

植物生态学报 ›› 2010, Vol. 34 ›› Issue (8): 946-956.DOI: 10.3773/j.issn.1005-264x.2010.08.007
刘万德1, 臧润国2,*( ), 丁易2, 张炜银2, 苏建荣1, 杨民3, 蔡笃磊3, 李儒财3
), 丁易2, 张炜银2, 苏建荣1, 杨民3, 蔡笃磊3, 李儒财3
收稿日期:2010-01-19
接受日期:2010-05-07
出版日期:2010-01-19
发布日期:2010-09-28
通讯作者:
臧润国
作者简介:* E-mail: zangrung@caf.ac.cn
LIU Wan-De1, ZANG Run-Guo2,*( ), DING Yi2, ZHANG Wei-Yin2, SU Jian-Rong1, YANG Min3, CAI Du-Lei3, LI Ru-Cai3
), DING Yi2, ZHANG Wei-Yin2, SU Jian-Rong1, YANG Min3, CAI Du-Lei3, LI Ru-Cai3
Received:2010-01-19
Accepted:2010-05-07
Online:2010-01-19
Published:2010-09-28
Contact:
ZANG Run-Guo
摘要:
树木死亡是调节群落组成和结构的一种方式, 在森林生态系统动态中发挥着重要作用。该文在对海南岛霸王岭热带季雨林群落大量调查和树种功能群划分研究的基础上, 探索了热带季雨林群落及其不同功能群树木的死亡率及其随径级和环境条件的变化规律。结果表明: 海南岛霸王岭热带季雨林群落内树木死亡率的变化范围为3.42%-18.71%, 平均值为7.60%。按照功能群比较, 乔木死亡率显著高于灌木, 落叶树种死亡率高于常绿树种, 但具刺树种死亡率显著低于无刺树种。按照树木径级划分, 5-30 cm径级范围的死亡率均超过10%, 最高值出现在5-10 cm径级范围, 在相同径级范围内比较时, 落叶树种在 胸径(DBH ) < 5 cm时的死亡率显著高于常绿树种, 而在其他径级范围内, 二者之间无显著差异, 具刺和无刺树种的死亡率在所有径级范围内均无显著差异。乔木死亡率与群落距河流距离具有显著的相关性, 距离河流越远死亡率越高; 落叶树种和无刺树种则与坡位存在显著的相关性, 坡上部的死亡率显著高于坡中部。热带季雨林内树木的死亡率与其所处的群落生境有关, 较干旱的生境是导致树木死亡率较高的重要原因。
