

植物生态学报 ›› 2020, Vol. 44 ›› Issue (6): 677-686.DOI: 10.17521/cjpe.2020.0058
收稿日期:2020-03-06
接受日期:2020-04-23
出版日期:2020-06-20
发布日期:2020-06-12
通讯作者:
万贤崇
基金资助:
LIU Li-Yan1,2, FENG Jin-Xia1, LIU Wen-Xin3, WAN Xian-Chong1,*( )
)
Received:2020-03-06
Accepted:2020-04-23
Online:2020-06-20
Published:2020-06-12
Contact:
WAN Xian-Chong
Supported by:摘要:
为研究水通道蛋白PtPIP2;8基因功能, 了解其不同表达水平的转基因84K杨(Populus alba × P. glandulossa)应对干旱胁迫的响应, 该文以转PtPIP2;8 84K杨抑制表达株系(抑制表达)、野生型(WT)和转PtPIP2;8 84K杨超表达株系(超表达)为试验材料, 测定PtPIP2;8表达水平、根系导度、光响应曲线、气体交换参数、生长及根系形态指标。结果显示: (1) WT植株PtPIP2;8仅在根系表达; 超表达植株PtPIP2;8除在根部显著表达外, 在茎和叶片中也显著表达; 抑制表达植株PtPIP2;8仅在根部有微量表达, 表达量分别是WT和超表达植株的1/20和1/80。(2)根系结构分析发现, 超表达植株总根长、总根表面积、总根体积、总根尖数显著低于WT和抑制表达植株, 根系导水率显著高于WT和抑制表达植株, 表明PtPIP2;8参与了植物根系水分运输, 提高了水分运输效率。(3)正常水分条件下, 抑制表达植株苗高、叶面积显著低于WT和超表达植株, 根冠比显著高于WT和超表达植株。干旱胁迫后, 抑制表达植株净光合速率(Pn)、气孔导度(Gs)下降幅度小, 仍能维持较高的Pn。气体交换参数显示抑制表达植株Pn、Gs日变化为“单峰”型, 属气孔因素引起的净光合速率下降;WT和超表达植株Pn、Gs日变化为“双峰”型, 干旱胁迫后, 抑制表达植株Pn略微下降, WT和超表达植株Pn均下降, 尤其是13:00、15:00下降显著, 表明WT和超表达植株对干旱胁迫更加敏感, 干旱对其影响更大。(4)干旱胁迫后, 抑制表达植株相对生长速率、总生物量降低的最少, 根冠比最高; 总根表面积、总根体积、总根尖数显著高于WT植株。表明PtPIP2;8直接参与水分运输并提高水分运输效率, 其转化影响了植株根系发育和生长。超表达植株根系发育的下降和叶面积的增大减弱了它的抗旱性, 而抑制表达植株矮小, 降低的叶面积, 增加的根系生长和根冠比提高了它的抗旱能力。从研究结果来看, 水通道蛋白提高了水分跨膜运输效率, 而非水通道蛋白导水机制对干旱有较强的耐受性。
刘丽燕, 冯锦霞, 刘文鑫, 万贤崇. 干旱胁迫对转PtPIP2;8基因84K杨苗木光合、生长和根系结构的影响. 植物生态学报, 2020, 44(6): 677-686. DOI: 10.17521/cjpe.2020.0058
