

Chin J Plant Ecol ›› 2016, Vol. 40 ›› Issue (9): 893-901.DOI: 10.17521/cjpe.2016.0163
Special Issue: 青藏高原植物生态学:群落生态学
• Research Articles • Previous Articles Next Articles
Kai YUE1, Wan-Qin YANG1,2, Yan PENG1, Chun-Ping HUANG1,3, Chuan ZHANG1, Fu-Zhong WU1,2,*( )
)
Received:2016-05-09
Accepted:2016-07-23
Online:2016-09-10
Published:2016-09-29
Contact:
Fu-Zhong WU
Kai YUE, Wan-Qin YANG, Yan PENG, Chun-Ping HUANG, Chuan ZHANG, Fu-Zhong WU. Effects of streams on lignin degradation during foliar litter decomposition in an alpine forest[J]. Chin J Plant Ecol, 2016, 40(9): 893-901.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2016.0163
| 生境 Habitat | AT (℃) | C (g·kg-1) | N (g·kg-1) | P (g·kg-1) | pH | ||
|---|---|---|---|---|---|---|---|
| 林下 Forest floor | 2.0 ± 5.2 | 126 ± 26 | 5.8 ± 1.1 | 1.2 ± 0.2 | 6.6 ± 0.02 | ||
| 生境 Habitat | AT (°C) | HCO3- (mg·L-1) | NH4+ (mg·L-1) | NO3-(mg·L-1) | PO43- (μg·L-1) | pH | FV (m·s-1) |
| 溪流 Stream | 5.1 ± 2.6 | 13.9 ± 1.96 | 0.10 ± 0.05 | 0.29 ± 0.07 | 7.85 ± 0.38 | 6.6 ± 0.4 | 0.53 ± 0.15 |
| 河岸带 Riparian zone | 4.8 ± 3.4 | 19.7 ± 1.33 | 0.04 ± 0.02 | 0.34 ± 0.08 | 7.84 ± 0.41 | 6.9 ± 0.3 | 0.05 ± 0.01 |
Table 1 Characteristics of environmental conditions of different habitats during the process of foliar litter decomposition (mean ± SD, n = 90)
| 生境 Habitat | AT (℃) | C (g·kg-1) | N (g·kg-1) | P (g·kg-1) | pH | ||
|---|---|---|---|---|---|---|---|
| 林下 Forest floor | 2.0 ± 5.2 | 126 ± 26 | 5.8 ± 1.1 | 1.2 ± 0.2 | 6.6 ± 0.02 | ||
| 生境 Habitat | AT (°C) | HCO3- (mg·L-1) | NH4+ (mg·L-1) | NO3-(mg·L-1) | PO43- (μg·L-1) | pH | FV (m·s-1) |
| 溪流 Stream | 5.1 ± 2.6 | 13.9 ± 1.96 | 0.10 ± 0.05 | 0.29 ± 0.07 | 7.85 ± 0.38 | 6.6 ± 0.4 | 0.53 ± 0.15 |
| 河岸带 Riparian zone | 4.8 ± 3.4 | 19.7 ± 1.33 | 0.04 ± 0.02 | 0.34 ± 0.08 | 7.84 ± 0.41 | 6.9 ± 0.3 | 0.05 ± 0.01 |
| 物种 Species | C (%) | N (%) | P (%) | 木质素 Lignin (%) | C:N | C:P | N:P | Lignin:N |