刘万德, 臧润国, 丁易, 张炜银, 苏建荣, 杨民, 蔡笃磊, 李儒财. 海南岛霸王岭热带季雨林树木的死亡率. 植物生态学报, 2010, 34(8): 946-956. DOI: 10.3773/j.issn.1005-264x.2010.08.007
LIU Wan-De, ZANG Run-Guo, DING Yi, ZHANG Wei-Yin, SU Jian-Rong, YANG Min, CAI Du-Lei, LI Ru-Cai. Mortality of woody plants in tropical monsoon rainforests of Bawangling National Nature Reserve on Hainan Island, South China. Chinese Journal of Plant Ecology, 2010, 34(8): 946-956. DOI: 10.3773/j.issn.1005-264x.2010.08.007
| 指标 Index | 最大值 Maximum | 最小值 Minimum | 平均值 Mean |
|---|---|---|---|
| 死亡株数 No. of dead individuals (ind.·hm-2) | 2 136 | 164 | 679.6 |
| 树木死亡率 Stem mortality rate (%) | 18.71 | 3.42 | 7.60 |
表1 热带季雨林内树木死亡株数及死亡率
Table 1 Number of dead individuals and stem mortality rate in the tropical monsoon rainforest
| 指标 Index | 最大值 Maximum | 最小值 Minimum | 平均值 Mean |
|---|---|---|---|
