

Chin J Plant Ecol ›› 2017, Vol. 41 ›› Issue (8): 815-825.DOI: 10.17521/cjpe.2017.0018
Special Issue: 菌根真菌
• Research Articles • Next Articles
Li-Jiao XU1,2, Xue-Lian JIANG1,3, Zhi-Peng HAO1, Tao LI1, Zhao-Xiang WU1,4, Bao-Dong CHEN1,2,*( )
)
Online:2017-08-10
Published:2017-09-29
Contact:
Bao-Dong CHEN
About author:KANG Jing-yao(1991-), E-mail: Li-Jiao XU, Xue-Lian JIANG, Zhi-Peng HAO, Tao LI, Zhao-Xiang WU, Bao-Dong CHEN. Arbuscular mycorrhiza improves plant adaptation to phosphorus deficiency through regulating the expression of genes relevant to carbon and phosphorus metabolism[J]. Chin J Plant Ecol, 2017, 41(8): 815-825.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2017.0018
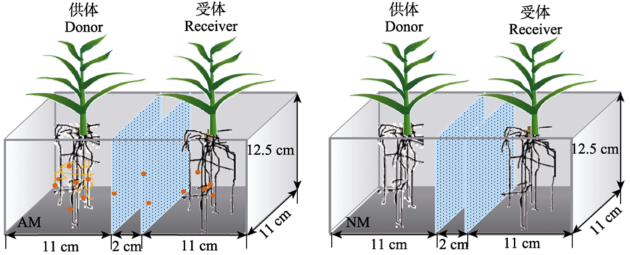
Fig. 1 Diagram of the compartment cultivation system. Different compartments were separated by microporous filter with pore size of 0.45 μm. AM and NM represent inoculation of donor plants with AM fungus and the non-mycorrhizal control respectively. There are two phosphorus levels (10 mg?kg-1 and 100 mg?kg-1), and three replications for each treatment (n = 3).
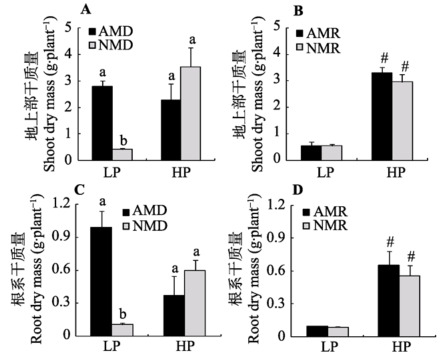
Fig. 2 Effects of mycorrhizal inoculation on maize dry mass under different P levels (mean ± SD). LP and HP refer to low P level (10 mg·kg-1) and high P level (100 mg·kg-1) respectively. AMD and NMD represent donor plants with and without AM fungus incubation, while AMR and NMR represent receiver plants with and without AM exudates respectively. Different letters above the columns indicate significant difference (p < 0.05) between corresponding treatments. # indicates significant difference (p < 0.05) between different P levels under the same inoculation treatment.
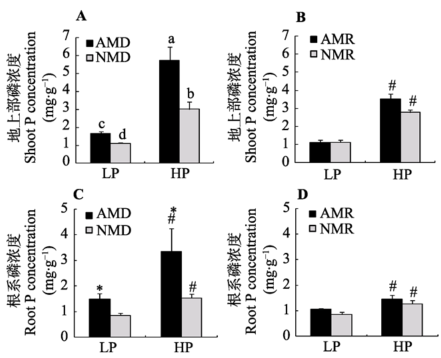
Fig. 3 Effects of inoculation with AM fungus on maize P concentrations under different P levels (mean ± SD). LP and HP refer to low P level (10 mg·kg-1) and high P level (100 mg·kg-1) respectively. AMD and NMD represent donor plants with and without AM fungus incubation, while AMR and NMR represent receiver plants with and without AM exudates respectively. The different letters indicates significant difference (p < 0.05) between corresponding treatments. # indicates significant difference (p < 0.05) between different P levels under the same inoculation treatment; * indicates significant difference (p < 0.05) between inoculation treatments under the same P level.