|---|---|---|---|---|---|---|---|---|
| 康定柳 S. paraplesia | 34.8 ± 0.9c | 2.64 ± 0.15a | 0.17 ± 0.01a | 24.7 ± 1.3d | 13.2 ± 0.8d | 207 ± 19.7c | 15.7 ± 1.7a | 9.38 ± 0.8c |
| 高山杜鹃 R. lapponicum | 38.6 ± 1.1b | 0.69 ± 0.10d | 0.10 ± 0.02d | 29.8 ± 0.8b | 57.2 ± 10.2a | 375 ± 53.6a | 6.75 ± 1.5c | 44.3 ± 8.3a |
| 方枝柏 S. saltuaria | 46.9 ± 1.8a | 1.05 ± 0.06c | 0.15 ± 0.01b | 28.1 ± 0.8c | 45.1 ± 3.9b | 304 ± 12.6b | 6.79 ± 0.7c | 26.9 ± 1.8b |
| 四川红杉 L. mastersiana | 37.5 ± 0.5b | 1.59 ± 0.11b | 0.12 ± 0.01c | 37.8 ± 1.0a | 23.6 ± 1.8c | 320 ± 24.6b | 13.6 ± 0.8b | 30.1 ± 2.1b |
Table 2 Initial chemical properties of Salix paraplesia, Rhododendron lapponicum, Sabina saltuaria, and Larix mastersiana foliar litters (mean ± SD, n = 9)
| 物种 Species | C (%) | N (%) | P (%) | 木质素 Lignin (%) | C:N | C:P | N:P | Lignin:N |
|---|---|---|---|---|---|---|---|---|
| 康定柳 S. paraplesia | 34.8 ± 0.9c | 2.64 ± 0.15a | 0.17 ± 0.01a | 24.7 ± 1.3d | 13.2 ± 0.8d | 207 ± 19.7c | 15.7 ± 1.7a | 9.38 ± 0.8c |
| 高山杜鹃 R. lapponicum | 38.6 ± 1.1b | 0.69 ± 0.10d | 0.10 ± 0.02d | 29.8 ± 0.8b | 57.2 ± 10.2a | 375 ± 53.6a | 6.75 ± 1.5c | 44.3 ± 8.3a |
| 方枝柏 S. saltuaria | 46.9 ± 1.8a | 1.05 ± 0.06c | 0.15 ± 0.01b | 28.1 ± 0.8c | 45.1 ± 3.9b | 304 ± 12.6b | 6.79 ± 0.7c | 26.9 ± 1.8b |
| 四川红杉 L. mastersiana | 37.5 ± 0.5b | 1.59 ± 0.11b | 0.12 ± 0.01c | 37.8 ± 1.0a | 23.6 ± 1.8c | 320 ± 24.6b | 13.6 ± 0.8b | 30.1 ± 2.1b |
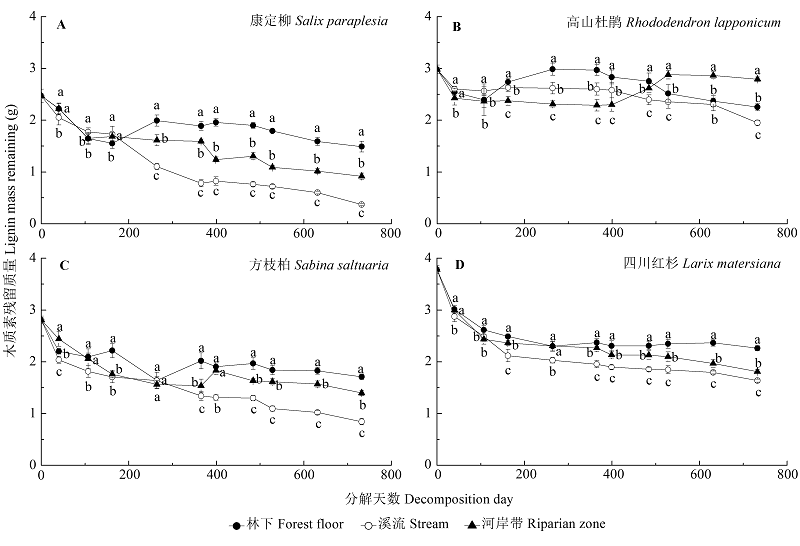
Fig. 1 Dynamics of lignin mass remaining (g) in the decomposing foliar litter of Salix paraplesia (A), Rhododendron lapponicum (B), Sabina saltuaria (C), and Larix mastersiana (D) under different habitat conditions (mean ± SD, n = 9). Different lowercase letters indicate significant (p < 0.05) differences of lignin mass remaining for a given litter species in a specific decomposition period under different habitat conditions.
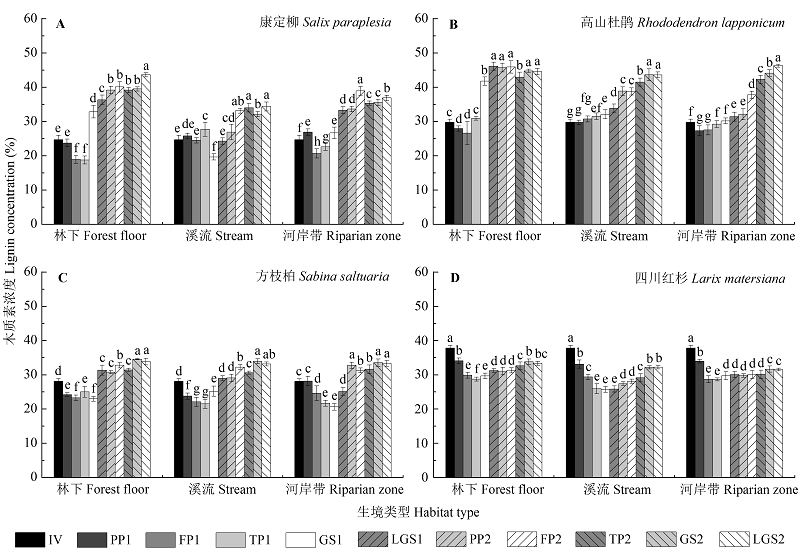
Fig. 2 Dynamics of lignin concentration (percent litter mass, %) during Salix paraplesia (A), Rhododendron lapponicum (B), Sabina saltuaria (C), and Larix mastersiana (D) foliar litter decomposition (p < 0.05) under different habitat conditions (mean ± SD, n = 9). Different lowercase letters indicate significant (p < 0.05) differences of lignin concentration among different decomposition periods for a given litter species incubated in a specific type of habitat. FP, freezing period; GS, growing season; IV, initial value; LGS, late growing season; PP, pre-freezing period; TP, thawing period; 1, first year; 2, second year.
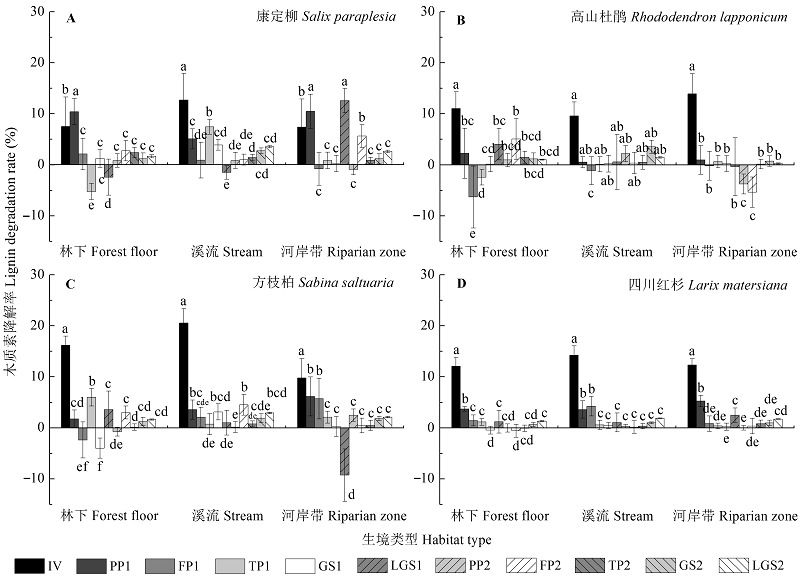
Fig. 3 Dynamics of lignin degradation rate (%/month) during Salix paraplesia (A), Rhododendron lapponicum (B), Sabina saltuaria (C), and Larix mastersiana (D) foliar litter decomposition (p < 0.05) under different habitat conditions (mean ± SD, n = 9). Different lowercase letters indicate significant (p < 0.05) differences of lignin degradation rate among different decomposition periods for a given litter species incubated in a specific type of habitat. FP, freezing period; GS, growing season; IV, initial value; LGS, late growing season; PP, pre-freezing period; TP, thawing period; 1, first year; 2, second year.
| 影响因子 Influence factor | 自由度 Degree of freedom | F | p |
|---|---|---|---|
| 物种 Species | 3 | 165.753 | < 0.001 |
| 生境 Habitat | 2 | 75.197 | < 0.001 |
| 时期 Period | 9 | 504.141 | < 0.001 |
| 物种×生境 Species × habitat | 6 | 40.353 | < 0.001 |
| 物种×时期 Species × period | 27 | 17.003 | < 0.001 |
| 生境×时期 Habitat × period | 18 | 18.317 | < 0.001 |
| 物种×生境×时期 Species × habitat × period | 54 | 12.020 | < 0.001 |
Table 3 Repeated-measure ANOVA analysis on the effects of litter species, habitat type, and decomposition period on lignin degradation rate during litter decomposition process
| 影响因子 Influence factor | 自由度 Degree of freedom | F | p |
|---|---|---|---|
| 物种 Species | 3 | 165.753 | < 0.001 |
| 生境 Habitat | 2 | 75.197 | < 0.001 |
| 时期 Period | 9 | 504.141 | < 0.001 |
| 物种×生境 Species × habitat | 6 | 40.353 | < 0.001 |
| 物种×时期 Species × period | 27 | 17.003 | < 0.001 |
| 生境×时期 Habitat × period | 18 | 18.317 | < 0.001 |
| 物种×生境×时期 Species × habitat × period | 54 | 12.020 | < 0.001 |
| 生境 Habitat | 回归式 Regression model | ||||
|---|---|---|---|---|---|
| a0 | a1X1 | a2X2 | a3X3 | a4X4 | |
| 林下 Forest floor | ŷ = 0.424 | -0.017 C:N (0.353) | +0.042 C (0.607) | ||
| 溪流 Stream | ŷ = 1.602 | -0.031 Lignin:N (0.785) | +13.231 P (0.874) | ||
| 河岸带 Riparian zone | ŷ = -7.311 | +0.032 Lignin (0.783) | +20.108 P (0.888) | +0.164 N:P (0.932) | +0.090 C (0.940) |
Table 4 Stepwise regression analysis between lignin degradation rate (%/month) of the 2 years and foliar litter initial chemical properties
| 生境 Habitat | 回归式 Regression model | ||||
|---|---|---|---|---|---|
| a0 | a1X1 | a2X2 | a3X3 | a4X4 | |
| 林下 Forest floor | ŷ = 0.424 | -0.017 C:N (0.353) | +0.042 C (0.607) | ||
| 溪流 Stream | ŷ = 1.602 | -0.031 Lignin:N (0.785) | +13.231 P (0.874) | ||
| 河岸带 Riparian zone | ŷ = -7.311 | +0.032 Lignin (0.783) | +20.108 P (0.888) | +0.164 N:P (0.932) | +0.090 C (0.940) |
| 林下 Forest floor | AT | C | N | P | pH | ||
|---|---|---|---|---|---|---|---|
| 康定柳 Salix paraplesia | 26.925*** | 35.094*** | 1.987 | 0.340 | 6.194* | ||
| 高山杜鹃 Rhododendron lapponicum | 16.022*** | 0.064 | 5.700* | 17.816*** | 2.431 | ||
| 方枝柏 Sabina saltuaria | 10.134** | 1.037 | 23.348*** | 23.681*** | 8.314** | ||
| 四川红杉 Larix mastersiana | 30.336*** | 7.748** | 32.560*** | 10.076** | 13.489*** | ||
| 溪流 Stream | AT | HCO3- | NH4+ | NO3- | PO43- | pH | FV |
| 康定柳 Salix paraplesia | 0.001 | 0.572 | 2.692 | 13.248*** | 0.522 | 6.208* | 0.385 |
| 高山杜鹃 Rhododendron lapponicum | 1.286 | 1.722 | 6.088* | 8.832** | 1.612 | 1.652 | 1.590 |
| 方枝柏 Sabinasaltuaria | 1.245 | 4.809* | 7.579** | 8.964** | 0.001 | 6.454* | 0.103 |
| 四川红杉 Larix mastersiana | 2.815 | 2.179 | 4.681* | 11.866** | 0.063 | 5.594* | 0.053 |
| 河岸带 Riparian zone | AT | HCO3- | NH4+ | NO3- | PO43- | pH | FV |