| 死亡株数 No. of dead individuals (ind.·hm-2) | 2 136 | 164 | 679.6 |
| 树木死亡率 Stem mortality rate (%) | 18.71 | 3.42 | 7.60 |
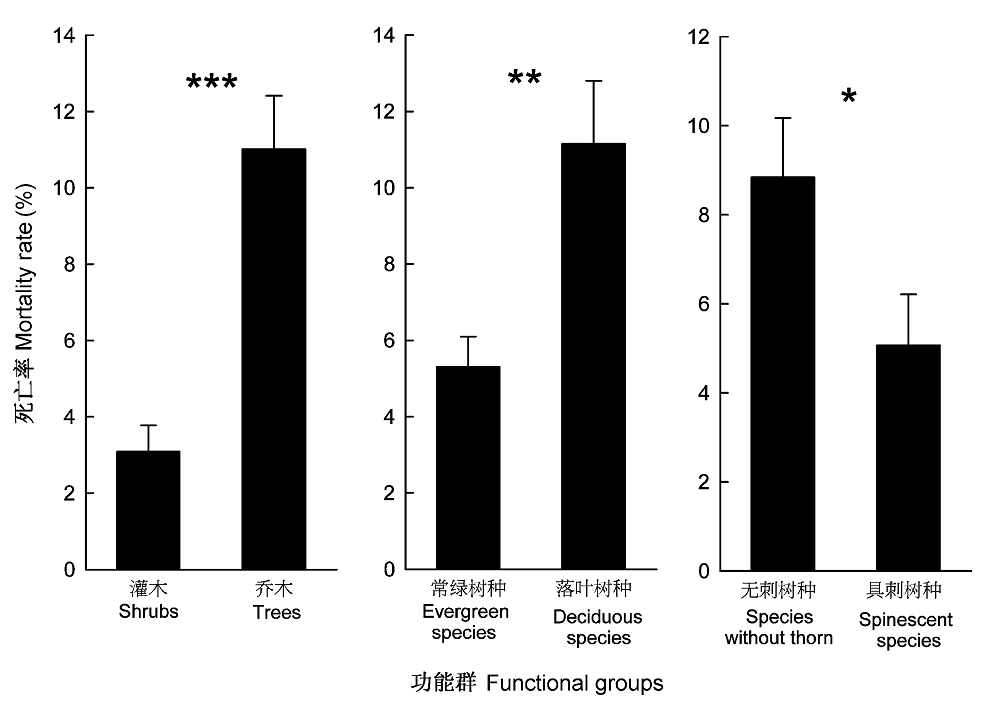
图1 热带季雨林内不同功能群的死亡率。
Fig. 1 Mortality rates of different functional groups in the tropical monsoon rainforest. * , p < 0.05, **, p < 0.01, *** , p < 0.001.
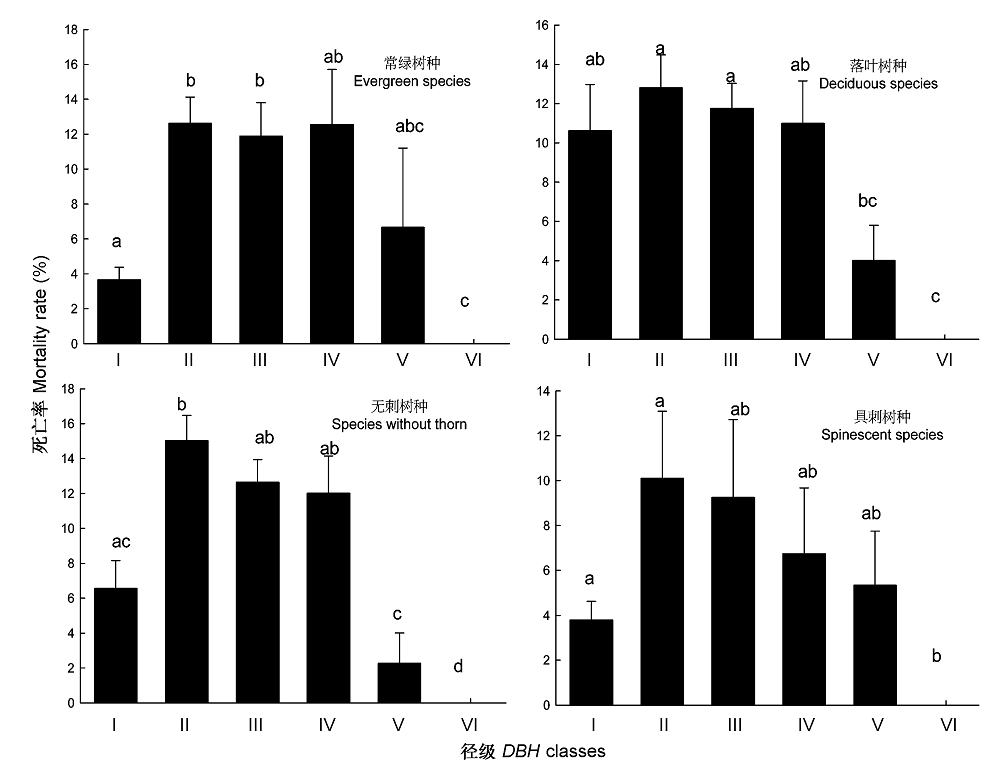
图2 各功能群树种在不同径级范围的死亡率。 柱状图顶部字母的不同表示存在显著性差异(p < 0.05)。DBH, 胸径。
Fig. 2 Mortality rate of different functional groups in different diameter classes. Different letters at the tops of bars indicate significant difference (p < 0.05). DBH, diameter of breast height. I, 1 cm ≤ DBH < 5 cm; II, 5 cm ≤ DBH < 10 cm; III, 10 cm ≤ DBH < 20 cm; IV, 20 cm ≤ DBH < 30 cm; V, 30 cm ≤ DBH < 40 cm; VI, DBH ≥ 40 cm.
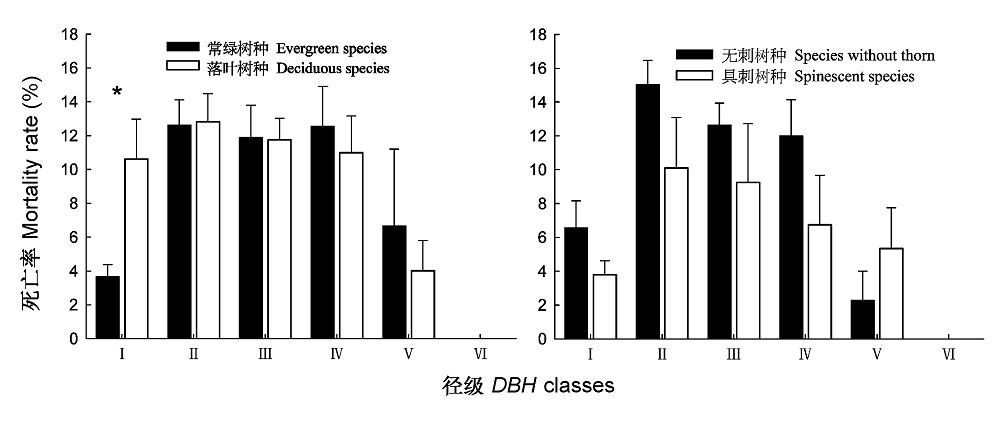
图3 不同功能群树种在相同径级范围的死亡率。 DBH、I-VI同图2。
Fig. 3 Mortality rates of different functional groups species in the same DBH classes. DBH, I-VI see Fig. 2. * , p < 0.05.