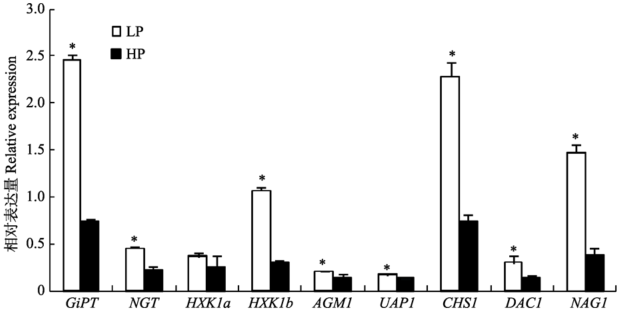
Fig. 4 Expression of AM fungal genes relevant to C and P metabolisms under different P levels (mean ± SD). LP refers to low P treatments, HP refers to high P treatments, * indicates significant difference (p < 0.05) between different P levels. GiPT, AM fungal P transporter gene; NGT1, GlcNAc transporter gene, HXK1b, GlcNAc kinase gene; AGM1, GlcNAc phosphomutase gene; UAP1, UDP GlcNAc pyrophosphorylase gene; CHS1, chitin synthase gene; DAC1, GlcNAc-6-phosphate deacetylase gene; NAG1, glucosamine- 6-phosphate isomerase gene.
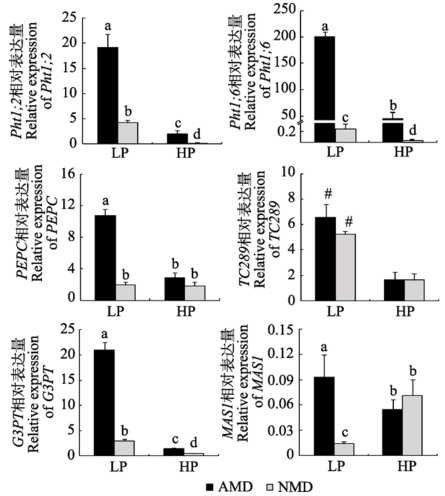
Fig. 5 Expression of genes relevant to C and P metabolism in maize roots from donor compartment under different P levels (mean ± SD). LP and HP refer to low P level (10 mg?kg-1) and high P level (100 mg?kg-1) respectively. AMD and NMD represent donor plants with and without AM fungus. Different letters above the columns indicate significant difference (p < 0.05) between corresponding treatments. # indicates significant difference (p < 0.05) between different P levels. Pht1;2, Pht1;6, P transporter genes; PEPC, phosphoenolpiruvate carboxylase gene; TC289, inorganic pyrophosphatase gene; G3PT, glycerol- 3-phosphate transporter gene; MAS1, malate synthase gene.
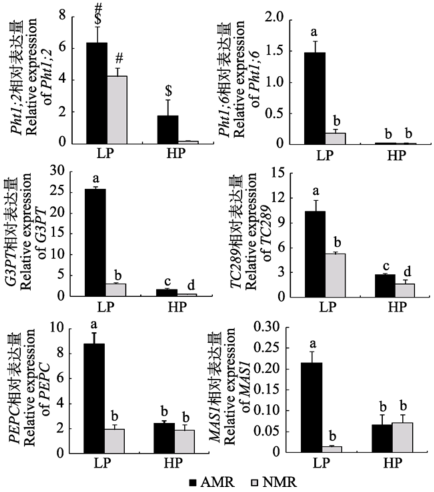
Fig. 6 Expression of genes relevant to C and P metabolism in maize roots from receiver compartment under different P levels (mean ± SD). LP and HP refer to low P level (10 mg?kg-1) and high P level (100 mg?kg-1) respectively. AMR and NMR represent receiver plants with and without AM exudates respectively. # indicates significant difference (p < 0.05) between different P levels, while $ indicates significant difference (p < 0.05) between inoculation treatments under the same P level. Pht1;2, Pht1;6, P transporter genes; PEPC, phosphoenolpiruvate carboxylase gene; TC289, inorganic pyrophosphatase gene; G3PT, glycerol-3-phosphate transporter gene; MAS1, malate synthase gene.
