

Chin J Plan Ecolo ›› 2017, Vol. 41 ›› Issue (7): 787-794.DOI: 10.17521/cjpe.2016.0322
• Method and Technology • Previous Articles Next Articles
Feng-Jiao SHEN, Qian-Qian REN, Qi DONG, Li ZHU, Jian-Fang ZHANG, Jing YANG, Ran ZHANG, Hong-Zhu LIANG, Jian-Cheng ZHAO, Shuo SHI*( )
)
Received:2016-10-17
Accepted:2017-06-01
Online:2017-07-10
Published:2017-08-21
Contact:
Shuo SHI
About author:KANG Jing-yao(1991-), E-mail:
Feng-Jiao SHEN, Qian-Qian REN, Qi DONG, Li ZHU, Jian-Fang ZHANG, Jing YANG, Ran ZHANG, Hong-Zhu LIANG, Jian-Cheng ZHAO, Shuo SHI. A new angiosperms molecular specimen treatment method for field use[J]. Chin J Plan Ecolo, 2017, 41(7): 787-794.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2016.0322
| 干燥方式 Drying method | 日本晚樱 Prunus serrulata var. lannesiana | 山麦冬 Liriope spicata | ||||
|---|---|---|---|---|---|---|
| OD260/280 | DNA (ng·μL-1) | PCR (ng·μL-1) | OD260/280 | DNA (ng·μL-1) | PCR (ng·μL-1) | |
| 150 | 1.83 ± 0.17a | 451.24 ± 150.91a | 13.94 ± 3.97c | 2.03 ± 0.04a | 211.43 ± 64.31a | 0.74 ± 2.30d |
| 80 | 1.80 ± 0.13a | 376.13 ± 121.04a | 34.48 ± 9.42b | 1.83 ± 0.16b | 80.64 ± 48.16c | 16.51 ± 17.49c |
| 40 | 1.74 ± 0.16ab | 470.44 ± 228.50a | 49.07 ± 4.83a | 1.90 ± 0.08ab | 241.41 ± 88.07a | 57.64 ± 6.51a |
| Y | 1.73 ± 0.09ab | 291.64 ± 90.77b | 38.38 ± 4.75b | 1.93 ± 0.14ab | 160.01 ± 22.95b | 46.98 ± 8.22b |
| S | 1.64 ± 0.10b | 432.59 ± 167.67a | 39.95 ± 5.82b | 1.90 ± 0.24ab | 252.33 ± 61.74a | 43.19 ± 8.72b |
Table 1 Comparisons of DNA purity, concentration and the concentration of PCR products in the specimens of Prunus serrulata var. lannesiana and Liriope spicata obtained with different drying methods (mean ± SD)
| 干燥方式 Drying method | 日本晚樱 Prunus serrulata var. lannesiana | 山麦冬 Liriope spicata | ||||
|---|---|---|---|---|---|---|
| OD260/280 | DNA (ng·μL-1) | PCR (ng·μL-1) | OD260/280 | DNA (ng·μL-1) | PCR (ng·μL-1) | |
| 150 | 1.83 ± 0.17a | 451.24 ± 150.91a | 13.94 ± 3.97c | 2.03 ± 0.04a | 211.43 ± 64.31a | 0.74 ± 2.30d |
| 80 | 1.80 ± 0.13a | 376.13 ± 121.04a | 34.48 ± 9.42b | 1.83 ± 0.16b | 80.64 ± 48.16c | 16.51 ± 17.49c |
| 40 | 1.74 ± 0.16ab | 470.44 ± 228.50a | 49.07 ± 4.83a | 1.90 ± 0.08ab | 241.41 ± 88.07a | 57.64 ± 6.51a |
| Y | 1.73 ± 0.09ab | 291.64 ± 90.77b | 38.38 ± 4.75b | 1.93 ± 0.14ab | 160.01 ± 22.95b | 46.98 ± 8.22b |
| S | 1.64 ± 0.10b | 432.59 ± 167.67a | 39.95 ± 5.82b | 1.90 ± 0.24ab | 252.33 ± 61.74a | 43.19 ± 8.72b |
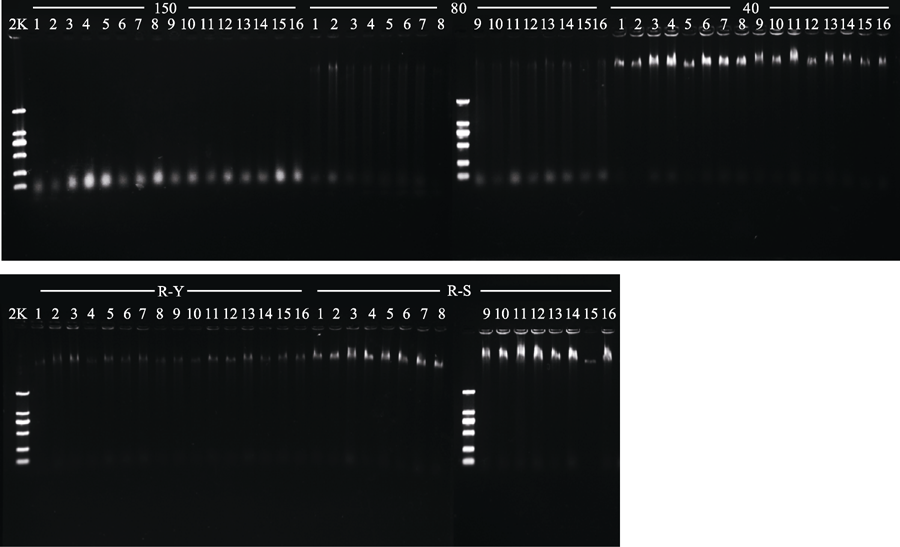
Fig. 1 Genomic DNA in specimens of Prunus serrulata var. lannesiana (R) obtained with five different drying methods. 150, drying at 150 °C; 80, drying at 80 °C; 40, drying at 40 °C; Y, natural drying; S, silica gel drying; 2K, 2 kb plus DNA ladder.
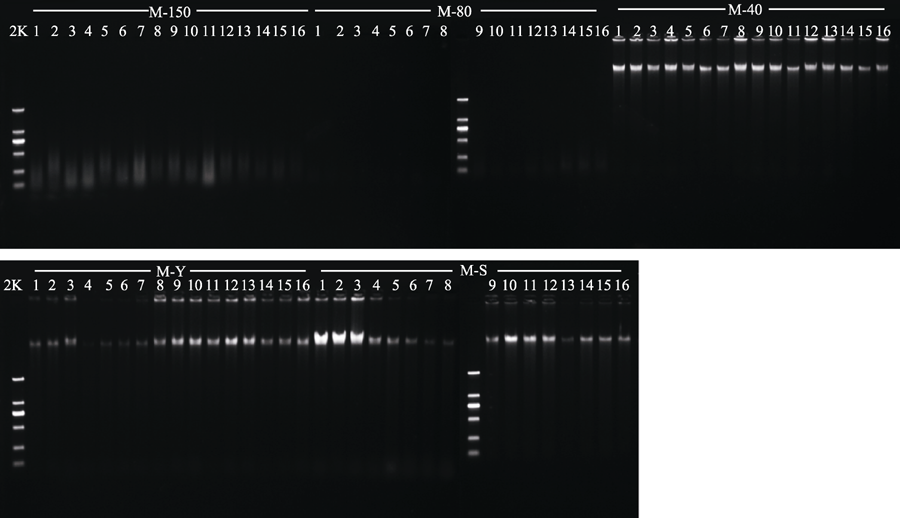
Fig. 2 Genomic DNA in specimens of Liriope spicata (M) obtained with five different drying methods. 50, drying at 150 °C; 80, drying at 80 °C; 40, drying at 40 °C; Y, natural drying; S, silica gel drying; 2K, 2 kb plus DNA ladder.
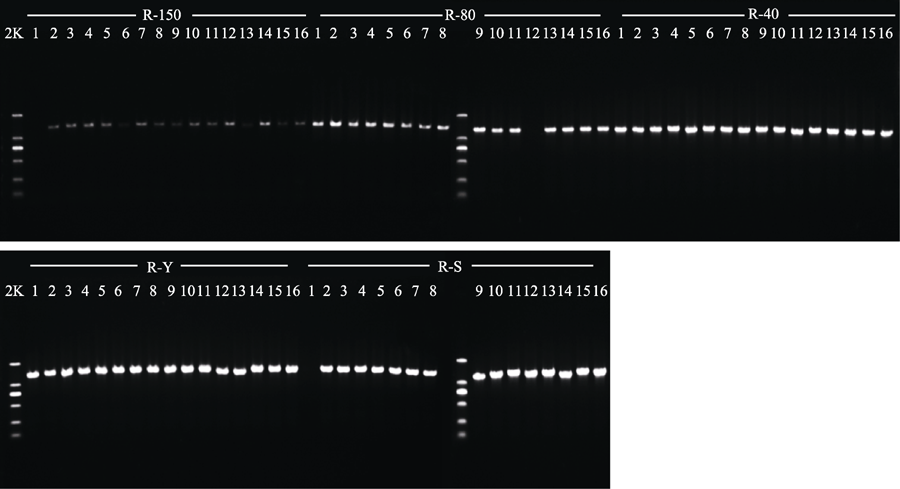
Fig. 3 Agarose gel electrophoresis of PCR products in specimens of Prunus serrulata var. lannesiana (R) obtained with five different drying methods. 150, drying at 150 °C; 80, drying at 80 °C; 40, drying at 40 °C; Y, natural drying; S, silica gel drying; 2K, 2kb plus DNA ladder.
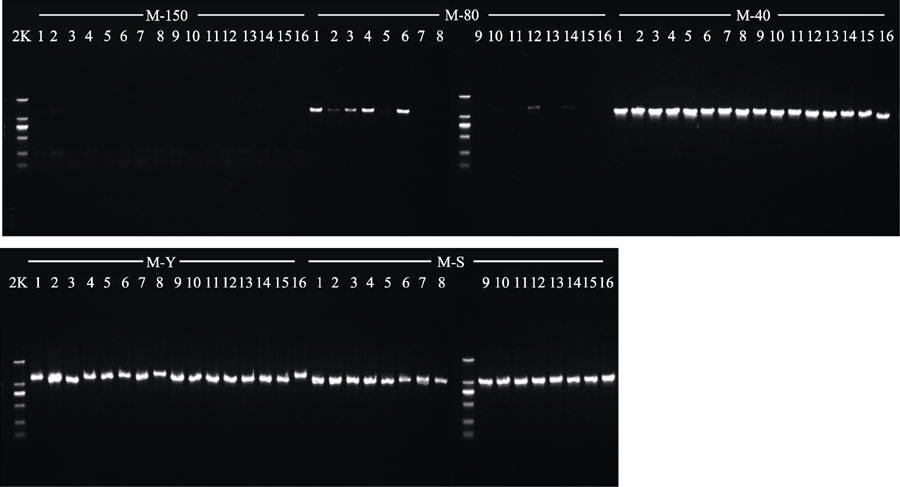
Fig. 4 Agarose gel electrophoresis of PCR products in specimens of Liriope spicata (M) obtained with five different drying methods. 150, drying at 150 °C; 80, drying at 80 °C; 40, drying at 40 °C; Y, natural drying; S, silica gel drying; 2K, 2 kb plus DNA ladder.
| [1] | Alexander PJ, Rajanikanth G, Bacon CD, Donovanbailey C (2007). Recovery of plant DNA using a reciprocating saw and silica-based columns.Molecular Ecology Notes, 7, 5-9. |
| [2] | Cai XZ, Liu KM, Long CL (2008). DNA extraction from dried leaves and PCR amplification of Colocasia.Chinese Wild Plant Resources, 27(1), 51-57. (in Chinese with English abstract)[蔡秀珍, 刘克明, 龙春林 (2008). 野生芋属植物干叶片DNA的提取及PCR扩增. 中国野生植物资源, 27(1), 51-57.] |
| [3] | Chase MW, Hills HH (1991). Silica gel: An ideal material for field preservation of leaf samples for DNA studies.Taxon, 40, 215-220. |
| [4] | Cliquet S, Jackson MA (1997). Comparison of air-drying methods for evaluating the desiccation tolerance of liquid culture-produced blastospores of Paecilomyces fumosoroseus. World Journal of Microbiology and Biotechnology, 13, 299-303. |
| [5] | Doyle JJ, Dickson EE (1987). Preservation of plant samples for DNA restriction endonuclease analysis.Taxon, 36, 715-722. |
| [6] | He TM, Chen XS, Wu Y (2004). Preparation of DNA from silica gel dried leaves of Rosaceae. Journal of Shihezi University (Natural Science), 22, 316-319. (in Chinese with English abstract)[何天明, 陈学森, 吴燕 (2004). 从蔷薇科果树硅胶干燥叶片中制备DNA. 石河子大学学报(自然科学版), 22, 316-319.] |
| [7] | Heenan PB, Goeke DF, Houliston GJ, Lysak MA (2012). Phylogenetic analyses of ITS and rbcL DNA sequences for sixteen genera of Australian and New Zealand Brassicaceae result in the expansion of the tribe Microlepidieae.Taxon, 61, 970-979. |
| [8] | Jing XM, Chu YX, Tang GG, Zhang YC, Liu Z, Zhang H (2008). Effects of different sample preserving methods on genomic DNA extraction of Chimonanthus praecox and their ISSR-PCR validation.Molecular Plant Breeding, 6, 387-392. (in Chinese with English abstract)[靖相密, 褚云霞, 汤庚国, 张永春, 刘忠, 张慧 (2008). 不同保存方法对蜡梅总DNA提取效果的影响及ISSR-PCR验证. 分子植物育种, 6, 387-392.] |
| [9] | Li JL, Wang S, Yu J, Wang L, Zhou SL (2013). A modified CTAB protocol for plant DNA extraction.Chinese Bulletin of Botany, 48, 72-78. (in Chinese with English abstract)[李金璐, 王硕, 于婧, 王玲, 周世良 (2013). 一种改良的植物DNA提取方法. 植物学报, 48, 72-78.] |
| [10] | Liston A, Rieseberg LH, Adams RP, Do N, Zhu GL (1990). A method for collecting dried plant specimens for DNA and isozyme analyses, and the results of a field test in Xinjiang, China.Annals of the Missouri Botanical Garden, 77, 859-863. |
| [11] | Qiu NW, Liu Q, Liu H (2015). Multiple comparison data in statistical analysis.Journal of Biomathematics, 30, 535-541. (in Chinese with English abstract)[邱念伟, 刘倩, 刘慧 (2015). 生物学实验数据统计分析中的多重比较法. 生物数学学报, 30, 535-541.] |
| [12] | Särkinen T, Staats M, Richardson JE, Cowan RS, Bakker FT (2012). How to open the treasure chest? Optimising DNA extraction from herbarium specimens.PLOS ONE, 7, e43808. doi: 10.1371/journal.pone.0043808. |
| [13] | Staats M, Cuenca A, Richardson JE, Ginkel RV, Petersen G, Seberg O, Bakker FT (2011). DNA damage in plant herbarium tissue.PLOS ONE, 6, e28448. doi: 10.1371/journal. pone.0028448. |
| [14] | Taggart JB, Hynes RA, Prodöuhl PA, Ferguson A (1991). A simplified protocol for routine total DNA isolation from salmonid fishes.Journal of Fish Biology, 40, 963-965. |
| [15] | Wang XD, Wang ZP, Zou YP (1996). An improved procedure for the isolation of nuclear DNA from leaves of wild grapevine dried with silica gel.Plant Molecular Biology Reporter, 14, 369-373. |
| [16] | Wei CX, Xie PS, Zhou WD, Chen YF, Zhang J, Huai HY (2008). Observation on morphological structure of leaf epidermis of Ophiopogon japonicus, Liriope spicata and L. platyphylla.Journal of Plant Resources and Environment, 17(4), 9-15. (in Chinese with English abstract)[韦存虚, 谢佩松, 周卫东, 陈义芳, 张军, 淮虎银 (2008). 麦冬、土麦冬和阔叶土麦冬叶表皮形态结构的观察. 植物资源与环境学报, 17(4), 9-15.] |
| [17] | Xie Z, Ge S, Hong D (1998). Preparation of DNA from silica gel dried mini-amount of leaves of Oryza rufipogon for RAPD study and total DNA bank construction.Acta Botanica Sinica, 41, 807-812. |
| [18] | Xu C, Dong W, Shi S, Cheng T, Li CH, Liu YL, Wu P, Wu HK, Gao P (2015). Accelerating plant DNA barcode reference library construction using herbarium specimens: Improved experimental techniques.Molecular Ecology Resources, 15, 1366-1374. |
| [1] | YANG An-Na, LI Zeng-Yan, MOU Ling, YANG Bai-Yu, SAI Bi-Le, ZHANG Li, ZHANG Zeng-Ke, WANG Wan-Sheng, DU Yun-Cai, YOU Wen-Hui, YAN En-Rong. Variation in soil bacterial community across vegetation types in Dajinshan Island, Shanghai [J]. Chin J Plant Ecol, 2024, 48(3): 377-389. |
| [2] | ZHAO Xiao-Xiang, ZHU Bin-Bin, TIAN Qiu-Xiang, LIN Qiao-Ling, CHEN Long, LIU Feng. Research progress on home-field advantage of leaf litter decomposition [J]. Chin J Plant Ecol, 2023, 47(5): 597-607. |
| [3] | WU Kai, LI Kai, JIA Wei-Han, LIAO Meng-Na, NI Jian. Modern processes of lacustrine plant sedimentary ancient DNA [J]. Chin J Plant Ecol, 2022, 46(7): 735-752. |
| [4] | XIAO Wen-Hong, ZHOU Qing-Song, ZHU Chao-Dong, WU Dong-Hui, XIAO Zhi-Shu. Advances in techniques and methods of wildlife monitoring [J]. Chin J Plant Ecol, 2020, 44(4): 409-417. |
| [5] | SHEN Jia-Yan, LI Shuai-Feng, HUANG Xiao-Bo, LEI Zhi-Quan, SHI Xing-Quan, SU Jian-Rong. Radial growth responses to climate warming and drying in Pinus yunnanensis in Nanpan River Basin [J]. Chin J Plant Ecol, 2019, 43(11): 946-958. |
| [6] | Jing-Xin XU, You-Fei ZHENG, Bo-Ru MAI, Hui ZHAO, Zhong-Fang CHU, Ji-Qing HUANG, Yue YUAN. Characteristics and partitioning of ozone dry deposition measured by eddy-covariance technology in a winter wheat field [J]. Chin J Plant Ecol, 2017, 41(6): 670-682. |
| [7] | ZHANG Feng, ZHOU Guang-Sheng. Estimating canopy photosynthetic parameters in maize field based on multi-spectral remote sensing [J]. Chin J Plant Ecol, 2014, 38(7): 710-719. |
| [8] | Jannathan MAMUT, TAN Dun-Yan. Gynomonoecy in angiosperms: phylogeny, sex expression and evolutionary significance [J]. Chin J Plant Ecol, 2014, 38(1): 76-90. |
| [9] | HOU Yan-Hui, ZHOU Guang-Sheng, XU Zhen-Zhu. An overview of research progress on responses of grassland ecosystems to global warming based on infrared heating experiments [J]. Chin J Plant Ecol, 2013, 37(12): 1153-1167. |
| [10] | LIU Xiao-Mei, FANG Jian, ZHANG Jing, LIN Wu-Ying, FAN Ting-Lu, FENG Hu-Yuan. EFFECTS OF LONG-TERM FERTILIZATION ON VERTICAL DISTRIBUTION OF MICROORGANISMS IN WHEAT FIELD SOIL [J]. Chin J Plant Ecol, 2009, 33(2): 397-404. |
| [11] | CHENG Kai, SUN Kun, WEN Hong-Yan, ZHANG Min, JIA Dong-Rui, LIU Jian-Quan. MATERNAL DIVERGENCE AND PHYLOGEOGRAPHICAL RELATIONSHIPS BETWEEN HIPPOPHAE GYANTSENSIS AND H. RHAMNOIDES SUBSP. YUNNANENSIS [J]. Chin J Plant Ecol, 2009, 33(1): 1-11. |
| [12] | ZHANG Yun-Xia, LI Xiao-Bing, ZHANG Yun-Fei. DETERMINING VEGETATION COVER BASED ON FIELD DATA AND MULTI-SCALE REMOTELY SENSED DATA [J]. Chin J Plant Ecol, 2007, 31(5): 842-849. |
| [13] | LI Jun, ZHOU Shou-Biao, WANG Chun-Jing, LI Jin-Hua. COMPARATIVE STUDY OF DROUGHT TOLERANCE AND TOLERANCE MECHANISMS IN WILD AND CULTIVATED DICHONDRA REPENS [J]. Chin J Plant Ecol, 2007, 31(3): 521-527. |
| [14] | HAN Guang-Xuan, ZHU Bo, JIANG Chang-Sheng. SOIL RESPIRATION AND ITS CONTROLLING FACTORS IN RICE FIELDS IN THE HILL REGION OF THE CENTRAL SICHUAN BASIN [J]. Chin J Plant Ecol, 2006, 30(3): 450-456. |
| [15] | QIU Guo_Yu, WANG Shuai, WU Xiao. THREE TEMPERATURE (3T) MODEL——A METHOD TO ESTIMATE EVAPOTRANSPIRATION AND EVALUATE ENVIRONMENTAL QUALITY [J]. Chin J Plant Ecol, 2006, 30(2): 231-238. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
![]()