| 功能群 Functional group | 距河流距离 Distance to rivers | 坡向 Slope aspect | 坡位 Slope position | 坡度 Slope gradient | 郁闭度 Canopy density | 裸岩面积 Area of exposed rock | 海拔 Elevation |
|---|---|---|---|---|---|---|---|
| 灌木 Shrub | 0.47 | -0.24 | 0.05 | 0.05 | 0.20 | 0.22 | 0.27 |
| 乔木 Tree | 0.83** | -0.48 | -0.14 | 0.02 | 0.29 | 0.03 | 0.54 |
| 常绿树种 Evergreen species | 0.34 | -0.28 | -0.09 | -0.02 | 0.31 | -0.39 | 0.41 |
| 落叶树种 Deciduous species | 0.21 | -0.05 | -0.52* | 0.36 | 0.03 | 0.42 | -0.12 |
| 无刺树种 Species without thorn | 0.38 | -0.35 | -0.54* | 0.16 | 0.18 | 0.13 | 0.01 |
| 具刺树种 Spinescent species | 0.42 | 0.12 | -0.15 | 0.49 | 0.27 | 0.23 | 0.25 |
| 合计 Total | 0.37 | -0.28 | -0.41 | 0.27 | 0.13 | -0.12 | 0.19 |
表2 树木死亡率和环境变量之间的相关性
Table 2 Correlation analysis between mortality rates and environment variables
| 功能群 Functional group | 距河流距离 Distance to rivers | 坡向 Slope aspect | 坡位 Slope position | 坡度 Slope gradient | 郁闭度 Canopy density | 裸岩面积 Area of exposed rock | 海拔 Elevation |
|---|---|---|---|---|---|---|---|
| 灌木 Shrub | 0.47 | -0.24 | 0.05 | 0.05 | 0.20 | 0.22 | 0.27 |
| 乔木 Tree | 0.83** | -0.48 | -0.14 | 0.02 | 0.29 | 0.03 | 0.54 |
| 常绿树种 Evergreen species | 0.34 | -0.28 | -0.09 | -0.02 | 0.31 | -0.39 | 0.41 |
| 落叶树种 Deciduous species | 0.21 | -0.05 | -0.52* | 0.36 | 0.03 | 0.42 | -0.12 |
| 无刺树种 Species without thorn | 0.38 | -0.35 | -0.54* | 0.16 | 0.18 | 0.13 | 0.01 |
| 具刺树种 Spinescent species | 0.42 | 0.12 | -0.15 | 0.49 | 0.27 | 0.23 | 0.25 |
| 合计 Total | 0.37 | -0.28 | -0.41 | 0.27 | 0.13 | -0.12 | 0.19 |
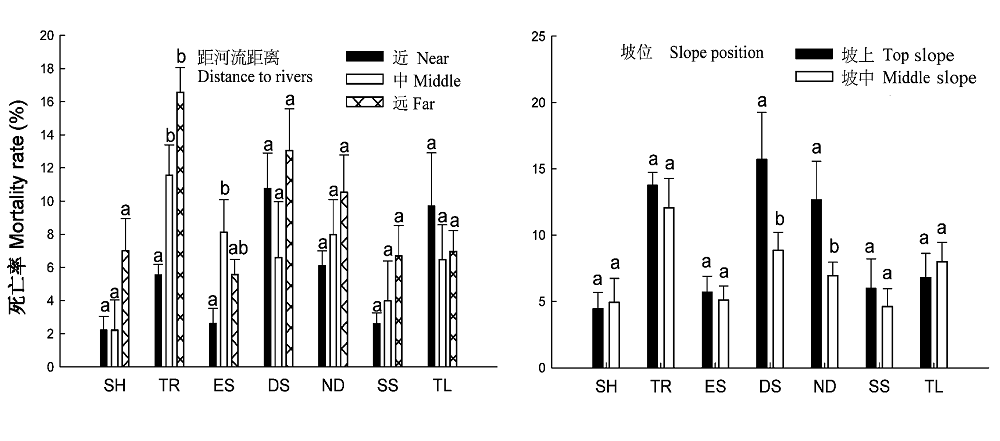
图4 不同功能群随距河流距离及坡位的死亡率变化。 柱状图顶部字母的不同表示存在显著性差异(p < 0.05)。SH, 灌木; TR, 乔木; ES, 常绿树种; DS, 落叶树种; ND, 无刺树种; SS, 具刺树种; TL, 合计。
Fig. 4 Variations of mortality rates of different functional groups with site conditions and slops position. Different letters at the tops of bars indicate significant difference (p < 0.05). SH, shrub; TR, tree; ES, evergreen species; DS, deciduous species; ND, species without thorn; SS, spinescent species; TL, total.