| 康定柳 Salix paraplesia | 35.148*** | 5.748* | 12.267** | 0.256 | 1.305 | 16.431*** | 2.540 |
| 高山杜鹃 Rhododendron lapponicum | 3.702 | 2.822 | 2.029 | 1.369 | 0.001 | 7.300** | 5.752* |
| 方枝柏 Sabina saltuaria | 1.564 | 4.024* | 6.775* | 4.609* | 0.115 | 3.545 | 6.232* |
| 四川红杉 Larix mastersiana | 36.978*** | 15.189*** | 19.985*** | 4.305* | 0.055 | 0.371 | 11.602** |
Table 5 F-value for the regression analysis between lignin degradation rate (%/month) and environmental factors under different habitats during foliar litter decomposition
| 林下 Forest floor | AT | C | N | P | pH | ||
|---|---|---|---|---|---|---|---|
| 康定柳 Salix paraplesia | 26.925*** | 35.094*** | 1.987 | 0.340 | 6.194* | ||
| 高山杜鹃 Rhododendron lapponicum | 16.022*** | 0.064 | 5.700* | 17.816*** | 2.431 | ||
| 方枝柏 Sabina saltuaria | 10.134** | 1.037 | 23.348*** | 23.681*** | 8.314** | ||
| 四川红杉 Larix mastersiana | 30.336*** | 7.748** | 32.560*** | 10.076** | 13.489*** | ||
| 溪流 Stream | AT | HCO3- | NH4+ | NO3- | PO43- | pH | FV |
| 康定柳 Salix paraplesia | 0.001 | 0.572 | 2.692 | 13.248*** | 0.522 | 6.208* | 0.385 |
| 高山杜鹃 Rhododendron lapponicum | 1.286 | 1.722 | 6.088* | 8.832** | 1.612 | 1.652 | 1.590 |
| 方枝柏 Sabinasaltuaria | 1.245 | 4.809* | 7.579** | 8.964** | 0.001 | 6.454* | 0.103 |
| 四川红杉 Larix mastersiana | 2.815 | 2.179 | 4.681* | 11.866** | 0.063 | 5.594* | 0.053 |
| 河岸带 Riparian zone | AT | HCO3- | NH4+ | NO3- | PO43- | pH | FV |
| 康定柳 Salix paraplesia | 35.148*** | 5.748* | 12.267** | 0.256 | 1.305 | 16.431*** | 2.540 |
| 高山杜鹃 Rhododendron lapponicum | 3.702 | 2.822 | 2.029 | 1.369 | 0.001 | 7.300** | 5.752* |
| 方枝柏 Sabina saltuaria | 1.564 | 4.024* | 6.775* | 4.609* | 0.115 | 3.545 | 6.232* |
| 四川红杉 Larix mastersiana | 36.978*** | 15.189*** | 19.985*** | 4.305* | 0.055 | 0.371 | 11.602** |
| 1 | Berg B (2014). Decomposition patterns for foliar litter—A theory for influencing factors.Soil Biology & Biochem- istry, 78, 222-232. |
| 2 | Berg B, Kjønaas O, Johansson M-B, Erhagen B, Åkerblom S (2015). Late stage pine litter decomposition: Relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors.Forest Ecology and Management, 358, 41-47. |
| 3 | Berg B, McClaugherty C (2014). Plant Litter: Decomposition, Humus Formation, Carbon Sequestration. 3rd edn. Springer, Berlin. |
| 4 | Boyero L, Pearson RG, Gessner MO, Barmuta LA, Ferreira V, Graça MAS, Dudgeon D, Boulton AJ, Callisto M, Chauvet E, Helson JE, Bruder A, Albariño RJ, Yule CM, Arunachalam M, Davies JN, Figueroa R, Flecker AS, Ramírez A, Death RG, Iwata T, Mathooko JM, Mathuriau C, Gonçalves JF, Moretti MS, Jinggut T, Lamothe S, M’Erimba C, Ratnarajah L, Schindler MH, Castela J, Buria LM, Cornejo A, Villanueva VD, West DC (2011). A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration.Ecology Letters, 14, 289-294. |
| 5 | Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA (2016). Understanding the dominant controls on litter decomposition.Journal of Ecology, 104, 229-238. |
| 6 | Bradford MA, Warren II RJ, Baldrian P, Crowther TW, Maynard DS, Oldfield EE, Wieder WR, Wood SA, King JR (2014). Climate fails to predict wood decomposition at regional scales.Nature Climate Change, 4, 625-630. |
| 7 | Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008). Plant species traits are the predominant control on litter decomposition rates within biomes worldwide.Ecology Letters, 11, 1065-1071. |
| 8 | Ferreira V, Raposeiro PM, Pereira A, Cruz AM, Costa AC, Graça MAS, Gonçalves V (2016). Leaf litter decomposi- tion in remote oceanic island streams is driven by microbes and depends on litter quality and environmental conditions.Freshwater Biology, 61, 783-799. |
| 9 | García-Palacios P, Prieto I, Ourcival J-M, Hättenschwiler S (2016a). Disentangling the litter quality and soil microbial contribution to leaf and fine root litter decomposition responses to reduced rainfall.Ecosystems, 19, 490-503. |
| 10 | García-Palacios P, Shaw EA, Wall DH, Hättenschwiler S (2016b). Temporal dynamics of biotic and abiotic drivers of litter decomposition.Ecology Letters, 19, 554-563. |
| 11 | Gessner MO, Chauvet E, Dobson M (1999). A perspective on leaf litter breakdown in streams.Oikos, 85, 377-384. |
| 12 | Graça MA, Ferreira V, Canhoto C, Encalada AC, Guerrero- Bolaño F, Wantzen KM, Boyero L (2015). A conceptual model of litter breakdown in low order streams.International Review of Hydrobiology, 100, 1-12. |
| 13 | He W, Wu FZ, Yang WQ, Tan B, Zhao YY, Wu QQ, He M (2016). Lignin degradation in foliar litter of two shrub species from the gap center to the closed canopy in an alpine fir forest.Ecosystems, 19, 115-128. |
| 14 | He W, Wu FZ, Yang WQ, Wu QQ, He M, Zhao YY (2013). Effect of snow patches on leaf litter mass loss of two shrubs in an alpine forest.Chinese Journal of Plant Ecology, 37, 306-316. (in Chinese with English abstract)[何伟, 吴福忠, 杨万勤, 武启骞, 何敏, 赵野逸 (2013). 雪被斑块对高山森林两种灌木凋落叶质量损失的影响. 植物生态学报, 37, 306-316.] |
| 15 | Klotzbücher T, Kaiser K, Guggenberger G, Gatzek C, Kalbitz K (2011). A new conceptual model for the fate of lignin in decomposing plant litter.Ecology, 92, 1052-1062. |
| 16 | Li H, Wu FZ, Yang WQ, Xu LY, Ni XY, He J, Tan B, Hu Y (2016). Effects of forest gaps on litter lignin and cellulose dynamics vary seasonally in an alpine forest.Forests, 7, 27. |
| 17 | Martínez A, Larrañaga A, Pérez J, Descals E, Pozo J (2014). Temperature affects leaf litter decomposition in low- order forest streams: Field and microcosm approaches.FEMS Microbiology Ecology, 87, 257-267. |
| 18 | Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC (2007). Global-scale similarities in nitrogen release patterns during long-term decomposition.Science, 315, 361-364. |
| 19 | Prescott CE (2005). Do rates of litter decomposition tell us anything we really need to know?Forest Ecology and Management, 220, 66-74. |
| 20 | Wallace JB, Eggert S, Meyer JL, Webster J (1999). Effects of resource limitation on a detrital-based ecosystem.Ecological Monographs, 69, 409-442. |
| 21 | Yue K, Yang WQ, Peng CH, Peng Y, Zhang C, Huang CP, Tan Y, Wu FZ (2016). Foliar litter decomposition in an alpine forest meta-ecosystem on the eastern Tibetan Plateau. Science of the Total Environment, 566-567, 279-287. |
| 22 | Yue K, Yang WQ, Peng Y, Zhang C, Huang CP, Wu FZ (2015a). Carbon, nitrogen and phosphorus dynamics during winter foliar litter decomposition in an alpine forest river in the upper reaches of the Minjiang River.Chinese Journal of Applied and Environmental Biology, 21, 301-307. (in Chinese with English abstract)[岳楷, 杨万勤, 彭艳, 张川, 黄春萍, 吴福忠 (2015a). 岷江上游高山森林冬季河流中凋落叶碳氮和磷元素动态特征. 应用与环境生物学报, 21, 301-307.] |
| 23 | Yue K, Yang WQ, Peng Y, Zhang C, Huang CP, Wu FZ (2015b). Foliar litter mass loss in winter in an alpine forest river in the upper reaches of the Minjiang River.Resources and Environment in the Yangtze Basin, 24, 1177-1184. (in Chinese with English abstract)[岳楷, 杨万勤, 彭艳, 张川, 黄春萍, 吴福忠 (2015b). 岷江上游高山森林凋落叶在冬季河流中的质量损失特征. 长江流域资源与环境, 24, 1177-1184.] |
| 24 | Zhang C, Yang WQ, Yue K, Huang CP, Peng Y, Wu FZ (2015). Soluble nitrogen and soluble phosphorus dynamics during foliar litter decomposition in winter in alpine forest streams.Chinese Journal of Applied Ecology, 26, 1601-1608. (in Chinese with English abstract)[张川, 杨万勤, 岳楷, 黄春萍, 彭艳, 吴福忠 (2015). 高山森林溪流冬季不同时期凋落物分解中水溶性氮和磷的动态特征. 应用生态学报, 26, 1601-1608.] |
| 25 | Zhu JX, He XH, Wu FZ, Yang WQ, Tan B (2012). Decomposi- tion of Abies faxoniana litter varies with freeze-thaw stages and altitudes in subalpine/alpine forests of southwest China.Scandinavian Journal of Forest Research, 27, 586-596. |
| [1] | Shan-Shan LI xueqin liu. Analysis of functional traits of wetland plants in floodplains in the middle reaches of the Yangtze River [J]. Chin J Plant Ecol, 2024, 48(5): 601-611. |
| [2] | DONG Shao-Qiong, HOU Dong-Jie, QU Xiao-Yun, GUO Ke. A plot-based dataset of plant communities on the Qaidam Basin, China [J]. Chin J Plant Ecol, 2024, 48(4): 534-540. |
| [3] | PAN Yuan-Fang, PAN Liang-Hao, QIU Si-Ting, QIU Guang-Long, SU Zhi-Nan, SHI Xiao-Fang, FAN Hang-Qing. Variations in tree height among mangroves and their environmental adaptive mechanisms in China’s coastal areas [J]. Chin J Plant Ecol, 2024, 48(4): 483-495. |
| [4] | XUE Zhi-Fang, LIU Tong, WANG Li-Sheng, SONG Ji-Hu, CHEN Hong-Yang, XU Ling, YUAN Ye. Community structure and characteristics of plain valley forests in main tributaries of Ertix River Basin, China [J]. Chin J Plant Ecol, 2024, 48(3): 390-402. |
| [5] | NIU Yi-Di, CAI Ti-Jiu. Changes in species diversity and influencing factors in secondary forest succession in northern Da Hinggan Mountains [J]. Chin J Plant Ecol, 2024, 48(3): 349-363. |
| [6] | WANG Li-Ping, WU Jun-Jie, CHAI Yong, LI Jia-Hua, YANG Chang-Ji, ZHAO Shi-Jie. Spatial patterns and associations of dominant species in a subtropical mid-mountain moist evergreen broadleaf forest in Gaoligong Mountains, Southwest China [J]. Chin J Plant Ecol, 2024, 48(2): 180-191. |
| [7] | CHEN Yu-Ting, MA Song-Mei, ZHANG Dan, ZHANG Lin, WANG Chun-Cheng. Diversity pattern and formation mechanism of sympatric Haloxylon ammodendron and Haloxylon persicum in Xinjiang, China [J]. Chin J Plant Ecol, 2024, 48(1): 56-67. |
| [8] | XIAO Lan, DONG Biao, ZHANG Lin-Ting, DENG Chuan-Yuan, LI Xia, JIANG De-Gang, LIN Yong-Ming. Characteristics of main plant communities on uninhabited islands in Bohai Sea, China [J]. Chin J Plant Ecol, 2024, 48(1): 127-134. |
| [9] | WANG Yu-Ting, LIU Xu-Jing, TANG Chi-Fei, CHEN Wei-Yu, WANG Mei-Juan, XIANG Song-Zhu, LIU Mei, YANG Lin-Sen, FU Qiang, YAN Zhao-Gui, MENG Hong-Jie. Community characteristics and population dynamics of Acer miaotaiense, an extremely small population species in Shennongjia, China [J]. Chin J Plant Ecol, 2024, 48(1): 80-91. |
| [10] | CHEN Zhao-Quan, WANG Ming-Hui, HU Zi-Han, LANG Xue-Dong, HE Yun-Qiong, LIU Wan-De. Mechanisms of seedling community assembly in a monsoon evergreen broadleaf forest in Pu’er, Yunnan, China [J]. Chin J Plant Ecol, 2024, 48(1): 68-79. |
| [11] | CHEN Bao-Dong, FU Wei, WU Song-Lin, ZHU Yong-Guan. Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling [J]. Chin J Plant Ecol, 2024, 48(1): 1-20. |
| [12] | YUAN Ya-Ni, ZHOU Zhe, CHEN Bin-Zhou, GUO Yao-Xin, YUE Ming. Differential ecological strategies in functional traits among coexisting tree species in a Quercus aliena var. acuteserrata forest [J]. Chin J Plant Ecol, 2023, 47(9): 1270-1277. |
| [13] | LI Na, TANG Shi-Ming, GUO Jian-Ying, TIAN Ru, WANG Shan, HU Bing, LUO Yong-Hong, XU Zhu-Wen. Meta-analysis of effects of grazing on plant community properties in Nei Mongol grassland [J]. Chin J Plant Ecol, 2023, 47(9): 1256-1269. |
| [14] | REN Yue, GAO Guang-Lei, DING Guo-Dong, ZHANG Ying, ZHAO Pei-Shan, LIU Ye. Species composition and driving factors of the ectomycorrhizal fungal community associated with Pinus sylvestris var. mongolica at different growth periods [J]. Chin J Plant Ecol, 2023, 47(9): 1298-1309. |
| [15] | LI An-Yan, HUANG Xian-Fei, TIAN Yuan-Bin, DONG Ji-Xing, ZHENG Fei-Fei, XIA Pin-Hua. Chlorophyll a variation and its driving factors during phase shift from macrophyte- to phytoplankton-dominated states in Caohai Lake, Guizhou, China [J]. Chin J Plant Ecol, 2023, 47(8): 1171-1181. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn