

植物生态学报 ›› 2025, Vol. 49 ›› Issue (9): 1434-1447.DOI: 10.17521/cjpe.2025.0005 cstr: 32100.14.cjpe.2025.0005
朱瑞德1, 杨俊薇1, 刘宵含1, 陈冰瑞1, 池秀莲2, 田地1,*( )(
)( ), 杨光2, 程蒙2, 戴亚峰3, 王诗文3
), 杨光2, 程蒙2, 戴亚峰3, 王诗文3
收稿日期:2025-01-02
接受日期:2025-04-08
出版日期:2025-09-20
发布日期:2025-09-01
通讯作者:
*田地: ORCID: 0000-0002-0389-8683 (tiandi@bjfu.edu.cn)基金资助:
ZHU Rui-De1, YANG Jun-Wei1, LIU Xiao-Han1, CHEN Bing-Rui1, CHI Xiu-Lian2, TIAN Di1,*( )(
)( ), YANG Guang2, CHENG Meng2, DAI Ya-Feng3, WANG Shi-Wen3
), YANG Guang2, CHENG Meng2, DAI Ya-Feng3, WANG Shi-Wen3
Received:2025-01-02
Accepted:2025-04-08
Online:2025-09-20
Published:2025-09-01
Supported by:摘要: 霍山石斛(Dendrobium huoshanense)是兰科国家一级保护野生濒危植物, 也是一种药食兼用的中药资源植物。虽然设施和林下栽培已经成为霍山石斛的主要栽培模式, 但两种栽培模式下霍山石斛地上部生物量与基质/土壤中微生物群落间的关联特征尚不清楚, 限制了关于霍山石斛地上-地下过程与机制的理解。鉴于此, 该研究在安徽大别山霍山石斛种植基地开展了野外实验与随机采样, 旨在探究霍山石斛设施和林下栽培模式中养分对植物-微生物关联的调控。结果发现: 设施和林下栽培模式下, 基质与土壤中的微生物生物量、多样性与群落组成均存在显著的差异, 具体表现在基质中微生物生物量碳、氮含量显著高于林下土壤, 且具有更丰富的细菌多样性与丰度更高的外生菌根真菌。林下种植霍山石斛显著改变了土壤中的微生物群落, 而在设施栽培模式下种植霍山石斛却没有改变基质中的微生物群落。基质中霍山石斛植株生物量显著高于林下。此外, 结构方程模型结果表明, 基质/土壤中的养分含量和微生物群落组成与霍山石斛植株生物量之间存在密切的调控关系, 且不同栽培模式下这种关系有所不同。具体地, 在设施栽培的富养条件下, 外生菌根真菌直接促进了霍山石斛植株的地上部生物量; 而在林下栽培的贫养条件下, 病原菌的增殖可能会抑制霍山石斛植株的地上部生长。该研究理清了设施和林下栽培霍山石斛模式中植株生物量与微生物群落之间的关联性, 为开发促进植株生长的功能菌剂、保护霍山石斛这一珍稀濒危植物资源提供了科学依据。
朱瑞德, 杨俊薇, 刘宵含, 陈冰瑞, 池秀莲, 田地, 杨光, 程蒙, 戴亚峰, 王诗文. 霍山石斛设施和林下栽培模式中养分对植物-微生物关联的调控. 植物生态学报, 2025, 49(9): 1434-1447. DOI: 10.17521/cjpe.2025.0005
ZHU Rui-De, YANG Jun-Wei, LIU Xiao-Han, CHEN Bing-Rui, CHI Xiu-Lian, TIAN Di, YANG Guang, CHENG Meng, DAI Ya-Feng, WANG Shi-Wen. Nutrient regulation of plant-microbial association in Dendrobium huoshanense facilities and understory cultivation patterns. Chinese Journal of Plant Ecology, 2025, 49(9): 1434-1447. DOI: 10.17521/cjpe.2025.0005
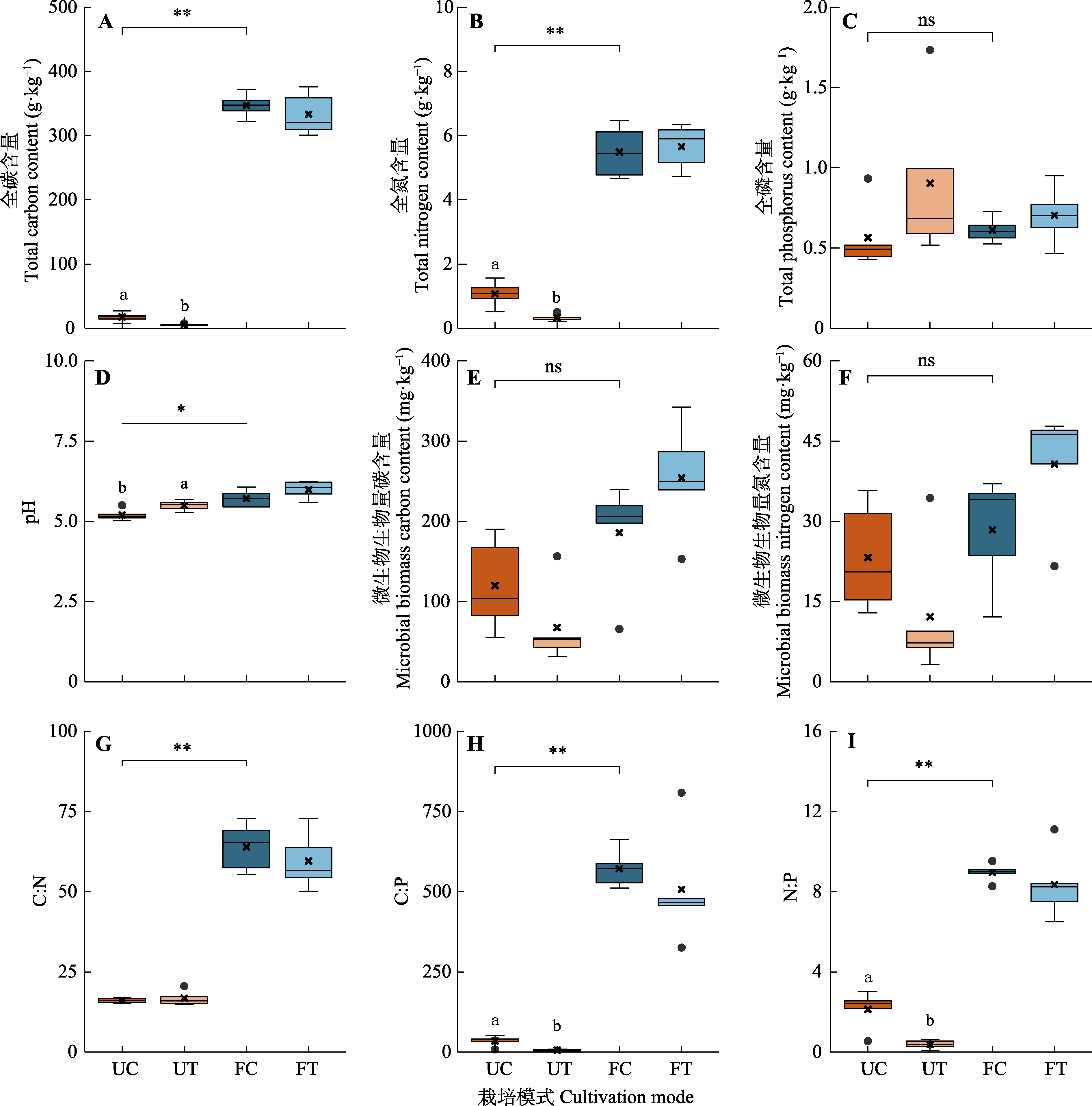
图1 不同栽培模式下基质与土壤间理化性质的差异。“×”表示该组数据的平均值点。FC, 设施对照; FT, 设施栽培; UC, 林下对照; UT, 林下栽培。ns, p > 0.05; *, p < 0.05; **, p < 0.01。不同小写字母表示林下对照与林下栽培处理间差异显著(p < 0.05)。
Fig. 1 Difference of physical and chemical properties between substrate and soil in different cultivation modes. “×” represents the mean value for each group. FC, facility control; FT, facility cultivation treatment; UC, understory control; UT, understory cultivation treatment. ns, p > 0.05; *, p < 0.05; **, p < 0.01. Different lowercase letters indicate significant difference between understory control and understory cultivation treatment.