| 基因 Gene | 正向引物 Forward primer | 反向引物 Reverse primer | 文献 Reference |
|---|---|---|---|
| Action | GTCCGTGCGTTTCCTTTTGT | AAACCGGCCTTGACCATTCC | Soderlund et al., 2009 |
| Pht1;2 | CCAACTTGCTTGGCTTTATCCT | AGCCTCCCCGGACATCTC | Schnable et al., 2009 |
| Pht1;6 | CTACAGCCAGAACCTGACCC | ACATGACGCCCATCAGTAGC | Schnable et al., 2009 |
| G3PT | TTCACCGCCTGCGTCCTT | TCGCTGGGCTCCTCTTGAG | Carlos et al., 2008 |
| PEPC | CACGCTGATCCTGACCATGA | TCGCAAACCGAGTATGTATCTT | Carlos et al., 2008 |
| TC289 | CCCTTGGCATGATCTGGAGAT | CCTTGCTGCCCCTTGGTAT | Carlos et al., 2008 |
| MAS1 | TGGACGCGTACAACCTCATC | CTGACTCCACTGCCGACAAA | Carlos et al., 2008 |
Appendix I The PCR primer sequences for functional genes in maize plants
| 基因 Gene | 正向引物 Forward primer | 反向引物 Reverse primer | 文献 Reference |
|---|---|---|---|
| Action | GTCCGTGCGTTTCCTTTTGT | AAACCGGCCTTGACCATTCC | Soderlund et al., 2009 |
| Pht1;2 | CCAACTTGCTTGGCTTTATCCT | AGCCTCCCCGGACATCTC | Schnable et al., 2009 |
| Pht1;6 | CTACAGCCAGAACCTGACCC | ACATGACGCCCATCAGTAGC | Schnable et al., 2009 |
| G3PT | TTCACCGCCTGCGTCCTT | TCGCTGGGCTCCTCTTGAG | Carlos et al., 2008 |
| PEPC | CACGCTGATCCTGACCATGA | TCGCAAACCGAGTATGTATCTT | Carlos et al., 2008 |
| TC289 | CCCTTGGCATGATCTGGAGAT | CCTTGCTGCCCCTTGGTAT | Carlos et al., 2008 |
| MAS1 | TGGACGCGTACAACCTCATC | CTGACTCCACTGCCGACAAA | Carlos et al., 2008 |
| 基因 Gene | 正向引物 Forward primer | 反向引物 Reverse primer | 文献 Reference |
|---|---|---|---|
| EF1β | CCCATGCAGCTCGATGGTA | TGCCAGGAAGTGAAGAAAATGA | Yoshihiro et al., 2015 |
| NGT1 | TGGCGCAGCACTTTTGTG | CGTTCGGTAGGGTAAGATAACATGA | Yoshihiro et al., 2015 |
| HXK1a | CGATTGCCAACTGGTATGGA | GCGCAAATTAGTCCCACCTAAG | Yoshihiro et al., 2015 |
| HXK1b | GGAATCCCAACTGGCAAAGA | ACATTCGTAAATTTGTACCTCCAAGA | Yoshihiro et al., 2015 |
| AGM1 | AAAACAATTCGATCTGCTGAAGGT | ATGCTCGTAATTTTTCGATTGCT | Yoshihiro et al., 2015 |
| UAP1 | TGAACGCGTCAACCGAATC | CGGTACCGGGAGCAATTTC | Yoshihiro et al., 2015 |
| CHS1 | CGGCACAATTTAGGGATATAGTGA | GGTTCCCCATGAATCAAACTAGTAA | Yoshihiro et al., 2015 |
| DAC1 | TTTGGAAGAGTTGGTTAATTTTGGT | AATACGGTCGCGGACGAA | Yoshihiro et al., 2015 |
| NAG1 | GGCGTTAGCTCTTGCCAAGT | CGCCGAAACGGTAAACATG | Yoshihiro et al., 2015 |
| GiPT | CTGCTGTTGATTATTGTTGGC | GAACGGTTCCCATAATAGTG | Maldonado-Mendoza et al., 2001 |
Appendix II The PCR primer sequences for AM fungal genes
| 基因 Gene | 正向引物 Forward primer | 反向引物 Reverse primer | 文献 Reference |
|---|---|---|---|
| EF1β | CCCATGCAGCTCGATGGTA | TGCCAGGAAGTGAAGAAAATGA | Yoshihiro et al., 2015 |
| NGT1 | TGGCGCAGCACTTTTGTG | CGTTCGGTAGGGTAAGATAACATGA | Yoshihiro et al., 2015 |
| HXK1a | CGATTGCCAACTGGTATGGA | GCGCAAATTAGTCCCACCTAAG | Yoshihiro et al., 2015 |
| HXK1b | GGAATCCCAACTGGCAAAGA | ACATTCGTAAATTTGTACCTCCAAGA | Yoshihiro et al., 2015 |
| AGM1 | AAAACAATTCGATCTGCTGAAGGT | ATGCTCGTAATTTTTCGATTGCT | Yoshihiro et al., 2015 |
| UAP1 | TGAACGCGTCAACCGAATC | CGGTACCGGGAGCAATTTC | Yoshihiro et al., 2015 |
| CHS1 | CGGCACAATTTAGGGATATAGTGA | GGTTCCCCATGAATCAAACTAGTAA | Yoshihiro et al., 2015 |
| DAC1 | TTTGGAAGAGTTGGTTAATTTTGGT | AATACGGTCGCGGACGAA | Yoshihiro et al., 2015 |
| NAG1 | GGCGTTAGCTCTTGCCAAGT | CGCCGAAACGGTAAACATG | Yoshihiro et al., 2015 |
| GiPT | CTGCTGTTGATTATTGTTGGC | GAACGGTTCCCATAATAGTG | Maldonado-Mendoza et al., 2001 |
| 地上部干质量 Shoot dry mass | 根系干质量 Root dry mass | 地上部磷浓度 Shoot P concentration | 根系磷浓度 Root P concentration | Pht1;2 | Pht1;6 | G3PT | PEPC | TC289 | MAS1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 供体植物 Donor | ||||||||||
| 接种处理 Inoculation treatment (I) | * | ** | * | ** | ** | ** | ** | ** | ns | ** |
| 磷水平 P levels (P) | ** | ns | ** | ** | ** | ** | ** | ** | ** | ns |
| 交互作用 I × P | ** | ** | ns | * | ** | ** | ** | ** | ns | ** |
| 受体植物 Receiver | ||||||||||
| 接种处理 Inoculation treatment (I) | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** |
| 磷水平 P levels (P) | * | * | * | * | ** | ** | ** | ** | ** | ** |
| 交互作用 I × P | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** |
Appendix III Two-way ANOVA of shoot and root dry mass, P concentrations and expression of genes related to C and P metabolisms as influenced by mycorrhizal inoculation and soil P levels
| 地上部干质量 Shoot dry mass | 根系干质量 Root dry mass | 地上部磷浓度 Shoot P concentration | 根系磷浓度 Root P concentration | Pht1;2 | Pht1;6 | G3PT | PEPC | TC289 | MAS1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 供体植物 Donor | ||||||||||
| 接种处理 Inoculation treatment (I) | * | ** | * | ** | ** | ** | ** | ** | ns | ** |
| 磷水平 P levels (P) | ** | ns | ** | ** | ** | ** | ** | ** | ** | ns |
| 交互作用 I × P | ** | ** | ns | * | ** | ** | ** | ** | ns | ** |
| 受体植物 Receiver | ||||||||||
| 接种处理 Inoculation treatment (I) | ns | ns | ns | ns | ** | ** | ** | ** | ** | ** |
| 磷水平 P levels (P) | * | * | * | * | ** | ** | ** | ** | ** | ** |
| 交互作用 I × P | ns | ns | ns | ns | ns | ** | ** | ** | ** | ** |
| [1] |
Bago B, Pfeffer PE, Shachar-Hill Y (2000). Carbon metabolism and transport in arbuscular mycorrhizas.Plant Physiology, 124, 949-958.
DOI URL |
| [2] | Bao SD (2000). Soil and Agricultural Chemistry Analysis. China Agriculture Press, Beijing. 81.(in Chinese)[鲍士旦 (2000). 土壤农化分析. 中国农业出版社, 北京. 81.] |
| [3] |
Barto EK, Hilker M, Müller F, Mohney BK, Weidenhamer JD, Rillig MC (2011). The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils.PLOS ONE, 6, e27195. doi: 10.1371/ journal.pone.0027195.
