毛白杨和元宝槭的水力学调控[J]. 植物生态学报, 2022, 46(9): 1086-1097. |
| [7] | 董涵君, 王兴昌, 苑丹阳, 柳荻, 刘玉龙, 桑英, 王晓春. 温带不同材性树种树干非结构性碳水化合物的径向分配差异[J]. 植物生态学报, 2022, 46(6): 722-734. |
| [8] | 李思源, 张照鑫, 饶良懿. 桑苗非结构性碳水化合物和生长激素对水淹胁迫的响应[J]. 植物生态学报, 2022, 46(3): 311-320. |
| [9] | 秦慧君, 焦亮, 周怡, 薛儒鸿, 柒常亮, 杜达石. 祁连山优势树木碳水化合物资源分配的海拔和树种效应[J]. 植物生态学报, 2022, 46(2): 208-219. |
| [10] | 韩聪, 刘鹏, 母艳梅, 原媛, 郝少荣, 田赟, 查天山, 贾昕. 黑沙蒿灌丛生态系统碳平衡对昼夜非对称增温的响应[J]. 植物生态学报, 2022, 46(12): 1473-1485. |
| [11] | 刘宁, 彭守璋, 陈云明. 气候因子对青藏高原植被生长的时间效应[J]. 植物生态学报, 2022, 46(1): 18-26. |
| [12] | 林夏珍, 刘林, 董婷婷, 方琦博, 郭庆学. 非结构性碳水化合物与氮分配对美洲黑杨和青杨耐盐能力的影响[J]. 植物生态学报, 2021, 45(9): 961-971. |
| [13] | 吴秋霞, 吴福忠, 胡仪, 康自佳, 张耀艺, 杨静, 岳楷, 倪祥银, 杨玉盛. 亚热带同质园11个树种新老叶非结构性碳水化合物含量比较[J]. 植物生态学报, 2021, 45(7): 771-779. |
| [14] | 倪铭, 张曦月, 姜超, 王鹤松. 中国西南部地区植被对极端气候事件的响应[J]. 植物生态学报, 2021, 45(6): 626-640. |
| [15] | 宋琳, 雒文涛, 马望, 何鹏, 梁潇洒, 王正文. 极端干旱对草甸草原优势植物非结构性碳水化合物的影响[J]. 植物生态学报, 2020, 44(6): 669-676. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19