| [1] | Bebi P, Kulakowski D, Veblen TT (2003). Interactions between fire and spruce beetles in a subalpine Rocky Mountain forest landscape. Ecology, 84, 362-371. |
| [2] | Bigler C, Bräker OU, Bugmann H, Dobbertin M, Rigling A (2006). Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems, 9, 330-343. |
| [3] | Bigler C, Gavin DG, Gunning C, Veblen TT (2007). Drought induces lagged tree mortality in a subalpine forest in the Rocky Mountains. Oikos, 116, 1983-1994. |
| [4] | Bigler C, Kulakowski D, Veblen TT (2005). Multiple disturbance interactions and drought influence fire severity in Rocky Mountain subalpine forests. Ecology, 86, 3018-3029. |
| [5] | Bohlman SA, Adams JB, Smith MO, Peterson DL (1998). Seasonal foliage changes in the Eastern Amazon basin detected from Landsat thematic mapped images. Biotropica, 30, 376-391. |
| [6] | Boisvenue C, Running SW (2006). Impacts of climate change on natural forest productivity―evidence since the middle of the 20th century. Global Change Biology, 12, 1-12. |
| [7] |
Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG, Romme WH, Kastens JH, Floyd ML, Belnap J, Anderson JJ, Myers OB, Meyer CW (2005). Regional vegetation die-off in response to global-change-type drought. Proceedings of the National Academy of Sciences of the United States of America, 102, 15144-15148.
DOI URL PMID |
| [8] | Bullock SH, Mooney HA, Medina E (1995). Seasonally Dry Tropical Forests. Cambridge University Press, Cambridge, UK. 68-95. |
| [9] | Chao KJ, Phillips OL, Gloor E, Monteagudo A, Torres-Lezama A, Martinez RV (2008). Growth and wood density predict tree mortality in Amazon forests. Journal of Ecology, 96, 281-292. |
| [10] | Condit R, Hubbell SP, Foster RB (1995). Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecological Monographs, 65, 419-439. |
| [11] | Condit R, Watts K, Bohlman SA, Perez R, Hubbell SP, Foster RB (2000). Quantifying the deciduousness of tropical forest canopies under varying climates. Journal of Vegetation Science, 11, 649-658. |
| [12] | Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003). A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany, 51, 335-380. |
| [13] |
Dale VH, Joyceb LA, McNultyc S, Neilsond RP (2000). The interplay between climate change, forest, and disturbances. The Science of the Total Environment, 262, 201-204.
URL PMID |
| [14] |
Daniel CN, Ingrid MT, David R, Paulo M, Georgina C (2007). Mortality of large trees and lianas following experimental drought in Amazon forest. Ecology, 88, 2259-2269.
URL PMID |
| [15] |
Das A, Battles J, van Mantgem PJ, Stephenson NL (2008). Spatial elements of mortality risk in old-growth forests. Ecology, 89, 1744-1756.
DOI URL PMID |
| [16] | Diffenbaugh NS, Pal JS, Trapp RJ, Giorgi F (2005). Fine-scale processes regulate the response of extreme events to global climate change. Proceedings of the National Academy of Sciences of the United States of the America, 102, 15774-15778. |
| [17] | Franklin JF, Shugart HH, Harmon ME (1987). Tree death as an ecological process. BioScience, 37, 550-556. |
| [18] |
Frazier MR, Huey RB, Berrigan D (2006). Thermodynamics constrains the evolution of insect population growth rates: “Warmer is better”. The American Naturalist, 168, 512-520.