DOI URL PMID |
| [4] |
Biermann B, Linderman RG (1981). Quantifying vesicular- arbuscular mycorrhizae: A proposed method towards standardization.New Phytologist, 87, 63-67.
DOI URL |
| [5] |
Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, Hause B, Bucher M, Kretzschmar T, Bossolini C, Kuhlemeier C, Martinoia E, Franken P, Scholz U, Reinhardt D (2010). Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning.The Plant Journal, 64, 1002-1017.
DOI URL PMID |
| [6] |
Carlos CV, Enrique IL, Juan CP, Herrera-Estrella L (2008). Transcript profiling of Zea mays roots reveals gene responses to phosphate deficiency at the plant-and species- specific levels.Journal of Experimental Botany, 59, 2479-2497.
DOI URL PMID |
| [7] |
Casieri L, Lahmidi NA, Doidy J, Veneault-Fourrey C, Migeon A, Bonneau L, Courty P, Garcia K, Charbonnier M, Delteil A, Brun A, Zimmermann S, Plassard C, Wipf D (2013). Biotrophic transportome in mutualistic plant-fungal interactions.Mycorrhiza, 23, 597-625.
DOI URL PMID |
| [8] |
Chabaud M, Genre A, Sieberer BJ, Faccio A, Fournier J, Novero M, Barker DG, Bonfante P (2011). Arbuscular mycorrhizal hyphopodia and germinated spore exudates trigger Ca2+ spiking in the legume and nonlegume root epidermis.New Phytologist, 189, 347-355.
DOI URL PMID |
| [9] |
Chen A, Hu J, Sun S, Xu G (2007). Conservation and divergence of both phosphate- and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in solanaceous species.New Phytologist, 173, 817-831.
DOI URL |
| [10] | Cunningham JE, Kuiack C (1992). Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii.Applied and Environmental Microbiology, 58, 1451-1458. |
| [11] | Fixen P (2002). Soil test levels in North America.Better Crops, 86, 12-15. |
| [12] |
Fukayama H, Hatch MD, Tamai T, Tsuchida H, Sudoh S, Furbank RT, Miyao M (2003). Activity regulation and physiological impacts of maize C4-specific phosphoenolpyruvate carboxylase overproduced in transgenic rice plants.Photosynthesis Research, 77, 227-239.
DOI URL PMID |
| [13] |
Gardner WK, Barber DA, Parbery DG (1983). The acquisition of phosphorus by Lupinus albus L.: 3. The probable mechanism by which phosphorus movement in the soil/ root interface is enhanced.Plant Soil, 70, 107-124.
DOI URL |
| [14] |
Gu M, Chen AQ, Dai XL, Liu W, Xu G (2011). How does phosphate status influence the development of the arbuscular mycorrhizal symbiosis?Plant Signalling and Behavior, 6, 1300-1304.
DOI URL PMID |
| [15] |
Guimil S, Chang HS, Zhu T, Sesma A, Osbourn A, Roux C, Ioannidis V, Oakeley EJ, Docquier M, Descombes P, Briggs SP, Paszkowski U (2005). Comparative transcriptomics of rice reveals an ancient pattern of response to microbial colonization.Proceedings of the National Academy of Sciences of the United States of America, 102, 8066-8070.
DOI URL PMID |
| [16] |
Gutjahr C, Casieri L, Paszkowski U (2009). Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling.New Phytologist, 182, 829-837.
DOI URL PMID |
| [17] |
Harrison MJ, Dewbre GR, Liu J (2002). A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi.Plant Cell, 14, 2413-2429.
DOI URL PMID |
| [18] | Harrison MJ, Pumplin N, Breuillin FJ, Noar RD, Park HJ (2010). Phosphate transporters in arbuscular mycorrhizal symbiosis. In: Koltai H, Kapulnik Y eds. Arbuscular Mycorrhizas: Physiology and Function. Springer, Dordrecht, The Netherlands. 117-135. |
| [19] |
Javot H, Pumplin N, Harrison M (2007). Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles.Plant, Cell & Environment, 30, 310-322.
DOI URL PMID |
| [20] |
Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, Barker DG, Bécard G (2003). A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula.Plant Physiology, 131, 952-962.