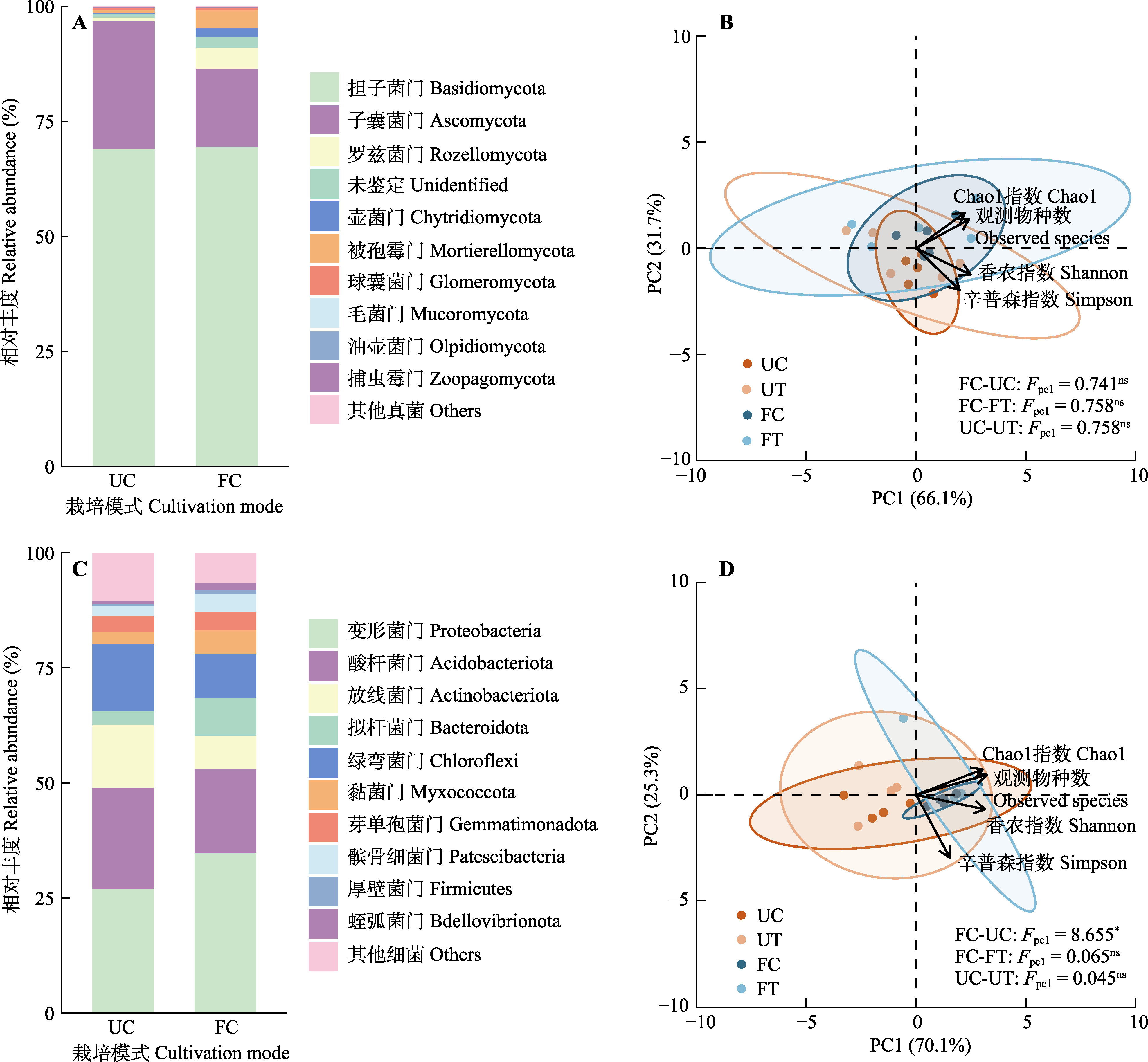
图2 不同栽培模式下微生物群落组成与多样性的差异。A, 真菌优势菌门组成。B, 真菌多样性。C, 细菌优势菌门组成。D, 细菌多样性。FC, 设施对照; FT, 设施栽培; UC, 林下对照; UT, 林下栽培。PC1, 主成分1; PC2, 主成分2。ns, p > 0.05; *, p < 0.05。
Fig. 2 Difference of microbial community composition and diversity in different cultivation modes. A, Composition of fungal dominant phyla. B, Fungal diversity. C, Composition of bacterial dominant phyla. D, Bacterial diversity. FC, facility control; FT, facility cultivation treatment; UC, understory control; UT, understory cultivation treatment. PC1, principal component 1; PC2, principal component 2. ns, p > 0.05; *, p < 0.05.
| 微生物类群 Microbiome | 分组 Group | R2 | p |
|---|---|---|---|
| 真菌 Fungi | UC-FC | 0.513 | 0.012* |
| UC-UT | 0.252 | 0.008** | |
| FC-FT | 1.480 | 0.053 | |
| 细菌 Bacteria | UC-FC | 0.567 | 0.005** |
| UC-UT | 0.590 | 0.009** | |
| FC-FT | 0.870 | 0.639 |
表1 不同栽培模式下微生物群落结构差异显著性检验
Table 1 Significance of microbial community structure difference under different cultivation modes
| 微生物类群 Microbiome | 分组 Group | R2 | p |
|---|---|---|---|
| 真菌 Fungi | UC-FC | 0.513 | 0.012* |
| UC-UT | 0.252 | 0.008** | |
| FC-FT | 1.480 | 0.053 | |
| 细菌 Bacteria | UC-FC | 0.567 | 0.005** |
| UC-UT | 0.590 | 0.009** | |
| FC-FT | 0.870 | 0.639 |
| 不同类群 Different taxa | 微生物类群 Microbiome | 分组 Group | 相对丰度对比 Relative abundance contrast (%) | 显著性 Statistical significance |
|---|---|---|---|---|
| 分类群 Taxonomic group | 被孢霉门 Mortierellomycota | FC-UC | 4.08 ± 1.50 vs 0.63 ± 0.01 | *** |
| 放线菌门 Actinobacteriota | FC-UC | 7.31 ± 1.74 vs 13.67 ± 2.87 | ** | |
| 捕虫霉门 Zoofagomycota | UC-UT | 0.21 ± 0.00 vs 0.01 ± 0.00 | * | |
| 芽单孢菌门 Gemmatimonadota | UC-UT | 3.87 ± 1.46 vs 0.40 ± 0.00 | * | |
| 功能群 Functional group | 外生菌根真菌 Ectomycorrhizal | FC-UC | 58.96 ± 6.14 vs 27.64 ± 5.60 | ** |
| 丛枝菌根真菌 Arbuscular Mycorrhizal | FC-UC | 0.23 ± 0.09 vs 0.27 ± 0.12 | ns | |
| 内生真菌 Endophyte | FC-UC | 0.20 ± 0.08 vs 0.15 ± 0.07 | ns | |
| 兰花菌根真菌 Orchid Mycorrhizal | FC-UC | 0.81 ± 0.16 vs 0.56 ± 0.16 | ns | |
| 病原菌 Pathogens | UC-UT | 0.60 ± 0.11 vs 1.34 ± 0.23 | * |
表2 不同栽培模式下微生物群落组成的差异
Table 2 Difference of microbial community composition in different cultivation modes
| 不同类群 Different taxa | 微生物类群 Microbiome | 分组 Group | 相对丰度对比 Relative abundance contrast (%) | 显著性 Statistical significance |
|---|---|---|---|---|
| 分类群 Taxonomic group | 被孢霉门 Mortierellomycota | FC-UC | 4.08 ± 1.50 vs 0.63 ± 0.01 | *** |
| 放线菌门 Actinobacteriota | FC-UC | 7.31 ± 1.74 vs 13.67 ± 2.87 | ** | |
| 捕虫霉门 Zoofagomycota | UC-UT | 0.21 ± 0.00 vs 0.01 ± 0.00 | * | |
| 芽单孢菌门 Gemmatimonadota | UC-UT | 3.87 ± 1.46 vs 0.40 ± 0.00 | * | |
| 功能群 Functional group | 外生菌根真菌 Ectomycorrhizal | FC-UC | 58.96 ± 6.14 vs 27.64 ± 5.60 | ** |
| 丛枝菌根真菌 Arbuscular Mycorrhizal | FC-UC | 0.23 ± 0.09 vs 0.27 ± 0.12 | ns | |
| 内生真菌 Endophyte | FC-UC | 0.20 ± 0.08 vs 0.15 ± 0.07 | ns | |
| 兰花菌根真菌 Orchid Mycorrhizal | FC-UC | 0.81 ± 0.16 vs 0.56 ± 0.16 | ns | |
| 病原菌 Pathogens | UC-UT | 0.60 ± 0.11 vs 1.34 ± 0.23 | * |
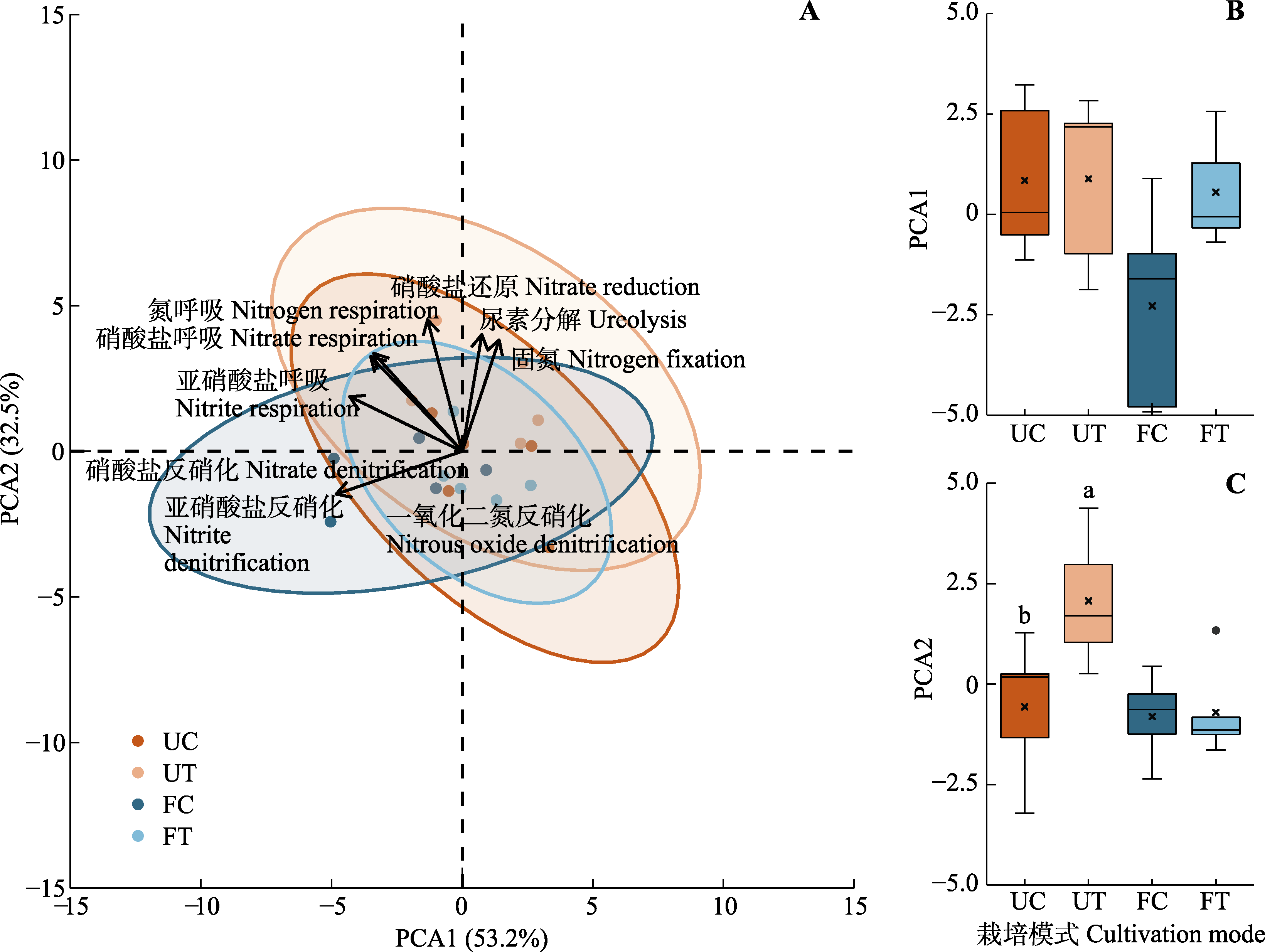
图3 不同栽培模式下氮代谢功能细菌的主成分分析(PCA) (A)以及第一主成分与第二主成分在不同栽培模式间的差异比较(B、C)。A图中样本点越向一轴左侧偏移, 代表着与反硝化作用相关的氮代谢过程越强, 越向二轴上侧偏移, 代表着与固氮和尿素分解相关的氮代谢过程越强。B-C图中“×”表示该组数据的平均值点。不同小写字母间表示林下对照与林下栽培处理间差异显著(p < 0.05)。FC, 设施对照; FT, 设施栽培; UC, 林下对照; UT, 林下栽培。
Fig. 3 Principal component analysis (PCA) of nitrogen metabolic functional bacteria in different cultivation modes (A) and comparison of the differences in the first and second principal components across different cultivation modes (B, C). In A, the farther the sample points deviate to the left side of the first axis, the stronger the nitrogen metabolism processes associated with denitrification. Conversely, the more the sample points shift towards the upper side of the second axis, the more intense the nitrogen metabolism processes related to nitrogen fixation and urea decomposition. In B, C, the “×” symbol represents the mean value for each group. Different lowercase letters indicate significant difference between understory control and understory cultivation treatment (p < 0.05). FC, facility control; FT, facility cultivation treatment; UC, understory control; UT, understory cultivation treatment.
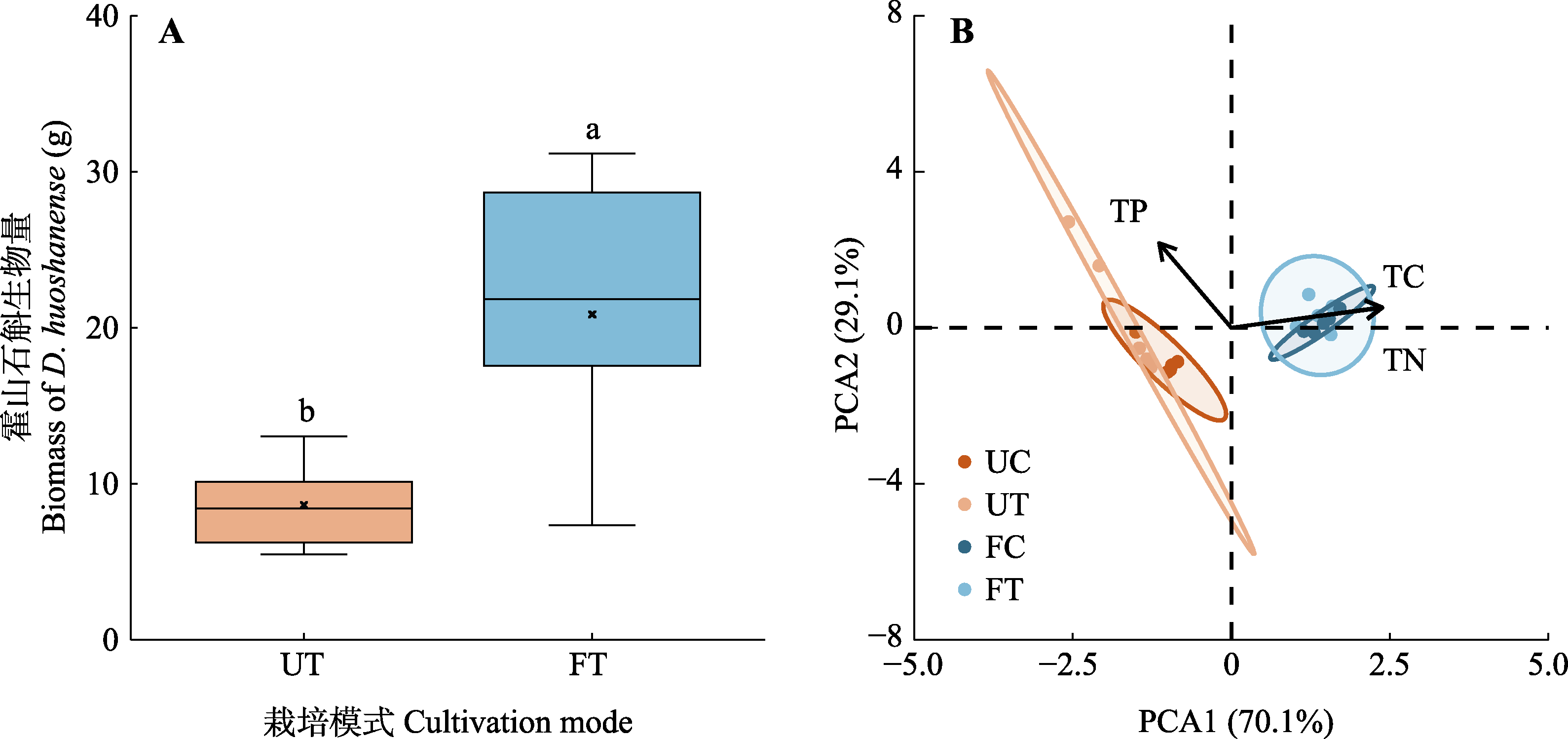
图4 不同栽培模式下霍山石斛生物量差异(A)以及不同栽培模式下养分状况的主成分分析(PCA) (B)。A中, “×”表示该组数据的平均值点。B中样本点越向一轴右侧偏移, 代表着养分状况中碳、氮含量越高。FC, 设施对照; FT, 设施栽培; UC, 林下对照; UT, 林下栽培。TC, 全碳含量; TN, 全氮含量; TP, 全磷含量。不同小写字母间表示设施栽培与林下栽培间差异显著(p < 0.05)。
Fig. 4 Differences in biomass of Dendrobium huoshanense under different cultivation modes (A) and principal component analysis (PCA) of nutrient status under different cultivation modes (B). In A, the “×” symbol represents the mean value for each group. In B, the further the sample points shift to the right on the first axis, the higher the carbon and nitrogen content in the nutrient status. FC, facility control; FT, facility cultivation treatment; UC, understory control; UT, understory cultivation treatment. TC, total carbon content; TN, total nitrogen content; TP, total phosphorus content. There is a significant difference (p < 0.05) between facility cultivation and understory cultivation between different lowercase letters.
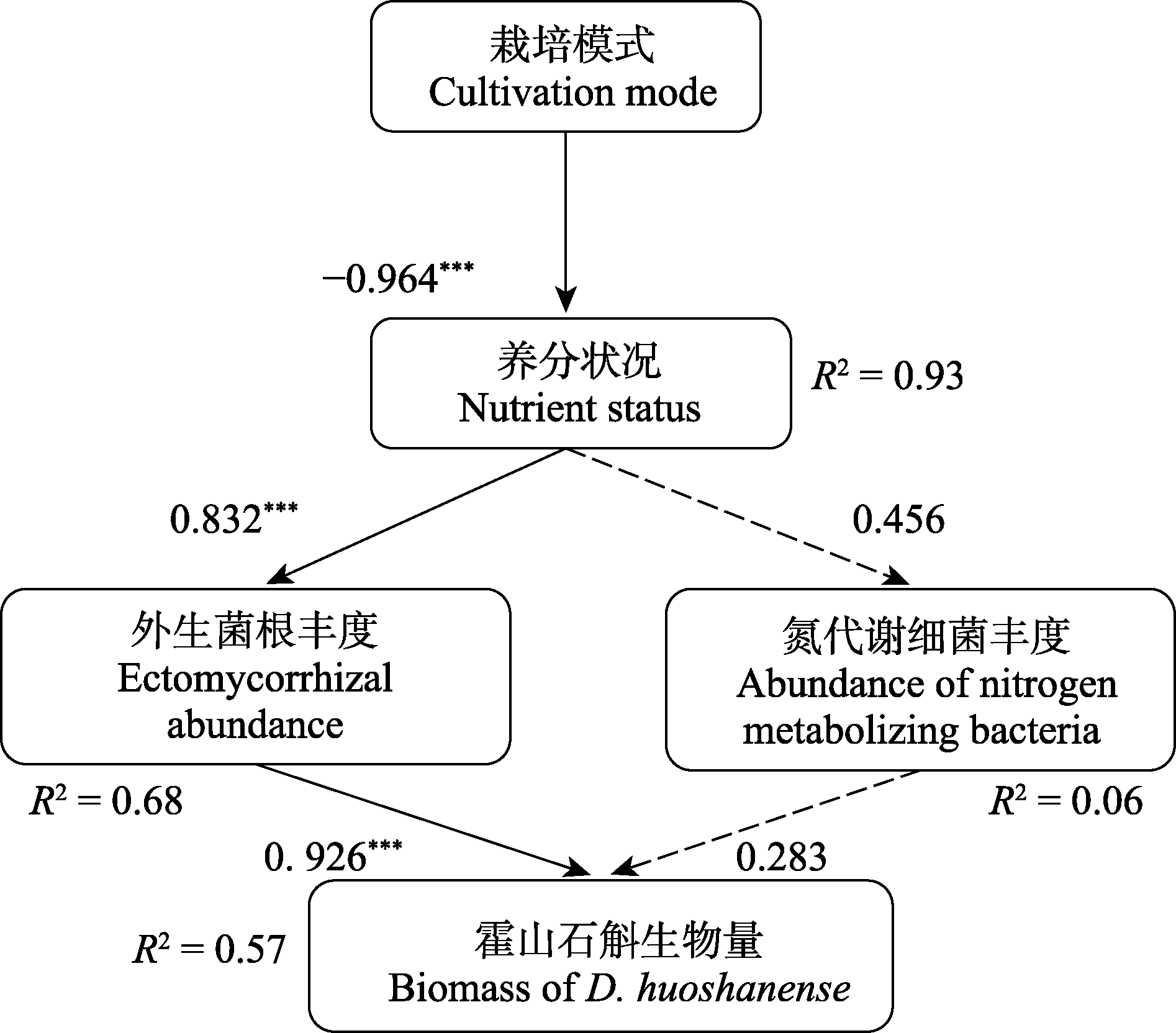
图5 霍山石斛植株生物量调控机制的结构方程模型。实线箭头代表显著的路径, 虚线箭头代表不显著的路径, 箭头上的数字为标准化路径系统。***, p < 0.001。
Fig. 5 Structural equation model of plant biomass regulation mechanism of Dendrobium huoshanense. Solid arrows represent significant paths, dashed arrows represent non-significant paths, and the numbers on the arrows are the standardized path coefficients. ***, p < 0.001.
| [1] | Aasfar A, Bargaz A, Yaakoubi K, Hilali A, Bennis I, Zeroual Y, Meftah Kadmiri I(2021). Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Frontiers in Microbiology, 12, 628379. DOI: 10.3389/fmicb.2021.628379. |
| [2] |
Ahsan T, Tian PC, Gao J, Wang C, Liu C, Huang YQ (2024). Effects of microbial agent and microbial fertilizer input on soil microbial community structure and diversity in a peanut continuous cropping system. Journal of Advanced Research, 64, 1-13.
DOI URL |
| [3] |
Bahram M, Netherway T, Hildebrand F, Pritsch K, Drenkhan R, Loit K, Anslan S, Bork P, Tedersoo L (2020). Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytologist, 227, 1189-1199.
DOI PMID |
| [4] | Baker NR, Zhalnina K, Yuan M, Herman D, Ceja-Navarro JA, Sasse J, Jordan JS, Bowen BP, Wu LY, Fossum C, Chew A, Fu Ying, Saha M, Zhou JZ, Pett-Ridge J, Northen TR, Firestone MK (2024). Nutrient and moisture limitations reveal keystone metabolites linking rhizosphere metabolomes and microbiomes. Proceedings of the National Academy of Sciences of the United States of America, 121, e2303439121. DOI: 10.1073/pnas.2303439121. |
| [5] |
Banerjee S, van der Heijden MGA(2023). Soil microbiomes and one health. Nature Reviews Microbiology, 21, 6-20.
DOI |
| [6] |
Berendsen RL, Pieterse CMJ, Bakker PAHM(2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17, 478-486.
DOI PMID |
| [7] |
Berg G (2009). Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Applied Microbiology and Biotechnology, 84, 11-18.
DOI PMID |
| [8] |
Bodelier PLE (2011). Toward understanding, managing, and protecting microbial ecosystems. Frontiers in Microbiology, 2, 80. DOI: 10.3389/fmicb.2011.00080.
PMID |
| [9] |
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985). Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology & Biochemistry, 17, 837-842.
DOI URL |
| [10] |
Burns RG, DeForest JL, Marxsen J, Sinsabaugh RL, Stromberger ME, Wallenstein MD, Weintraub MN, Zoppini A (2013). Soil enzymes in a changing environment: current knowledge and future directions. Soil Biology & Biochemistry, 58, 216-234.
DOI URL |
| [11] | Cai M, Cai JW, Yang QS, Yu J, Xie HQ, Han L, Peng DY (2024). Review of chemical constituents and pharmacological effects of Dendrobium huoshanense and prediction of its Q-markers. China Journal of Chinese Materia Medica, 49, 4860-4873. |
| [蔡明, 蔡静雯, 杨青山, 于娇, 解会群, 韩岚, 彭代银 (2024). 霍山石斛化学成分、药理作用研究进展及质量标志物的预测分析. 中国中药杂志, 49, 4860-4873.] | |
| [12] | Calderon RB, Dangi SR (2024). Arbuscular mycorrhizal fungi and rhizobium improve nutrient uptake and microbial diversity relative to dryland site-specific soil conditions. Microorganisms, 12, 667. DOI: 10.3390/microorganisms12040667. |
| [13] | Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America, 108, 4516-4522. |
| [14] | Chen Y, Jiang ZQ, Ou JM, Liu FD, Cai GY, Tan KM, Wang XL (2024). Nitrogen substitution practice improves soil quality of red soil (Ultisols) in South China by affecting soil properties and microbial community composition. Soil and Tillage Research, 240, 106089. DOI: 10.1016/j.still.2024.106089. |
| [15] |
Chen Y, Tian W, Shao Y, Li YJ, Lin LA, Zhang YJ, Han H, Chen ZJ (2020). Miscanthus cultivation shapes rhizosphere microbial community structure and function as assessed by Illumina MiSeq sequencing combined with PICRUSt and FUNGUIld analyses. Archives of Microbiology, 202, 1157-1171.
DOI PMID |
| [16] |
Chen YP, Chen GS, Robinson D, Yang ZJ, Guo JF, Xie JS, Fu SL, Zhou LX, Yang YS (2016). Large amounts of easily decomposable carbon stored in subtropical forest subsoil are associated with r-strategy-dominated soil microbes. Soil Biology & Biochemistry, 95, 233-242.
DOI URL |
| [17] |
Compant S, Cassan F, Kostić T, Johnson L, Brader G, Trognitz F, Sessitsch A (2025). Harnessing the plant microbiome for sustainable crop production. Nature Reviews Microbiology, 23, 9-23.
DOI |
| [18] |
Delgado-Baquerizo M, Eldridge DJ (2019). Cross-biome drivers of soil bacterial alpha diversity on a worldwide scale. Ecosystems, 22, 1220-1231.
DOI |
| [19] |
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013). Rhizosphere priming: a nutrient perspective. Frontiers in Microbiology, 4, 216. DOI: 10.3389/fmicb.2013.00216.
PMID |
| [20] |
Dineen SM, Aranda R 4th, Anders DL, Robertson JM(2010). An evaluation of commercial DNA extraction kits for the isolation of bacterial spore DNA from soil. Journal of Applied Microbiology, 109, 1886-1896.
DOI PMID |
| [21] | Ditta A, Mehmood S, Husen A (2024). Symbiotic Association of Microorganisms with Medicinal and Herbal Plants. CRC Press, Boca Raton. 224. |
| [22] | Feng WH, Sánchez-Rodríguez AR, Bilyera N, Wang JQ, Wang XQ, Han YH, Ma BX, Zhang HY, Li FYH, Zhou J, Li YY (2024a). Mechanisms of biochar-based organic fertilizers enhancing maize yield on a Chinese Chernozem: root traits, soil quality and soil microorganisms. Environmental Technology & Innovation, 36, 103756. DOI: 10.1016/j.eti.2024.103756. |
| [23] | Feng Y, Hu X, Guan YH, Chu ZX, Du XF, Xie YY, Yang SQ, Ye SR, Zhang L, Ma JY, Chen HM (2024b). Regulatory effects of different biochar on soil properties and microbial community structure in Chrysanthemum continuous cropping soil. Agronomy, 14, 2034. DOI: 10.3390/agronomy14092034. |
| [24] |
Fierer N, Bradford MA, Jackson RB (2007). Toward an ecological classification of soil bacteria. Ecology, 88, 1354-1364.
DOI PMID |
| [25] |
Fierer N, Leff JW, Adams BJ, Nielsen UN, Bates ST, Lauber CL, Owens S, Gilbert JA, Wall DH, Caporaso JG (2012). Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proceedings of the National Academy of Sciences of the United States of America, 109, 21390-21395.
DOI PMID |
| [26] | Ge JQ, Li D, Ding JX, Xiao X, Liang YT (2023). Microbial coexistence in the rhizosphere and the promotion of plant stress resistance: a review. Environmental Research, 222, 115298. DOI: 10.1016/j.envres.2023.115298. |
| [27] |
Han CL, Zhou WD, Gu YJ, Wang JQ, Zhou YF, Xue YY, Shi ZG, Siddique KHM (2024). Effects of tillage regime on soil aggregate-associated carbon, enzyme activity, and microbial community structure in a semiarid agroecosystem. Plant and Soil, 498, 543-559.
DOI |
| [28] | Hei JY, Li Y, Wang Q, Wang S, He XH (2024). Effects of exogenous organic acids on the soil metabolites and microbial communities of Panax notoginseng from the forest understory. Agronomy, 14, 601. DOI: 10.3390/agronomy14030601. |
| [29] |
Hemkemeyer M, Schwalb SA, Berendonk C, Geisen S, Heinze S, Joergensen RG, Li R, Lövenich P, Xiong W, Wichern F (2024). Potato yield and quality are linked to cover crop and soil microbiome, respectively. Biology and Fertility of Soils, 60, 525-545.
DOI |
| [30] |
Hill BH, Elonen CM, Jicha TM, Kolka RK, Lehto LLP, Sebestyen SD, Seifert-Monson LR (2014). Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry, 120, 203-224.
DOI URL |
| [31] | Hnini M, Rabeh K, Oubohssaine M (2024). Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: a systematic review. Plant Stress, 11, 100391. DOI: 10.1016/j.stress.2024.100391. |
| [32] |
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010). A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecology Letters, 13, 394-407.
DOI PMID |
| [33] |
Högberg P, Johannisson C, Yarwood S, Callesen I, Näsholm T, Myrold DD, Högberg MN (2011). Recovery of ectomycorrhiza after ‘nitrogen saturation’ of a conifer forest. New Phytologist, 189, 515-525.
DOI PMID |
| [34] | Hu MJ, Sardans J, Sun DY, Yan RB, Wu H, Ni RX, Peñuelas J (2024). Microbial diversity and keystone species drive soil nutrient cycling and multifunctionality following mangrove restoration. Environmental Research, 251, 118715. DOI: 10.1016/j.envres.2024.118715. |
| [35] |
Huo CF, Luo YQ, Cheng WX (2017). Rhizosphere priming effect: a meta-analysis. Soil Biology & Biochemistry, 111, 78-84.
DOI URL |
| [36] |
Jílková V, Frouz J, Mudrák O, Vohník M (2015). Effects of nutrient-rich substrate and ectomycorrhizal symbiosis on spruce seedling biomass in abandoned nests of the wood ant (Formica polyctena): a laboratory experiment. Geoderma, 259-260, 56-61.
DOI URL |
| [37] | Jin X, Cai JW, Yang SY, Li SP, Shao XJ, Fu CM, Li CZ, Deng Y, Huang JQ, Ruan YZ, Li CJ (2023). Partial substitution of chemical fertilizer with organic fertilizer and slow-release fertilizer benefits soil microbial diversity and pineapple fruit yield in the tropics. Applied Soil Ecology, 189, 104974. DOI: 10.1016/j.apsoil.2023.104974. |
| [38] |
Lefcheck JS (2016). piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution, 7, 573-579.
DOI URL |
| [39] |
Lewin GR, Carlos C, Chevrette MG, Horn HA, McDonald BR, Stankey RJ, Fox BG, Currie CR (2016). Evolution and ecology of Actinobacteria and their bioenergy applications. Annual Review of Microbiology, 70, 235-254.
DOI PMID |
| [40] |
Li H, Ye DD, Wang XG, Settles ML, Wang J, Hao ZQ, Zhou LS, Dong P, Jiang Y, Ma ZS (2014). Soil bacterial communities of different natural forest types in Northeast China. Plant and Soil, 383, 203-216.
DOI URL |
| [41] | Lian WH, Dong L, Li WJ (2021). Advances in rhizosphere microorganism and plant interaction in soil environment. Journal of Microbiology, 41(4), 74-83. |
| [连文慧, 董雷, 李文均 (2021). 土壤环境下的根际微生物和植物互作关系研究进展. 微生物学杂志, 41(4), 74-83.] | |
| [42] |
Liang SM, Zou YN, Shu B, Wu QS (2024). Arbuscular mycorrhizal fungi and endophytic fungi differentially modulate polyamines or proline of peach in response to soil flooding. Pedosphere, 34, 460-472.
DOI URL |
| [43] | Lin QH, Zhu JX, Wang QF, Zhang QY, Yu GR (2024). Patterns and drivers of atmospheric nitrogen deposition retention in global forests. Global Change Biology, 30, e17410. DOI: 10.1111/gcb.17410. |
| [44] |
Ling N, Chen DM, Guo H, Wei JX, Bai YF, Shen QR, Hu SJ (2017). Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma, 292, 25-33.
DOI URL |
| [45] | Liu T, Tong DD, Chen S, Ning C, Zhang XY, Filimonenko E, Aloufi AS, Cai WY, Farooq A, Liu GQ, Kuzyakov Y, Yan WD (2024). Fertilization shapes microbial life strategies, carbon and nitrogen metabolic functions in Camellia oleifera soil. Journal of Environmental Management, 370, 122896. DOI: 10.1016/j.jenvman.2024.122896. |
| [46] | Liu Y, Tan XP, Fu SL, Shen WJ (2022). Canopy and understory nitrogen addition alters organic soil bacterial communities but not fungal communities in a temperate forest. Frontiers in Microbiology, 13, 888121. DOI: 10.3389/fmicb.2022.888121. |
| [47] |
Louca S, Parfrey LW, Doebeli M (2016). Decoupling function and taxonomy in the global ocean microbiome. Science, 353, 1272-1277.
DOI PMID |
| [48] | Manici LM, Caputo F, de Sabata D, Fornasier F (2024). The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Applied Soil Ecology, 196, 105323. DOI: 10.1016/j.apsoil.2024.105323. |
| [49] |
Maslov MN, Maslova OA, Tokareva OA (2019). Changes in labile and microbial pools of carbon and nitrogen in forest litter samples under different methods of storage. Eurasian Soil Science, 52, 747-755.
DOI |
| [50] | Mennicken S, de Paula CCP, Vogt-Schilb H, Jersáková J (2024). Diversity of mycorrhizal fungi in temperate orchid species: comparison of culture-dependent and culture-independent methods. Journal of Fungi, 10, 92. DOI: 10.3390/jof10020092. |
| [51] |
Moore-Kucera J, Dick RP (2008). Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids. Soil Biology & Biochemistry, 40, 2485-2493.
DOI URL |
| [52] |
Nguyen NH, Song ZW, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016). FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecology, 20, 241-248.
DOI URL |
| [53] | Ning Q, Chen L, Li F, Zhang CZ, Ma DH, Cai ZJ, Zhang JB (2022). Effects of Mortierella on nutrient availability and straw decomposition in soil. Acta Pedologica Sinica, 59(1), 206-217. |
| [宁琪, 陈林, 李芳, 张丛志, 马东豪, 蔡泽江, 张佳宝 (2022). 被孢霉对土壤养分有效性和秸秆降解的影响. 土壤学报, 59(1), 206-217.] | |
| [54] | Nizamani MM, Hughes AC, Qureshi S, Zhang Q, Tarafder E, Das D, Acharya K, Wang Y, Zhang ZG (2024). Microbial biodiversity and plant functional trait interactions in multifunctional ecosystems. Applied Soil Ecology, 201, 105515. DOI: 10.1016/j.apsoil.2024.105515. |
| [55] | Parasar BJ, Sharma I, Agarwala N (2024). Root exudation drives abiotic stress tolerance in plants by recruiting beneficial microbes. Applied Soil Ecology, 198, 105351. DOI: 10.1016/j.apsoil.2024.105351. |
| [56] |
Philippot L, Chenu C, Kappler A, Rillig MC, Fierer N (2024). The interplay between microbial communities and soil properties. Nature Reviews Microbiology, 22, 226-239.
DOI |
| [57] | Qiao YZ, Wang TT, Huang QW, Guo HY, Zhang H, Xu QC, Shen QR, Ling N (2024). Core species impact plant health by enhancing soil microbial cooperation and network complexity during community coalescence. Soil Biology & Biochemistry, 188, 109231. DOI: 10.1016/j.soilbio.2023.109231. |
| [58] |
Schimel JP, Weintraub MN (2003). The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biology & Biochemistry, 35, 549-563.
DOI URL |
| [59] |
Shen CC, Xiong JB, Zhang HY, Feng YZ, Lin XG, Li XY, Liang WJ, Chu HY (2013). Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biology & Biochemistry, 57, 204-211.
DOI URL |
| [60] |
Singh M, Awasthi A, Soni SK, Singh R, Verma RK, Kalra A (2015). Complementarity among plant growth promoting traits in rhizospheric bacterial communities promotes plant growth. Scientific Reports, 5, 15500. DOI: 10.1038/srep15500.
PMID |
| [61] | Song XW, Zhu FC, Chen CW, Chen NF, Han BX (2019). Development status and existing problems of traditional Chinese medicine industry in Lu’an. Journal of West Anhui University, 35(5), 20-23. |
| [宋向文, 朱富成, 陈存武, 陈乃富, 韩邦兴 (2019). 六安市中药产业发展现状与存在问题研究. 皖西学院学报, 35(5), 20-23.] | |
| [62] |
Sun Y, Xu XL, Kuzyakov Y (2014). Mechanisms of rhizosphere priming effects and their ecological significance. Chinese Journal of Plant Ecology, 38, 62-75.
DOI URL |
|
[孙悦, 徐兴良, Kuzyakov Y (2014). 根际激发效应的发生机制及其生态重要性. 植物生态学报, 38, 62-75.]
DOI |
|
| [63] | Tang YJ, Zhou DY, Dai J, Li Y, Xing YM, Guo SX, Chen J (2022). Potential specificity between mycorrhizal fungi isolated from widespread Dendrobium spp. and rare D. huoshanense seeds. Current Microbiology, 79, 264. DOI: 10.1007/s00284-022-02952-z. |
| [64] |
Tian D, Yang JW, Chen BR, Chi XL, Hu YY, Tang SN, Yang G, Cheng M, Dai YF, Wang SW (2025). Carbon-friendly ecological cultivation mode of Dendrobium huoshanense based on greenhouse gas emission measurement. China Journal of Chinese Materia Medica, 50(1), 93-101.
DOI PMID |
| [田地, 杨俊薇, 陈冰瑞, 池秀莲, 胡严炎, 唐胜男, 杨光, 程蒙, 戴亚峰, 王诗文 (2025). 基于温室气体排放研究的霍山石斛碳友好型生态种植模式. 中国中药杂志, 50(1), 93-101.] | |
| [65] |
Toljander JF, Eberhardt U, Toljander YK, Paul LR, Taylor AFS (2006). Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytologist, 170, 873-884.
PMID |
| [66] |
Vance ED, Brookes PC, Jenkinson DS (1987). An extraction method for measuring soil microbial biomass C. Soil Biology & Biochemistry, 19, 703-707.
DOI URL |
| [67] | Wang SS, Liu JM, Sun J, Sun YF, Liu JN, Jia N, Fan B, Dai XF (2019). Diversity of culture-independent bacteria and antimicrobial activity of culturable endophytic bacteria isolated from different Dendrobium stems. Scientific Reports, 9, 10389. DOI: 10.1038/s41598-019-46863-9. |
| [68] | Wang SW, Huang YH, Shang LL, Zong RY, Yang YJ, Dai YF (2024). Planting technology of Dendrobium huoshanense and its comparative analysis. Modern Agricultural Science and Technology, (14), 209-212. |
| [王诗文, 黄跃华, 尚亮亮, 纵瑞叶, 杨燕杰, 戴亚峰 (2024). 霍山石斛种植技术及其比较分析. 现代农业科技, (14), 209-212.] | |
| [69] | Wang YX, Liu XY, Di HH, He XS, Sun Y, Xiang S, Huang ZB (2024). The mechanism of microbial community succession and microbial co-occurrence network in soil with compost application. Science of the Total Environment, 906, 167409. DOI: 10.1016/j.scitotenv.2023.167409. |
| [70] | Wang ZC, Liu H, Ren Y, Zhu XF, Fu SL (2022). Effects of the growth and major constituents of Dendrobium huoshanense under three cultivation modes. Journal of Anhui Agricultural University, 49, 527-532. |
| [王兆成, 刘华, 任媛, 朱先富, 傅松玲 (2022). 栽培模式对霍山石斛生长和主要内含物的影响. 安徽农业大学学报, 49, 527-532.] | |
| [71] |
Wei CB, Gu W, Tian R, Xu F, Han Y, Ji YY, Li T, Zhu Y, Lang PL, Wu WQ (2022). Comparative analysis of the structure and function of rhizosphere microbiome of the Chinese medicinal herb Alisma in different regions. Archives of Microbiology, 204, 448. DOI: 10.1007/s00203-022-03084-5.
PMID |
| [72] |
Weidner S, Koller R, Latz E, Kowalchuk G, Bonkowski M, Scheu S, Jousset A (2015). Bacterial diversity amplifies nutrient-based plant-soil feedbacks. Functional Ecology, 29, 1341-1349.
DOI URL |
| [73] | Whit TJ, Bruns TD, Lee SB, Taylor JW,(1990). Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics//Innis MA, Gelfand DH, Sninsky JJ, White TJ. PCR Protocols: a Guide to Methods and Applications. Academic Press, New York. 315-322. |
| [74] | Wu QC, Chen Y, Dou XH, Liao DX, Li KY, An CC, Li GH, Dong Z (2024). Microbial fertilizers improve soil quality and crop yield in coastal saline soils by regulating soil bacterial and fungal community structure. Science of the Total Environment, 949, 175127. DOI: 10.1016/j.scitotenv.2024.175127. |
| [75] | Xie GJ, Yin ZC, Zhang ZL, Wang XY, Sun CB (2024). Microbial diversity and potential functional dynamics within the rhizocompartments of Dendrobium huoshanense. Frontiers in Plant Science, 15, 1450716. DOI: 10.3389/fpls.2024.1450716. |
| [76] | Xu GT (2011). Study on methods of improving the survival rate of bionic cultivation of Dendrobium huoshanense. Anhui Forestry Science and Technology, 37(6), 62-64. |
| [徐光涛 (2011). 提高霍山石斛仿生态栽培成活率方法的研究. 安徽林业科技, 37(6), 62-64.] | |
| [77] | Xu LJ, He JD, Meng Y, Zheng YY, Lu B, Zhang JW, Zhou Y (2024). Enhancing drought resistance in Pinus tabuliformis seedlings through root symbiotic fungi inoculation. Frontiers in Plant Science, 15, 1446437. DOI: 10.3389/fpls.2024.1446437. |
| [78] |
Yadav AN (2022). Beneficial plant-microbe interactions for agricultural sustainability. Journal of Applied Biology and Biotechnology, 9(1), 1-4.
DOI URL |
| [79] |
Yafetto L (2018). The structure of mycelial cords and rhizomorphs of fungi: a minireview. Mycosphere, 9, 984-998.
DOI URL |
| [80] | Yan ZX, Li Y, Peng SY, Wei L, Zhang B, Deng XY, Zhong M, Cheng X (2024). Cadmium biosorption and mechanism investigation using two cadmium-tolerant microorganisms isolated from rhizosphere soil of rice. Journal of Hazardous Materials, 470, 134134. DOI: 10.1016/j.jhazmat.2024.134134. |
| [81] | Yuan XT, Wu MC (2016). Analysis of the characteristics of temperature and precipitation in recent 50 years in Huoshan County. Journal of Suzhou University, 31(12), 91-96. |
| [袁新田, 吴茂成 (2016). 霍山县近50年气温和降水变化特征研究. 宿州学院学报, 31(12), 91-96.] | |
| [82] | Yue JY, Yu Y (2022). Isolation and identification of endophytic fungi from Dendrobium huoshanense with their antibacterial and anti-inflammatory activities. Pakistan Journal of Pharmaceutical Sciences, 35, 1143-1151. |
| [83] | Zhang J (2012). Research on Diversity of Endosymbiotic Fungi in Roots of Cymbidium. Master degree dissertation, Chinese Academy of Forestry, Beijing. |
| [张辑 (2012). 中国兰属植物内生菌多样性研究. 硕士学位论文, 中国林业科学研究院, 北京.] | |
| [84] | Zhang WD, Wang XF, Wang SL (2013). Addition of external organic carbon and native soil organic carbon decomposition: a meta-analysis. PLoS ONE, 8, e54779. DOI: 10.1371/journal.pone.0054779. |
| [85] | Zhu KY, Wang QC, Zhang Y, Zarif N, Ma SJ, Xu LQ (2022). Variation in soil bacterial and fungal community composition at different successional stages of a broad-leaved Korean pine forest in the Lesser Hinggan Mountains. Forests, 13, 625. DOI: 10.3390/f13040625. |
| [1] | 王蓉钧, 吴福忠, 吴秋霞, 朱晶晶, 倪祥银. 不同生活型植物叶片氮重吸收效率的差异[J]. 植物生态学报, 2026, 50(化学计量与功能性状): 1-. |
| [2] | 张静, 陈洁, 李艳朋, 盘李军, 许涵, 李意德, 何海生. 南亚热带针阔混交人工林植物生物量比较及其影响因子分析[J]. 植物生态学报, 2026, 50(化学计量与功能性状): 0-. |
| [3] | 张法伟, 李红琴, 祝景彬, 樊博, 周华坤, 李英年, 梁乃申. 氮添加和降水改变对高寒草甸生态系统地上与地下碳储的影响[J]. 植物生态学报, 2025, 49(9): 1399-1409. |
| [4] | 邢强, 赵斌, 胡永红, 杨君, 秦俊, 刘何铭, 王红兵, 周鹏. 华东地区两种典型立体绿化植物根系性状特征及对新型土壤基质的响应[J]. 植物生态学报, 2025, 49(9): 1498-1514. |
| [5] | 贾紫璇, 方涛, 张舒欣, 刘一凡, 赵微, 王荣, 昌海超, 朱耀军, 罗芳丽, 郭允倩, 于飞海. 不同沼泽湿地芦苇地上-地下性状对水分变化的响应[J]. 植物生态学报, 2025, 49(9): 1448-1460. |
| [6] | 陈刚刚, 朱思洁, 郭亮娜, 付芳伟, 刘昱灼, 李江荣. 藏东南色季拉山高山树线乔灌地上-地下养分分配策略[J]. 植物生态学报, 2025, 49(9): 1515-1526. |
| [7] | 宋珊珊, 唐志尧. 河北塞罕坝草甸草原根际土壤真菌与植物地上生物量的关系[J]. 植物生态学报, 2025, 49(9): 1461-1471. |
| [8] | 樊月玲, 蒋正德, 叶佳舒, 郑立臣, 陈欣. 2005-2015年下辽河平原农田长期观测样地主要农作物收获期性状和产量数据集[J]. 植物生态学报, 2025, 49(8): 1271-1282. |
| [9] | 马腾飞, 郝杰, 刁华杰, 宁亚楠, $\boxed{\hbox{王常慧}}$, 董宽虎. 晋北农牧交错带草地土壤无机氮含量的季节变化及其对放牧强度的响应[J]. 植物生态学报, 2025, 49(6): 965-974. |
| [10] | 刘新月, 王立平, 刘春和, 孙艳丽, 刘鹏, 田赟, 贾昕, 查天山, 钱多. 北京不同林龄人工林生物量空间格局及其影响因素[J]. 植物生态学报, 2025, 49(6): 939-951. |
| [11] | 杜英杰, 范爱连, 王雪, 闫晓俊, 陈廷廷, 贾林巧, 姜琦, 陈光水. 亚热带天然常绿阔叶林乔木树种与林下灌木树种根-叶功能性状协调性及差异[J]. 植物生态学报, 2025, 49(4): 585-595. |
| [12] | 李欣怡, 张丽芳, 吴友贵, 郭静, 兰荣光, 吕洪飞, 于明坚. 不同海拔高度下百山祖冷杉幼苗的生长特征及其影响因素[J]. 植物生态学报, 2025, 49(4): 610-623. |
| [13] | 李梦琦, 苗灵凤, 李大东, 龙奕帆, 叶冰冰, 杨帆. 海南东寨港红树林植物细根功能性状对不同潮位沉积物养分变化的响应[J]. 植物生态学报, 2025, 49(4): 552-561. |
| [14] | 李冬梅, 孙龙, 韩宇, 胡同欣, 杨光, 蔡慧颖. 计划火烧对红松人工林生物多样性与生态系统多功能性关系的影响[J]. 植物生态学报, 2025, 49(3): 379-392. |
| [15] | 陈文义, 王智勇, 周梦岩, 麻文俊, 王军辉, 罗志斌, 周婧. 幼龄楸树生物量分配规律与异速生长模型[J]. 植物生态学报, 2025, 49(2): 356-366. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2026 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()