DOI URL PMID |
| [19] | Guarín A, Taylor AH (2005). Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. Forest Ecology and Management, 218, 229-244. |
| [20] | Hanson PJ, Weltzin JF (2000). Drought disturbance from climate change: response of United States forests. Science of the Total Environment, 262, 205-220. |
| [21] | Hughes RF, Archer SR, Asner GP, Wessman CA, McMurtry C, Nelson J, Ansley RJ (2006). Changes in aboveground primary production and carbon and nitrogen pools accompanying woody plant encroachment in a temperate savanna. Global Change Biology, 12, 1733-1747. |
| [22] | Hursh CR, Haasis FW (1931). Effects of 1925 summer drought on southern Appalachian hardwoods. Ecology, 12, 380-386. |
| [23] | Katzner TE, Bragin EA, Milner-Gulland EJ (2006). Modelling populations of long-lived birds of prey for conservation: a study of imperial eagles in Kazakhstan. Biological Conservation, 132, 322-335. |
| [24] | Kobe RK, Pacala SW, Silander JA, Canham CD (1995). Juvenile tree survivorship as a component of shade tolerance. Ecological Applications, 5, 517-532. |
| [25] | Kulakowski D, Veblen TT (2002). Influences of fire history and topography on the pattern of a severe wind blowdown in a Colorado subalpine forest. Journal of Ecology, 90, 806-819. |
| [26] | Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008). Mountain pine beetle and forest carbon feedback to climate change. Nature, 452, 987-990. |
| [27] | Lapenis A, Shvidenkow A, Shepascheko D, Ennilsson S, Aiyyer A (2005). Acclimation of Russian forests to recent changes in climate. Global Change Biology, 11, 2090-2102. |
| [28] |
Laurance WF, Oliveira AA, Laurance SG, Condit R, Nascimento HEM, Sanchez-Thorin AC, Lovejoy TE, Andrade A, D’Angelo S, Ribeiro JE, Dick CW (2004). Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature, 428, 171-175.
URL PMID |
| [29] |
Linares C, Doak DF, Coma R, Diaz D, Zabala M (2007). Life history and viability of a long-lived marine invertebrate: the octocoral Paramuricea clavata. Ecology, 88, 918-928.
DOI URL PMID |
| [30] | Lorimer CG, Dahir SE, Nordheim EV (2001). Tree mortality rates and longevity in mature and old-growth hemlock- hardwood forests. Journal of Ecology, 89, 960-971. |
| [31] | MacGregor SD, O’Connor TG (2002). Patch dieback of Colophospermum mopane in a dysfunctional semi-arid African savanna. Austral Ecology, 27, 385-395. |
| [32] | Marbà N, Duarte CM, Agustí S (2007). Allometric scaling of plant life history. Proceedings of the National Academy of Sciences of the United States of the America, 104, 15777-15780. |
| [33] |
McCoy MW, Gillooly JF (2008). Predicting natural mortality rates of plants and animals. Ecology Letters, 11, 710-716.
URL PMID |
| [34] | McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178, 719-739. |
| [35] | McDowell NG, Allen CD, Marshall L (2010). Growth, carbon- isotope discrimination, and drought-associated mortality across a Pinus ponderosa elevational transect. Global Change Biology, 16, 399-415. |
| [36] | Mueller RC, Scudder CM, Porter ME, Talbot Trotter III R, Gehring CA, Whitham TG (2005). Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. Journal of Ecology, 93, 1085-1093. |
| [37] | Nakagawa M, Tanaka K, Nakashizuka T, Ohkubo T, Kato T, Maeda T, Sato K, Miguchi H, Nagamasu H, Ogino K, Teo S, Hamid AA, Seng LH (2000). Impact of severe drought associated with the 1997-1998 El Niño in a tropical forest in Sarawak. Journal of Tropical Ecology, 16, 355-367. |
| [38] |
Newman MJH, Paredes GA, Sala E, Jackson JBC (2006). Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecology Letters, 9, 1216-1227.
DOI URL PMID |
| [39] | Ogle K, Whitham TG, Cobb NS (2000). Tree-ring variation in pinyon predicts likelihood of death following severe drought. Ecology, 81, 3237-3243. |
| [40] | Pacala SW, Canham CD, Saponara J, Silander JA, Kobe RK, Ribbens E (1996). Forest models defined by field measurements: estimation, error analysis and dynamics. Ecological Monographs, 66, 1-43. |
| [41] |
Rice KJ, Matzner SL, Byer W, Brown JR (2004). Patterns of tree dieback in Queensland, Australia: the importance of drought stress and the role of resistance to cavitation. Oecologia, 139, 190-198.
URL PMID |
| [42] |
Rich PM, Breshears DD, White AB (2008). Phenology of mixed woody-herbaceous ecosystems following extreme events: net and differential responses. Ecology, 89, 342-352.
URL PMID |
| [43] | Romme WH, Despain DG (1989). Historical perspective on the Yellowstone fires of 1988. BioScience, 39, 695-699. |
| [44] | Sibold JS, Veblen TT (2006). Relationships of subalpine forest fires in the Colorado Front Range with interannual and multi-decadal scale climatic variation. Journal of Biogeography, 33, 833-842. |
| [45] | Simonin K, Kolb T, Helu M, Koch G (2007). The influence of thinning on components of stand water balance in a ponderosa pine forest stand during and after extreme drought. Agricultural and Forest Meteorology, 143, 266-276. |
| [46] |
Slik JWF (2004). El Niño droughts and their effects on tree species composition and diversity in tropical rain forests. Oecologia, 141, 114-120.
DOI URL PMID |
| [47] | Stephenson NL (1998). Actual evapotranspiration and deficit: biologically meaningful correlates of vegetation distribution across spatial scales. Journal of Biogeography, 25, 855-870. |
| [48] | Sthultz CM, Gehring CA, Whitham TG (2009). Deadly combination of genes and drought: increased mortality of herbivore-resistant trees in a foundation species. Global Change Biology, 15, 1949-1961. |
| [49] | Suzuki RO, Kudoh H, Kachi N (2003). Spatial and temporal variations in mortality of the biennial plant, Lysimachia rubida: effects of intraspecific competition and environmental heterogeneity. Journal of Ecology, 91, 114-125. |
| [50] | Swaty RL, Deckert RJ, Whitham TG, Gehring CA (2004). Ectomycorrhizal abundance and community composition shifts with drought: predictions from tree rings. Ecology, 85, 1072-1084. |
| [51] |
van Mantgem PJ, Stephenson NL (2007). Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecology Letters, 10, 909-916.
DOI URL PMID |
| [52] | van Mantgem PJ, Stephenson NL, Keifer M, Keeley J (2004). Effects of an introduced pathogen and fire exclusion on the demography of sugar pine. Ecological Applications, 14, 1590-1602. |
| [53] |
van Nieuwstadt MGL, Sheil D (2005). Drought, fire and tree survival in a Borneo rain forest, East Kalimantan, Indonesia. Journal of Ecology, 93, 191-201.
URL PMID |
| [54] | Veblen TT, Hadley KS, Reid MS, Rebertus AJ (1989). Blowdown and stand development in a Colorado subalpine forest. Canadian Journal of Forest Research, 19, 1218-1225. |
| [55] | Whitmore TC (1984). Tropical Rain Forests of the Far East. 2nd edn. Clarendon Press, Oxford. 94-128. |
| [56] | Wilcox BP, Breshears DD, Allen CD (2003). Ecohydrology of a resource-conserving semiarid woodland: effects of scaling and disturbance. Ecological Monographs, 73, 223-239. |
| [57] | Williamson GB, Laurance WF, Oliveira AA, Delamônica P, Gascon C, Lovejoy TE, Pohl L (2000). Amazonia tree mortality during the 1997 El Niño drought. Conservation Biology, 14, 1538-1542. |
| [58] |
Woodman JN (1987). Pollution-induced injury in North American forests: facts and suspicions. Tree Physiology, 3, 1-15.
URL PMID |
| [59] | Wyckoff PH, Clark JS (2002). The relationship between growth and mortality for seven co-occurring tree species in the southern Appalachian Mountains. Journal of Ecology, 90, 604-615. |
| [60] | Yamasaki M, Sakimoto M (2009). Predicting oak tree mortality caused by the ambrosia beetle Platypus quercivorus in a cool-temperate forest. Journal of Applied Entomology, 133, 673-681. |
| [61] | Yarranton M, Yarranton GA (1975). Demography of a jack pine stand. Canadian Journal of Botany, 53, 310-314. |
| [62] | Young TP, Stanton ML, Christian CE (2003). Effects of natural and simulated herbivory on spine lengths of Acacia drepanolobium in Kenya. Oikos, 101, 171-179. |
| [63] |
Zhang YJ, Meinzer FC, Hao GY, Scholz FG, Bucci SJ, Takahashi FSC, Villalobos-Vega R, Giraldo JP, Cao KF, Hoffmann WA, Goldstein G (2009). Size-dependent mortality in a Neotropical savanna tree: the role of height-related adjustments in hydraulic architecture and carbon allocation. Plant, Cell & Environment, 32, 1456-1466.
URL PMID |
| [1] | 江康威 张青青 王亚菲 李宏 丁雨 杨永强 吐尔逊娜依·热依木. 放牧干扰下天山北坡中段植物功能群特征及其与土壤环境因子的关系[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 周建 王焓. 森林径级结构研究:从统计描述到理论演绎[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [3] | 盘远方, 潘良浩, 邱思婷, 邱广龙, 苏治南, 史小芳, 范航清. 中国沿海红树林树高变异与环境适应机制[J]. 植物生态学报, 2024, 48(4): 483-495. |
| [4] | 赵艳超, 陈立同. 土壤养分对青藏高原高寒草地生物量响应增温的调节作用[J]. 植物生态学报, 2023, 47(8): 1071-1081. |
| [5] | 李安艳, 黄先飞, 田源斌, 董继兴, 郑菲菲, 夏品华. 贵州草海草-藻型稳态转换过程中叶绿素a的变化及其影响因子[J]. 植物生态学报, 2023, 47(8): 1171-1181. |
| [6] | 赵孟娟, 金光泽, 刘志理. 阔叶红松林3种典型蕨类叶功能性状的垂直变异[J]. 植物生态学报, 2023, 47(8): 1131-1143. |
| [7] | 李伟, 张荣. 亚高寒草甸群落结构决定群落生产力实例验证[J]. 植物生态学报, 2023, 47(5): 713-723. |
| [8] | 杨丽琳, 邢万秋, 王卫光, 曹明珠. 新安江源区杉木树干液流速率变化及其对环境因子的响应[J]. 植物生态学报, 2023, 47(4): 571-583. |
| [9] | 何春梅, 李雨姗, 尹秋龙, 贾仕宏, 郝占庆. 秦岭皇冠暖温性落叶阔叶林优势树种的径级结构和数量特征[J]. 植物生态学报, 2023, 47(12): 1658-1667. |
| [10] | 张潇, 武娟娟, 贾国栋, 雷自然, 张龙齐, 刘锐, 吕相融, 代远萌. 降水控制对侧柏液流变化特征及其水分来源的影响[J]. 植物生态学报, 2023, 47(11): 1585-1599. |
| [11] | 赵镇贤, 陈银萍, 王立龙, 王彤彤, 李玉强. 河西走廊荒漠区不同功能类群植物叶片建成成本的比较[J]. 植物生态学报, 2023, 47(11): 1551-1560. |
| [12] | 郑宁, 李素英, 王鑫厅, 吕世海, 赵鹏程, 臧琛, 许玉珑, 何静, 秦文昊, 高恒睿. 基于环境因子对叶绿素影响的典型草原植物生活型优势研究[J]. 植物生态学报, 2022, 46(8): 951-960. |
| [13] | 彭鑫, 金光泽. 植物特性和环境因子对阔叶红松林暗多样性的影响[J]. 植物生态学报, 2022, 46(6): 656-666. |
| [14] | 王子龙, 胡斌, 包维楷, 李芳兰, 胡慧, 韦丹丹, 杨婷惠, 黎小娟. 西南干旱河谷植物群落组分生物量的纬度格局及其影响因素[J]. 植物生态学报, 2022, 46(5): 539-551. |
| [15] | 王俐爽, 同小娟, 孟平, 张劲松, 刘沛荣, 李俊, 张静茹, 周宇. 辽西半干旱地区两种典型人工林生态系统能量通量及蒸散特征[J]. 植物生态学报, 2022, 46(12): 1508-1522. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2026 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()