DOI URL PMID |
| [21] |
Li T, Chen BD (2012). Arbuscular mycorrhizal fungi improving drought tolerance of maize plants by up-regulation of aquaporin gene expressions in roots and the fungi themselves.Chinese Journal of Plant Ecology, 36, 973-981.(in Chinese with English abstract)[李涛, 陈保冬 (2012). 丛枝菌根真菌通过上调根系及自身水孔蛋白基因表达提高玉米抗旱性, 植物生态学报,36, 973-981.]
DOI URL |
| [22] |
Maillet F, Poinsot V, Andre O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, Martinez EA, Driguez H, Bécard G, Dénarié J (2011). Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza.Nature, 469, 58-63.
DOI URL PMID |
| [23] |
Maldonado-Mendoza IE, Dewbre GR, Harrison MJ (2001). A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment.Molecular Plant-Microbe Interactions, 14, 1140-1148.
DOI URL PMID |
| [24] | Marschner H (1995).Mineral Nutrition of Higher Plants. 2nd edn. Academic Pres. London. |
| [25] |
Nagy F, Karandashov V, Chague W, Kalinkevich K, Tamasloukht MB, Xu G, Jakobsen I, Levy AA, Amrhein N, Bucher M (2005). The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species.The Plant Journal, 42, 236-250.
DOI URL |
| [26] |
Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009). Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated.New Phytologist, 181, 950-959.
DOI URL PMID |
| [27] |
Oláh B, Brière C, Bécard G, Dénarié J, Gough C (2005). Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway.The Plant Journal, 44, 195-207.
DOI URL PMID |
| [28] |
Olsson PA, Hansson MC, Burleigh SH (2006). Effect of P availability on temporal dynamics of carbon allocation and Glomus intraradices high-affinity P transporter gene induction in arbuscular mycorrhiza.Applied and Environmental Microbiology, 72, 4115-4120.
DOI URL PMID |
| [29] |
Pfaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR.Nucleic Acids Research, 29, e45.
DOI URL PMID |
| [30] |
Phillips JM, Hayman DS (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection.Transactions of the British Mycological Society, 55, 158-161.
DOI URL |
| [31] |
Plénet D, Etchebest S, Mollier A, Pellerin S (2000). Growth analysis of maize field crops under phosphorus deficiency.Plant and Soil, 223, 119-132.
DOI URL |
| [32] |
Radchuk R, Radchuk V, G?tz KP, Weichert H, Richter A, Emery RJ, Winfriede W, Weber H (2007). Ectopic expression of phosphoenolpyruvate carboxylase in Vicia narbonensis seeds: Effects of improved nutrient status on seed maturation and transcriptional regulatory networks.The Plant Journal, 51, 819-839.
DOI URL PMID |
| [33] |
Ramaiah M, Jain A, Baldwin JC, Karthikeyan AS, Raghothama KG (2011). Characterization of the phosphate starvation- induced glycerol-3-phosphate permease gene family inArabidopsis. Plant physiology, 157, 279-291.
DOI URL PMID |
| [34] |
Rich MK, Schorderet M, Reinhardt D (2014). The role of the cell wall compartment in mutualistic symbioses of plants.Frontiers in Plant Science, 5, 238.
DOI URL PMID |
| [35] |
Rojas-Beltrán JA, Dubois F, Mortiaux F, Portetelle D, Gebhardt C, Sangwan RS, du Jardin P (1999). Identification of cytosolic Mg2+-dependent soluble inorganic pyrophosphatases in potato and phylogenetic analysis.Plant Molecular Biology, 39, 449-461.
DOI URL |
| [36] |
Rolletschek H, Borisjuk L, Radchuk R, Miranda M, Heim U, Wobus U, Weber H (2004). Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy.Plant Biotechnology Journal, 2, 211-219.