LIU Li-Yan, FENG Jin-Xia, LIU Wen-Xin, WAN Xian-Chong. Effects of drought stress on photosynthesis, growth and root structure of transgenic PtPIP2;8 poplar 84K (Populus alba × P. glandulosa). Chinese Journal of Plant Ecology, 2020, 44(6): 677-686. DOI: 10.17521/cjpe.2020.0058
| 基因名称 Gene name | 上游引物 Forward primer | 下游引物 Reverse primer |
|---|---|---|
| PtACTINrt | 5′-AAACTGTAATGGTCCTCCCTCCG-3′ | 5′-GCATCATCACAATCACTCTCCGA-3′ |
| PtPIP2;8 | 5′-GAGACTGTGAGGGACTACCAGGA-3′ | 5′-AATACCAAGAATGCCAACACCAC-3′ |
表1 实时荧光定量PCR引物序列
Table 1 Primer sequences for real-time PCR
| 基因名称 Gene name | 上游引物 Forward primer | 下游引物 Reverse primer |
|---|---|---|
| PtACTINrt | 5′-AAACTGTAATGGTCCTCCCTCCG-3′ | 5′-GCATCATCACAATCACTCTCCGA-3′ |
| PtPIP2;8 | 5′-GAGACTGTGAGGGACTACCAGGA-3′ | 5′-AATACCAAGAATGCCAACACCAC-3′ |
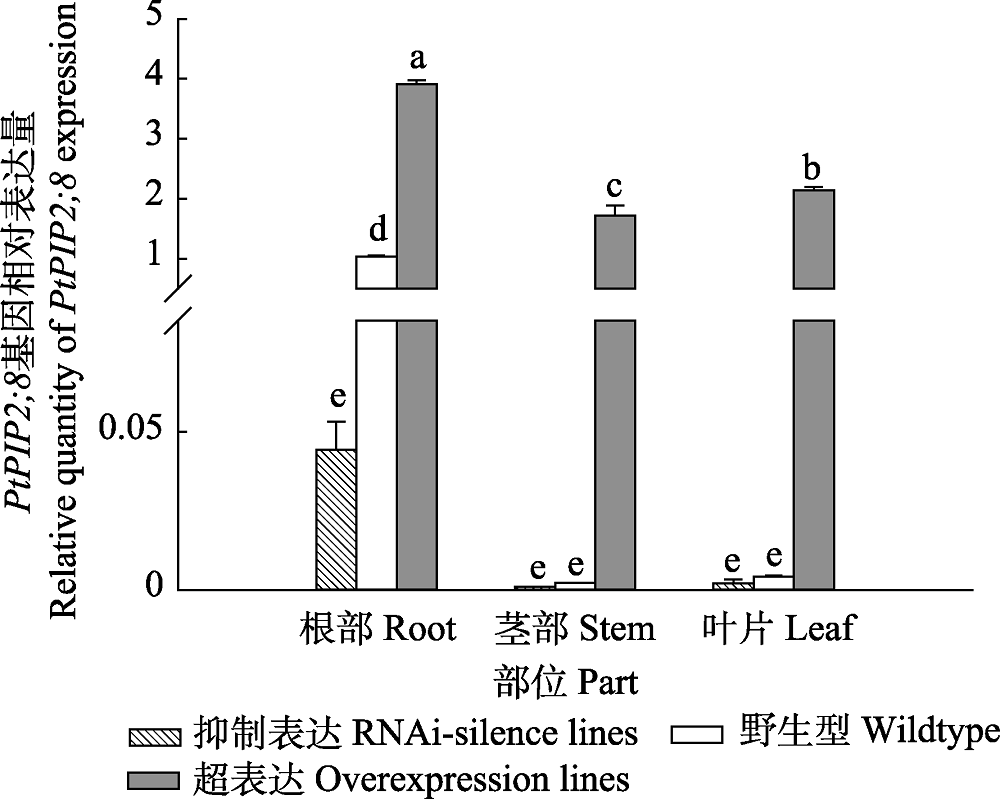
图1 84K杨转基因和野生型植株PtPIP2;8基因的相对表达量(平均值+标准偏差)。 不同小写字母表示转基因和野生型植株不同部位之间相对表达量差异显著(p < 0.05)。
Fig. 1 Relative quantity of PtPIP2;8 gene expression in transgenic and wildtype poplar 84K (mean + SD). Different lowercase letters indicate significant differences in relative expression levels between different parts of transgenic and wildtype plants (p < 0.05).
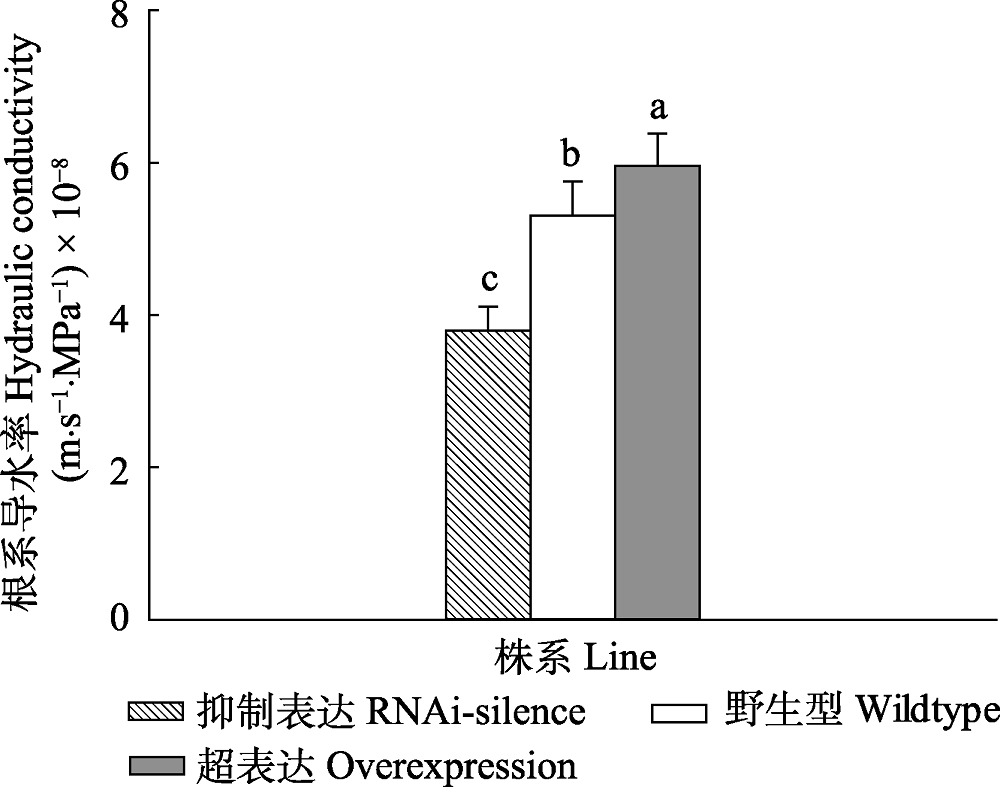
图2 正常水分条件下84K杨转基因和野生型植株根系导水率变化(平均值+标准偏差)。 不同小写字母表示转基因和野生型植株之间根系导水率存在显著差异(p < 0.05)。
Fig. 2 Changes of root hydraulic conductivity of transgenic and wildtype poplar 84K under normal watering condition (mean + SD). Different lowercase letters indicate significant differences in transgenic and wildtype plants (p < 0.05).
| 株系 Line | 基径 Basal diameter (mm) | 苗高 Height (cm) | 根冠比 Root-shoot ratio | 叶面积 Leaf area (cm2) | 比叶面积 Specific leaf area (cm2·g-1) |
|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 4.93 ± 0.26c | 71.60 ± 4.57b | 0.54 ± 0.05a | 534.35 ± 32.47b | 254.33 ± 14.32a |
| 野生型 Wildtype | 5.62 ± 0.43a | 82.55 ± 4.70a | 0.50 ± 0.03b | 565.23 ± 25.05a | 262.57 ± 20.49a |
| 超表达 Overexpression | 5.02 ± 0.46bc | 83.85 ± 4.99a | 0.32 ± 0.02c | 576.75 ± 13.31a | 240.61 ± 11.19a |
表2 正常水分条件下84K杨转基因和野生型植株生物量变化(平均值±标准偏差)
Table 2 The change of biomass of transgenic and wildtype poplar 84K under normal water condition (mean ± SD)
| 株系 Line | 基径 Basal diameter (mm) | 苗高 Height (cm) | 根冠比 Root-shoot ratio | 叶面积 Leaf area (cm2) | 比叶面积 Specific leaf area (cm2·g-1) |
|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 4.93 ± 0.26c | 71.60 ± 4.57b | 0.54 ± 0.05a | 534.35 ± 32.47b | 254.33 ± 14.32a |
| 野生型 Wildtype | 5.62 ± 0.43a | 82.55 ± 4.70a | 0.50 ± 0.03b | 565.23 ± 25.05a | 262.57 ± 20.49a |
| 超表达 Overexpression | 5.02 ± 0.46bc | 83.85 ± 4.99a | 0.32 ± 0.02c | 576.75 ± 13.31a | 240.61 ± 11.19a |
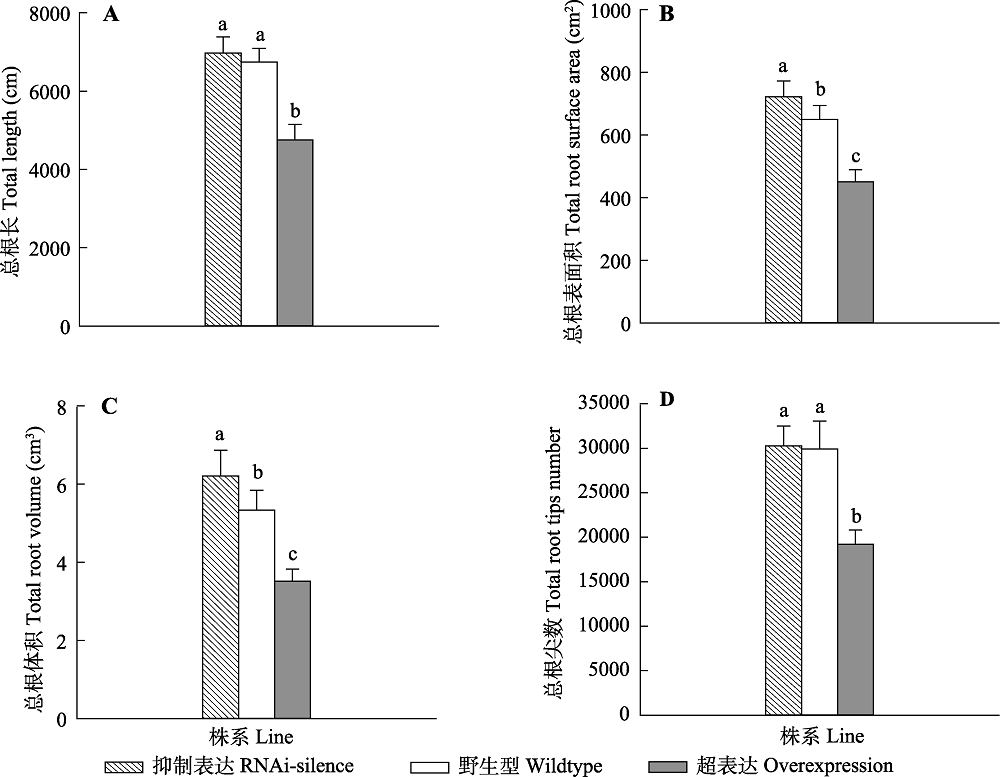
图3 正常水分条件下84K杨转基因和野生型植株根系形态变化(平均值+标准偏差)。 写字母表示转基因和野生型植株之间存在显著差异(p < 0.05)。
Fig. 3 Changes in root morphological traits of transgenic and wildtype poplar 84K under normal water condition (mean + SD). ferent lowercase letters indicate significant differences between transgenic and wildtype plants (p < 0.05).
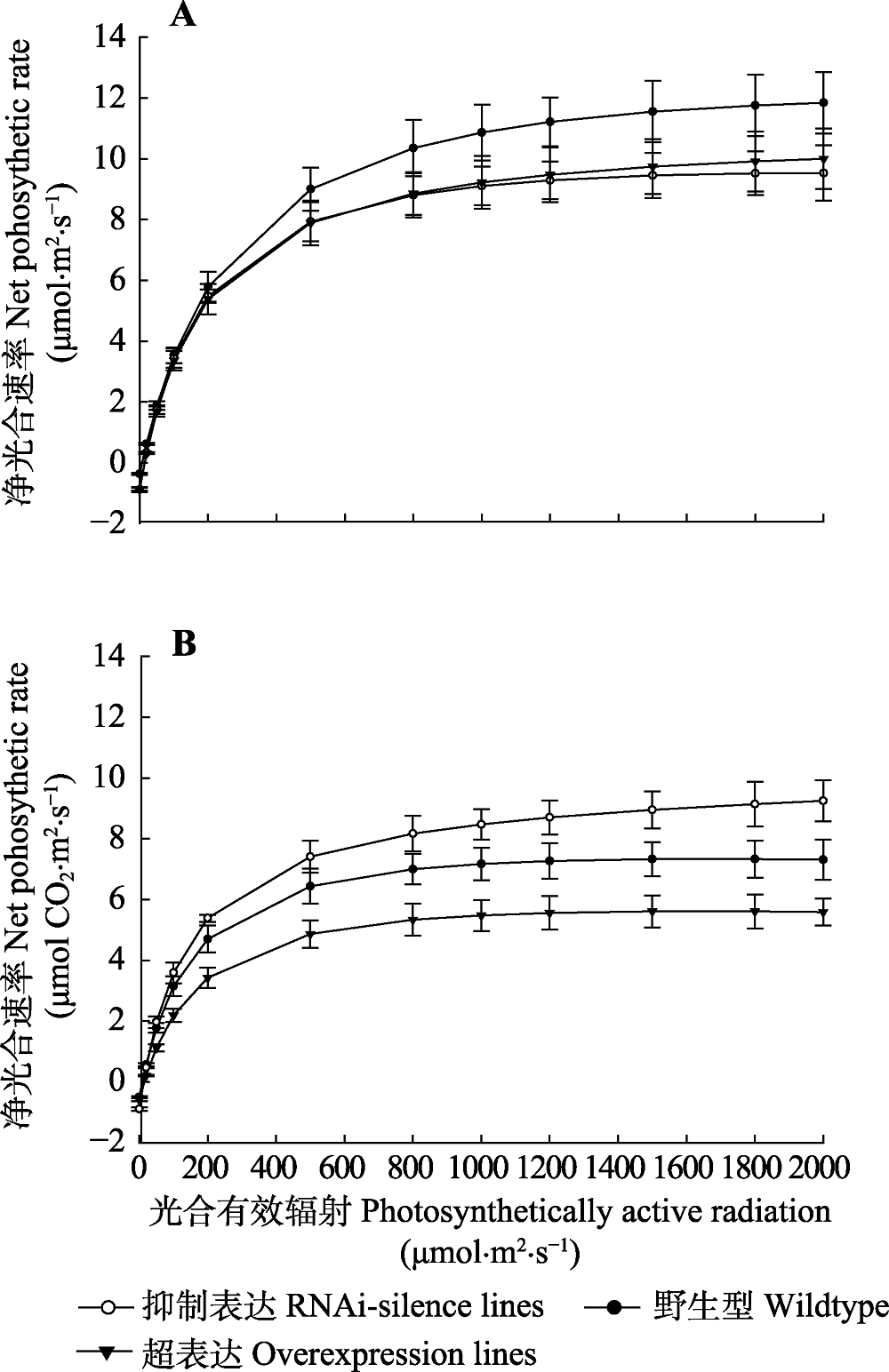
图4 84K杨转基因和野生型植株净光合速率的光响应曲线(平均值±标准偏差)。 正常水分条件下净光合速率的光响应曲线。B, 干旱胁迫后净光合速率的光响应曲线。
Fig. 4 Light response curve of net photosynthetic rate in transgenic and wildtype poplar 84K (mean ± SD). Light response of net photosynthetic rate under normal watering conditions. B, Light response of net photosynthetic rate after drought stress.
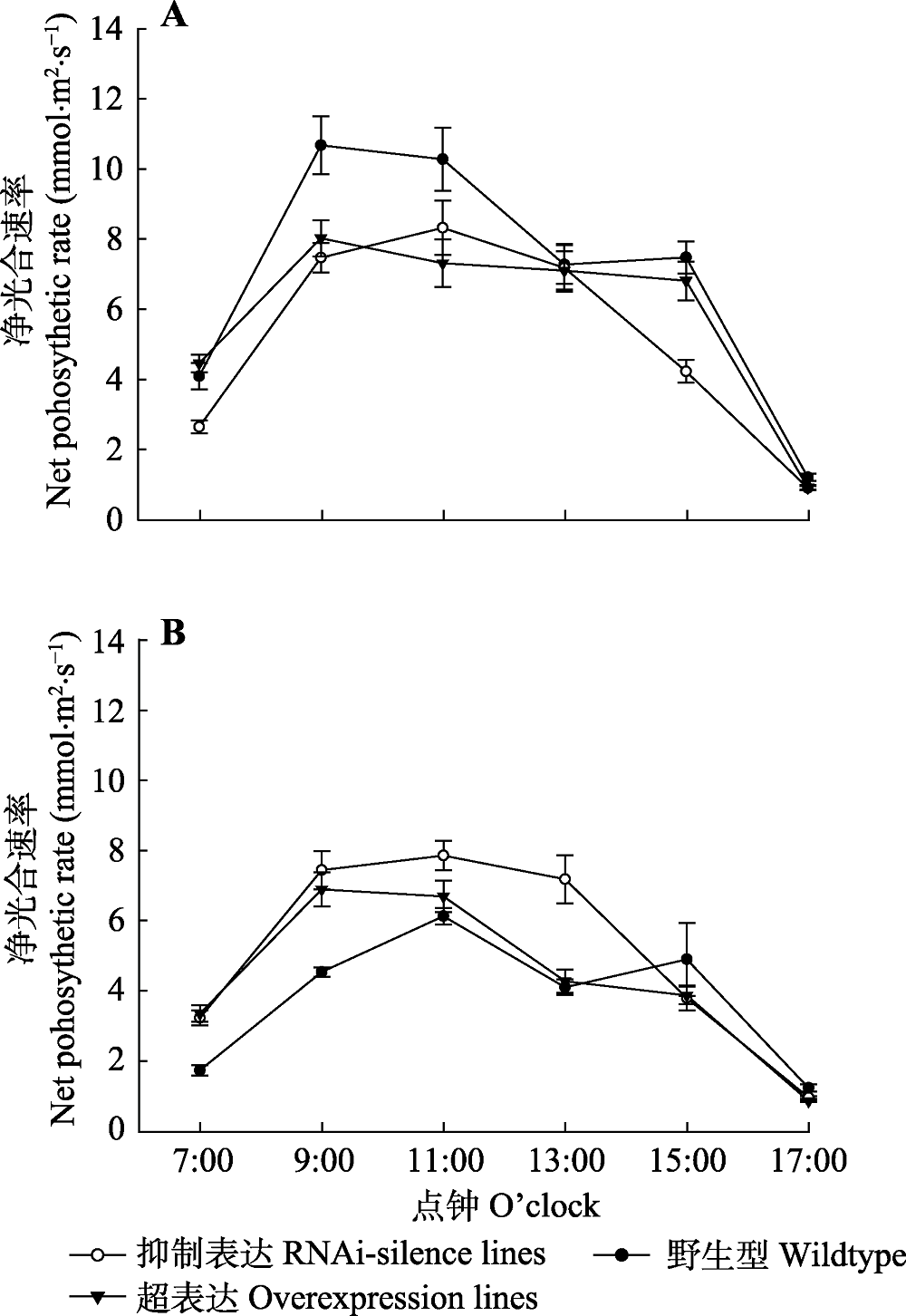
图5 84K杨转基因和野生型植株净光合速率日变化(平均值±标准偏差)。 A, 正常水分条件下净光合速率日变化。B, 干旱胁迫后净光合速率日变化。
Fig. 5 Diurnal variations of net photosynthetic rate in transgenic and wildtype poplar 84K (mean ± SD). A, Diurnal variations of net photosynthetic rate under normal watering conditions. B, Diurnal variations of net photosynthetic rate after drought stress.
| 株系 Line | 基径 Basal diameter (%) | 苗高 Height (%) | 地上生物量 Aboveground biomass (%) | 地下生物量 Belowground biomass (%) | 根冠比 Root shoot ratio | 叶面积 Leaf area (%) |
|---|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 97 ± 3a | 94 ± 3a | 93 ± 2a | 84 ± 5a | 0.5 ± 0.02a | 68 ± 4a |
| 野生型 Wildtype | 92 ± 3b | 88 ± 4b | 86 ± 7b | 77 ± 4b | 0.4 ± 0.03b | 44 ± 2b |
| 超表达 Overexpression | 92 ± 2b | 87 ± 4b | 77 ± 3c | 76 ± 5b | 0.3 ± 0.02c | 43 ± 2b |
表3 干旱胁迫后84K杨转基因和野生型植株相对生长速率及生物量变化(平均值±标准偏差)
Table 3 Changes of relative growth rate and biomass of transgenic and wildtype poplar 84K after drought stress (mean ± SD)
| 株系 Line | 基径 Basal diameter (%) | 苗高 Height (%) | 地上生物量 Aboveground biomass (%) | 地下生物量 Belowground biomass (%) | 根冠比 Root shoot ratio | 叶面积 Leaf area (%) |
|---|---|---|---|---|---|---|
| 抑制表达 RNAi-silence | 97 ± 3a | 94 ± 3a | 93 ± 2a | 84 ± 5a | 0.5 ± 0.02a | 68 ± 4a |
| 野生型 Wildtype | 92 ± 3b | 88 ± 4b | 86 ± 7b | 77 ± 4b | 0.4 ± 0.03b | 44 ± 2b |
| 超表达 Overexpression | 92 ± 2b | 87 ± 4b | 77 ± 3c | 76 ± 5b | 0.3 ± 0.02c | 43 ± 2b |
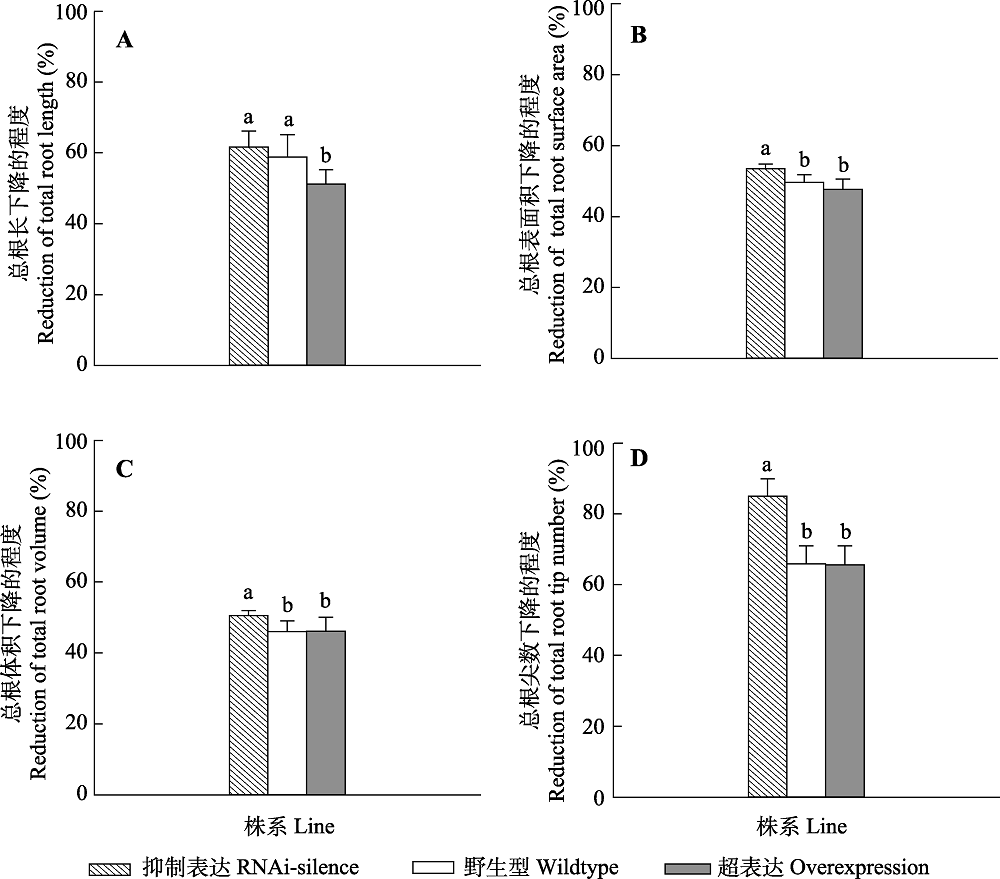
图6 干旱胁迫后转基因和野生型84K杨根系形态变化(平均值+标准偏差)。 不同小写字母表示转基因和野生型植株之间存在显著差异(p < 0.05)。
Fig. 6 Changes of root morphological traits of transgenic and wildtype poplar 84K after drought dress (mean + SD). Different lowercase letters indicate significant differences between transgenic and wildtype plants (p < 0.05).
| [1] |
Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G (2003). Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. The Plant Cell, 15, 1-9.
DOI URL PMID |
| [2] |
Alexandersson E, Fraysse L, Sjövall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005). Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology, 59, 469-484.
DOI URL PMID |
| [3] | Bao ZLT, Gao L, Wang SM (2017). Physiological functions of plant aquaporin. Plant Physiology Journal, 53, 1171-1178. |
| [ 包珠拉太, 高丽, 王锁民 (2017). 植物水通道蛋白及其生理功能. 植物生理学报, 53, 1171-1178.] | |
| [4] | Bots M, Feron R, Uehlein N, Weterings K, Kaldenhoff R, Mariani T (2005a). PIP1 and PIP2 aquaporins are differentially expressed during tobacco anther and stigma development. Journal of Experimental Botany, 56, 113-121. |
| [5] | Bots M, Vergeldt F, Wolters-Arts M, Weterings K, van As H, Mariani C (2005b). Aquaporins of the PIP2 class are required for efficient anther dehiscence in tobacco. Plant Physiology, 137, 1049-1056. |
| [6] |
Boughalleb F, Hajlaoui H (2011). Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiologiae Plantarum, 33, 53-65.
DOI URL |
| [7] | Cai Q, Ding GJ, Wen XP (2016). Cloning of thePmPIP1 gene from Pinus massoniana and its expression with drought stress. Journal of Zhejiang A&F University, 33, 191-200. |
| [ 蔡琼, 丁贵杰, 文晓鹏 (2016). 马尾松水通道蛋白PmPIP1基因克隆及在干旱胁迫下的表达分析. 浙江农林大学学报, 33, 191-200.] | |
| [8] |
Chaumont F, Tyerman SD (2014). Aquaporins: highly regulated channels controlling plant water relations. Plant Physiology, 164, 1600-1618.
DOI URL PMID |
| [9] |
Fotiadis D, Jenö P, Mini T, Wirtz S, Müller SA, Fraysse L, Kjellbom P, Engel A (2001). Structural characterization of two aquaporins isolated from native spinach leaf plasma membranes. Journal of Biological Chemistry, 276, 1707-1714.
DOI URL PMID |
| [10] | Huang J, Chen C, Zhang WX, Ding CJ, Su XH, Huang QJ (2017). Effects of drought stress on anatomical structure and photosynthetic characteristics of transgenic JERF36Populus alba × P. berolinensis seedling leaves. Scientia Silvae Sinicae, 53(5), 8-15. |
| [ 黄绢, 陈存, 张伟溪, 丁昌俊, 苏晓华, 黄秦军 (2017). 干旱胁迫对转JERF36银中杨苗木叶片解剖结构及光合特性的影响. 林业科学, 53(5), 8-15.] | |
| [11] |
Javot H, Maurel C (2002). The role of aquaporins in root water uptake. Annals of Botany, 90, 301-313.
DOI URL PMID |
| [12] | Jiang LJ, Chen CH, Yan X, Yang SM (2018). Research progress on responsive mechanism of aquaporins to drought stress in plants. Guihaia, 38, 672-680. |
| [ 江林娟, 陈春华, 颜旭, 杨世民 (2018). 植物水通道蛋白的干旱应答机制研究进展. 广西植物, 38, 672-680.] | |
| [13] |
Johansson I, Karlsson M, Johanson U, Larsson C, Kjellbom P (2000). The role of aquaporins in cellular and whole plant water balance. Biochimica et Biophysica Acta, 1465, 324-342.
DOI URL PMID |
| [14] | Kaldenhoff R, Grote K, Zhu JJ, Zimmermann U (1998). Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. The Plant Journal, 14, 121-128. |
| [15] |
Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994). Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression. The Plant Journal, 6, 187-199.
DOI URL PMID |
| [16] |
Katsuhara M, Koshio K, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K (2003). Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant and Cell Physiology, 44, 1378-1383.
DOI URL PMID |
| [17] |
Knapp AK, Smith MD (2001). Variation among biomes in temporal dynamics of aboveground primary production. Science, 291, 481-484.
DOI URL PMID |
| [18] | Knipfer T, Besse M, Verdeil JL, Fricke W (2011). Aquaporin- facilitated water uptake in barley (Hordeum vulgare L.) roots. Journal of Experimental Botany, 62, 4115-4126. |
| [19] | Leng HN (2012). Cloning and Expression of PIPs Gene in Populus and Aquaporins Role in Embolism Recovery. PhD dissertation, Chinese Academy of Forestry, Beijing. 23-95. |
| [ 冷华妮 (2012). 植物栓塞修复机制与质膜内在水通道蛋白基因的克隆、表达和转基因研究. 博士学位论文, 中国林业科学研究院, 北京. 23-95.] | |
| [20] | Lopez F, Bousser A, Sissoëff I, Gaspar M, Lachaise B, Hoarau J, Mahé A (2003). Diurnal regulation of water transport and aquaporin gene expression in maize roots: contribution of PIP2 proteins. Plant and Cell Physiology, 44, 1384-1395. |
| [21] | Lovisolo C, Secchi F, Nardini A, Salleo S, Buffa R, Schubert A (2007). Expression of PIP1 and PIP2 aquaporins is enhanced in olive dwarf genotypes and is related to root and leaf hydraulic conductance. Physiologia Plantarum, 130, 543-551. |
| [22] | Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ (2002). Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology, 130, 2101-2110. |
| [23] | Martre P, North GB, Nobel PS (2001). Hydraulic conductance and mercury-sensitive water transport for roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiology, 126, 352-362. |
| [24] | Matsunami M, Toyofuku K, Ishikawa-Sakurai J, Ogawa A, Matsunami T, Kokubun M (2016). Root development and the expression of aquaporin genes in rice seedlings under osmotic stress. Plant Production Science, 19, 315-322. |
| [25] | O’Brien M, Bertrand C, Matton DP (2002). Characterization of a fertilization-induced and developmentally regulated plasma-membrane aquaporin expressed in reproductive tissues, in the wild potato Solanum chacoense Bitt. Planta, 215, 485-493. |
| [26] |
Prieto I, Armas C, Pugnaire FI (2012). Water release through plant roots: new insights into its consequences at the plant and ecosystem level. New Phytologist, 193, 830-841.
DOI URL PMID |
| [27] | Schuurmans JAMJ, van Dongen JT, Rutjens BPW, Boonman A, Pieterse CMJ, Borstlap AC (2003). Members of the aquaporin family in the developing pea seed coat include representatives of the PIP, TIP, and NIP subfamilies. Plant Molecular Biology, 53, 655-667. |
| [28] |
Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002). PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. The Plant Cell, 14, 869-876.
DOI URL PMID |
| [29] | Steudle E, Peterson CA (1998). How does water get through roots? Journal of Experimental Botany, 49, 775-788. |
| [30] |
Suga S, Imagawa S, Maeshima M (2001). Specificity of the accumulation of mRNAs and proteins of the plasma membrane and tonoplast aquaporins in radish organs. Planta, 212, 294-304.
DOI URL PMID |
| [31] |
Weig A, Deswarte C, Chrispeels MJ (1997). The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiology, 114, 1347-1357.
DOI URL PMID |
| [32] | Yao QQ, Xie GS (2005). The photosynthetic stomatal and nonstomatal limitation under drought stress. Chinese Journal of Tropical Agriculture, 25(4), 80-85. |
| [ 姚庆群, 谢贵水 (2005). 干旱胁迫下光合作用的气孔与非气孔限制. 热带农业科学, 25(4), 80-85.] | |
| [33] |
Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F (2007). FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proceedings of the National Academy of Sciences of the United States of America, 104, 12359-12364.
DOI URL PMID |
| [34] | Zhao T, Cheng L, Wang C, You JQ, Zhu YF, Wang YX (2018). Effect of different apple scion-rootstock combinations on growth and photosynthesis characteristics. Acta Botanica Boreali-Occidentalia Sinica, 38, 1707-1716. |
| [ 赵通, 程丽, 王城, 游继权, 朱燕芳, 王延秀 (2018). 不同苹果砧穗组合的生长及光合特性. 西北植物学报, 38, 1707-1716.] | |
| [35] | Zhou XY (2013). Research on 84K Populus Regeneration and Genetic Transformation System. Master degree dissertation, Northeast Forestry University, Harbin. 1-8. |
| [ 周熙莹 (2013). 84K杨再生和遗传转化体系的研究. 硕士学位论文, 东北林业大学, 哈尔滨. 1-8.] |
| [1] | 范宏坤, 曾涛, 金光泽, 刘志理. 小兴安岭不同生长型阔叶植物叶性状变异及权衡[J]. 植物生态学报, 2024, 48(3): 364-376. |
| [2] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [3] | 张志山, 韩高玲, 霍建强, 黄日辉, 薛书文. 固沙灌木柠条锦鸡儿和中间锦鸡儿木质部导水与叶片光合能力对土壤水分的响应[J]. 植物生态学报, 2023, 47(10): 1422-1431. |
| [4] | 李变变, 张凤华, 赵亚光, 孙秉楠. 不同刈割程度对油莎豆非结构性碳水化合物代谢及生物量的影响[J]. 植物生态学报, 2023, 47(1): 101-113. |
| [5] | 周洁, 杨晓东, 王雅芸, 隆彦昕, 王妍, 李浡睿, 孙启兴, 孙楠. 梭梭和骆驼刺对干旱的适应策略差异[J]. 植物生态学报, 2022, 46(9): 1064-1076. |
| [6] | 徐丽娇, 郝志鹏, 谢伟, 李芳, 陈保冬. 丛枝菌根真菌根外菌丝跨膜H +和Ca 2+流对干旱胁迫的响应[J]. 植物生态学报, 2018, 42(7): 764-773. |
| [7] | 程汉亭, 李勤奋, 刘景坤, 严廷良, 张俏燕, 王进闯. 橡胶林下益智光合特性的季节动态变化[J]. 植物生态学报, 2018, 42(5): 585-594. |
| [8] | 王曦,胡红玲,胡庭兴,张城浩,王鑫,刘丹. 干旱胁迫对桢楠幼树渗透调节与活性氧代谢的影响及施氮的缓解效应[J]. 植物生态学报, 2018, 42(2): 240-251. |
| [9] | 罗丹丹, 王传宽, 金鹰. 植物水分调节对策: 等水与非等水行为[J]. 植物生态学报, 2017, 41(9): 1020-1032. |
| [10] | 翟占伟, 龚吉蕊, 罗亲普, 潘琰, 宝音陶格涛, 徐沙, 刘敏, 杨丽丽. 氮添加对内蒙古温带草原羊草光合特性的影响[J]. 植物生态学报, 2017, 41(2): 196-208. |
| [11] | 岑宇, 刘美珍. 凝结水对干旱胁迫下羊草和冰草生理生态特征及叶片形态的影响[J]. 植物生态学报, 2017, 41(11): 1199-1207. |
| [12] | 郭瑞, 周际, 杨帆, 李峰, 李昊如, 夏旭, 刘琪. 拔节孕穗期小麦干旱胁迫下生长代谢变化规律[J]. 植物生态学报, 2016, 40(12): 1319-1327. |
| [13] | 郭瑞, 李峰, 周际, 李昊儒, 夏旭, 刘琪. 亚麻响应盐、碱胁迫的生理特征[J]. 植物生态学报, 2016, 40(1): 69-79. |
| [14] | 邹长明, 王允青, 刘英, 张晓红, 唐杉. 四种豆科作物的光合生理和生长发育对弱光的响应[J]. 植物生态学报, 2015, 39(9): 909-916. |
| [15] | 安东升, 曹娟, 黄小华, 周娟, 窦美安. 基于Lake模型的叶绿素荧光参数在甘蔗苗期抗旱性研究中的应用[J]. 植物生态学报, 2015, 39(4): 398-406. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19