DOI URL PMID |
| [37] |
Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, Minx P, Reily AD, Courtney L, Kruchowski SS, Tomlinson C, Strong C, Delehaunty K, Fronick C, Courtney B, Rock SM, Belter E, Du F, Kim K, Abbott RM, Cotton M, Levy A, Marchetto P, Ochoa K, Jackson SM, Gillam B, Chen W, Yan L, Higginbotham J, Cardenas M, Waligorski J, Applebaum E, Phelps L, Falcone J, Kanchi K, Thane T, Scimone A, Thane N, Henke J, Wang T, Ruppert J, Shah N, Rotter K, Hodges J, Ingenthron E, Cordes M, Kohlberg S, Sgro J, Delgado B, Mead K, Chinwalla A, Leonard S, Crouse K, Collura K, Kudrna D, Currie J, He R, Angelova A, Rajasekar S, Mueller T, Lomeli R, Scara G, Ko A, Delaney K, Wissotski M, Lopez G, Campos D, Braidotti M, Ashley E, Golser W, Kim H, Lee S, Lin J, Dujmic Z, Kim W, Talag J, Zuccolo A, Fan C, Sebastian A, Kramer M, Spiegel L, Nascimento L, Zutavern T, Miller B, Ambroise C, Muller S, Spooner W, Narechania A, Ren L, Wei S, Kumari S, Faga B, Levy MJ, McMahan L, Van Buren P, Vaughn MW, Ying K, Yeh CT, Emrich SJ, Jia Y, Kalyanaraman A, Hsia AP, Barbazuk WB, Baucom RS, Brutnell TP, Carpita NC, Chaparro C, Chia JM, Deragon JM, Estill JC, Fu Y, Jeddeloh JA, Han Y, Lee H, Li P, Lisch DR, Liu S, Liu Z, Nagel DH, McCann MC, SanMiguel P, Myers AM, Nettleton D, Nguyen J, Penning BW, Ponnala L, Schneider KL, Schwartz DC, Sharma A, Soderlund C, Springer NM, Sun Q, Wang H, Waterman M, Westerman R, Wolfgruber TK, Yang L, Yu Y, Zhang L, Zhou S, Zhu Q, Bennetzen JL, Dawe RK, Jiang J, Jiang N, Presting GG, Wessler SR, Aluru S, Martienssen RA, Clifton SW, McCombie WR, Wing RA, Wilson RK (2009). The B73 maize genome: Complexity, diversity, and dynamics.Science, 326, 1112-1115.
DOI URL PMID |
| [38] | Smith SE, Read DJ (2008). Arbuscular mycorrhizas.Mycorrhizal Symbiosis, 3, 11-145. |
| [39] | Tyler G (1999). Plant distribution and soil-plant interactions on shallow soils.Acta Phytogeographica Suecica, 84, 21-32. |
| [40] |
van der Heijden MGA, Horton TR (2009). Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems.Journal of Ecology, 97, 1139-1150.
DOI URL |
| [41] |
Wright DP, Read DJ, Scholes JD (1998). Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L.Plant, Cell & Environment, 21, 881-891.
DOI URL |
| [42] |
Yoshihiro K, Miki K, Katsuharu S, Kikuchi Y, Ezawa T, Maeshima M, Hata S, Fujiwara T (2015). Up-regulation of genes involved in N-acetylglucosamine uptake and metabolism suggests a recycling mode of chitin in intraradical mycelium of arbuscular mycorrhizal fungi.Mycorrhiza, 25, 411-417.
DOI URL PMID |
| [1] | Ke-Yu CHEN Sen Xing Yu Tang Sun JiaHui Shijie Ren Bao-Ming JI. Arbuscular mycorrhizal fungal community characteristics and driving factors in different grassland types [J]. Chin J Plant Ecol, 2024, 48(5): 660-674. |
| [2] | Die Hu Xinqi Jiang DAI Zhicong Daiyi Chen Yu Zhang Shan-Shan Qi. Arbuscular mycorrhizal fungi enhance the herbicide tolerance of an invasive weed Sphagneticola trilobata [J]. Chin J Plant Ecol, 2024, 48(5): 651-659. |
| [3] | CHEN Bao-Dong, FU Wei, WU Song-Lin, ZHU Yong-Guan. Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling [J]. Chin J Plant Ecol, 2024, 48(1): 1-20. |
| [4] | YANG Jia-Rong, DAI Dong, CHEN Jun-Fang, WU Xian, LIU Xiao-Lin, LIU Yu. Insight into recent studies on the diversity of arbuscular mycorrhizal fungi in shaping plant community assembly and maintaining rare species [J]. Chin J Plant Ecol, 2023, 47(6): 745-755. |
| [5] | HE Fei, LI Chuan, Faisal SHAH, LU Xie-Min, WANG Ying, WANG Meng, RUAN Jia, WEI Meng-Lin, MA Xing-Guang, WANG Zhuo, JIANG Hao. Carbon transport and phosphorus uptake in an intercropping system of Robinia pseudoacacia and Amorphophallus konjac mediated by arbuscular mycorrhizal hyphal networks [J]. Chin J Plant Ecol, 2023, 47(6): 782-791. |
| [6] | XIE Wei, HAO Zhi-Peng, ZHANG Xin, CHEN Bao-Dong. Research progress and prospect of signal transfer among plants mediated by arbuscular mycorrhizal networks [J]. Chin J Plant Ecol, 2022, 46(5): 493-515. |
| [7] | MA Ju-Feng, XIN Min, XU Chen-Chao, ZHU Wan-Ying, MAO Chuan-Zao, CHEN Xin, CHENG Lei. Effects of arbuscular mycorrhizal fungi and nitrogen addition on nitrogen uptake of rice genotypes with different root morphologies [J]. Chin J Plant Ecol, 2021, 45(7): 728-737. |
| [8] | PANG Fang, XIA Wei-Kang, HE Min, QI Shan-Shan, DAI Zhi-Cong, DU Dao-Lin. Nitrogen-fixing bacteria alleviates competition between arbuscular mycorrhizal fungi and Solidago canadensis for nutrients under nitrogen limitation [J]. Chin J Plant Ecol, 2020, 44(7): 782-790. |
| [9] | CUI Li, GUO Feng, ZHANG Jia-Lei, YANG Sha, WANG Jian-Guo, MENG Jing-Jing, GENG Yun, LI Xin-Guo, WAN Shu-Bo. Improvement of continuous microbial environment in peanut rhizosphere soil by Funneliformis mosseae [J]. Chin J Plant Ecol, 2019, 43(8): 718-728. |
| [10] | GAO Wen-Tong, ZHANG Chun-Yan, DONG Ting-Fa, XU Xiao. Effects of arbuscular mycorrhizal fungi on the root growth of male and female Populus cathayana individuals grown under different sexual combination patterns [J]. Chin J Plant Ecol, 2019, 43(1): 37-45. |
| [11] | XU Li-Jiao, HAO Zhi-Peng, XIE Wei, LI Fang, CHEN Bao-Dong. Transmembrane H + and Ca 2+ fluxes through extraradical hyphae of arbuscular mycorrhizal fungi in response to drought stress [J]. Chin J Plant Ecol, 2018, 42(7): 764-773. |
| [12] | LIU Hai-Yue, LI Xin-Mei, ZHANG Lin-Lin, WANG Jiao-Jiao, HE Xue-Li. Eco-geographical distribution of arbuscular mycorrhizal fungi associated with Hedysarum scoparium in the desert zone of northwestern China [J]. Chin J Plant Ecol, 2018, 42(2): 252-260. |
| [13] | CHEN Bao-Ming, WEI Hui-Jie, CHEN Wei-Bin, ZHU Zheng-Cai, YUAN Ya-Ru, ZHANG Yong-Long, LAN Zhi-Gang. Effects of plant invasion on soil nitrogen transformation processes and its associated microbes [J]. Chin J Plant Ecol, 2018, 42(11): 1071-1081. |
| [14] | Ping SONG, Rui ZHANG, Zhi-Chun ZHOU, Jian-She TONG, Hui WANG. Effects of localized nitrogen supply treatments on growth and root parameters in Pinus massoniana families under phosphorus deficiency [J]. Chin J Plan Ecolo, 2017, 41(6): 622-631. |
| [15] | Liang-Hua CHEN, Juan LAI, Xiang-Wei HU, Wan-Qin YANG, Jian ZHANG, Xiao-Jun WANG, Ling-Jie TAN. Effects of inoculation with arbuscular mycorrhizal fungi on photosynthetic physiology in females and males of Populus deltoides exposed to cadmium pollution [J]. Chin J Plant Ecol, 2017, 41(4): 480-488. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn