

植物生态学报 ›› 2018, Vol. 42 ›› Issue (7): 764-773.DOI: 10.17521/cjpe.2018.0089
所属专题: 菌根真菌
徐丽娇1,2,郝志鹏1,谢伟1,2,李芳1,2,陈保冬1,2,*( )
)
出版日期:2018-07-20
发布日期:2018-06-11
通讯作者:
陈保冬
基金资助:
XU Li-Jiao1,2, HAO Zhi-Peng1, XIE Wei1,2, LI Fang1,2, CHEN Bao-Dong1,2,*( )
)
Online:2018-07-20
Published:2018-06-11
Contact:
Bao-Dong CHEN
Supported by:摘要:
丛枝菌根真菌(AMF)能够和大多数陆地植物形成共生体系, 对于植物生长发育和适应各种逆境胁迫具有重要作用。很多研究表明干旱胁迫下AMF能够促进宿主植物对水分的吸收从而增强植物抗旱能力, 但目前针对AMF根外菌丝响应水分胁迫的生理变化以及AMF与宿主植物逆境信号交流的研究并不多。该研究利用AMF Rhizophagus irregularis和胡萝卜(Daucus carota var. sativa)毛状根双重无菌培养体系获得纯净根外菌丝, 向培养基添加聚乙二醇(PEG)模拟干旱胁迫, 运用场发射扫描电子显微镜(FE-SEM-EDS)观察干旱胁迫对AMF根外菌丝形态的影响, 同时采用非损伤微测技术(NMT)观测根外菌丝跨膜H +和Ca 2+离子流变化。结果发现, PEG处理1 h后菌丝尖端和侧面发生H +外流和强烈的Ca 2+内流, 荧光探针分析也显示菌丝胞内pH值显著上升、Ca 2+浓度增加; PEG处理24 h后菌丝形态发生明显变化, 培养基pH值降低, P、Ca、Fe等元素在菌丝际积累。这些试验结果表明, 干旱胁迫下AMF根外菌丝跨膜H +和Ca 2+流发生变化, 促进了菌丝与环境之间的物质交换。菌丝酸化生长环境有利于养分吸收, 并促进AMF与宿主植物之间的信号交流以增强植物的耐旱性。
徐丽娇, 郝志鹏, 谢伟, 李芳, 陈保冬. 丛枝菌根真菌根外菌丝跨膜H +和Ca 2+流对干旱胁迫的响应. 植物生态学报, 2018, 42(7): 764-773. DOI: 10.17521/cjpe.2018.0089
XU Li-Jiao, HAO Zhi-Peng, XIE Wei, LI Fang, CHEN Bao-Dong. Transmembrane H + and Ca 2+ fluxes through extraradical hyphae of arbuscular mycorrhizal fungi in response to drought stress. Chinese Journal of Plant Ecology, 2018, 42(7): 764-773. DOI: 10.17521/cjpe.2018.0089
| 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) | 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) |
|---|---|---|---|
| Mg2+ | 70.8 | Fe2+ | 0.1 |
| SO42- | 280.1 | Mn2+ | 1.6 |
| K+ | 61 | Zn2+ | 0.6 |
| NO3- | 230 | BO33- | 0.24 |
| Cl- | 37.2 | 甘氨酸 Glycine | 3 |
| H2PO4- | 4.8 | 维生素B1 Vitamin B1 | 0.1 |
| Ca2+ | 49 | 维生素B6 Vitamin B6 | 0.1 |
| I- | 0.58 | 烟酸 Nicotinic acid | 0.5 |
| Na+ | 0.43 | 肌醇 Inositol | 50 |
表1 M培养基成分
Table 1 Formulation of the M medium
| 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) | 离子/化合物 Ion/Compound | 含量 Content (mg·L-1) |
|---|---|---|---|
| Mg2+ | 70.8 | Fe2+ | 0.1 |
| SO42- | 280.1 | Mn2+ | 1.6 |
| K+ | 61 | Zn2+ | 0.6 |
| NO3- | 230 | BO33- | 0.24 |
| Cl- | 37.2 | 甘氨酸 Glycine | 3 |
| H2PO4- | 4.8 | 维生素B1 Vitamin B1 | 0.1 |
| Ca2+ | 49 | 维生素B6 Vitamin B6 | 0.1 |
| I- | 0.58 | 烟酸 Nicotinic acid | 0.5 |
| Na+ | 0.43 | 肌醇 Inositol | 50 |
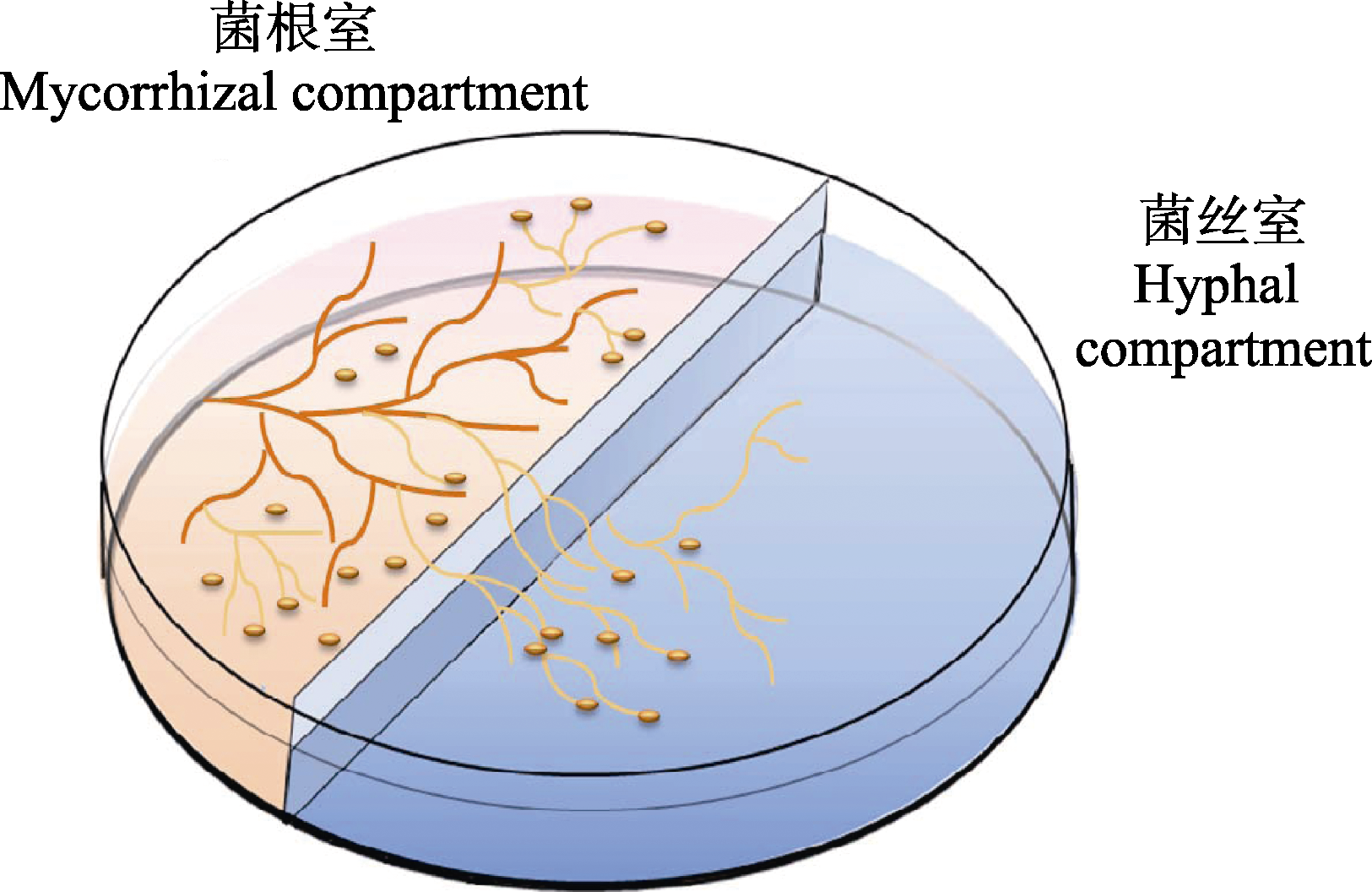
图1 丛枝菌根真菌-胡萝卜毛状根分室培养系统示意图。左边是菌根室(mycorrhizal compartment), 右边是菌丝室(hyphal compartment)。菌根室中加入用质量浓度0.4%的植物凝胶固化后的M培养基, 并接入菌根化的转移Ri T-DNA胡萝卜毛状根; 菌丝室加入液体M培养基, 以供根外菌丝生长(参考St-Arnaud et al., 1996)。
Fig. 1 Diagram of the two-compartments in vitro culture system of arbuscular mycorrhizal fungi with hairy carrot root. Mycorrhizal compartment was filled with solid M medium gelled with 0.4% phytagel, allowing development of mycorrhizal roots; extraradical mycelium ramified into hyphal compartment filled with liquid M medium without sucrose and phytagel, and the roots that crossed the central wall were trimmed to prevent their growth in hyphal compartment (referred to St-Arnaud et al., 1996).
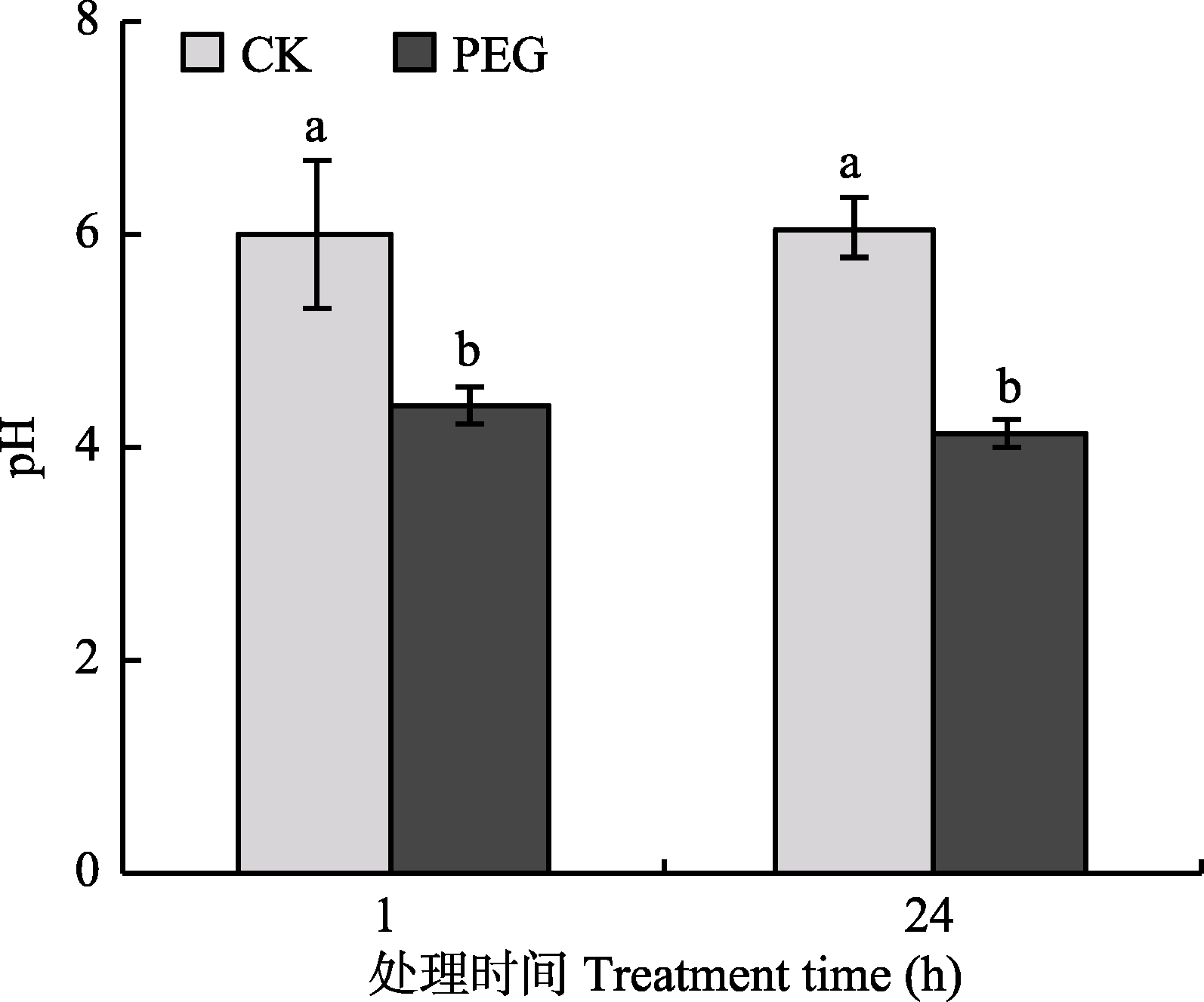
图2 PEG处理下菌丝室培养基的pH值(平均值±标准偏差, n = 6)。不同小写字母表示处理间差异显著 (Duncan’s多重比较, p < 0.05)。
Fig. 2 The pH value of culture medium in hyphal compartment after treatment by PEG (mean ± SD, n = 6). Different lowercase letters indicate significant difference between treatments (Duncan’s multiple range test, p < 0.05).
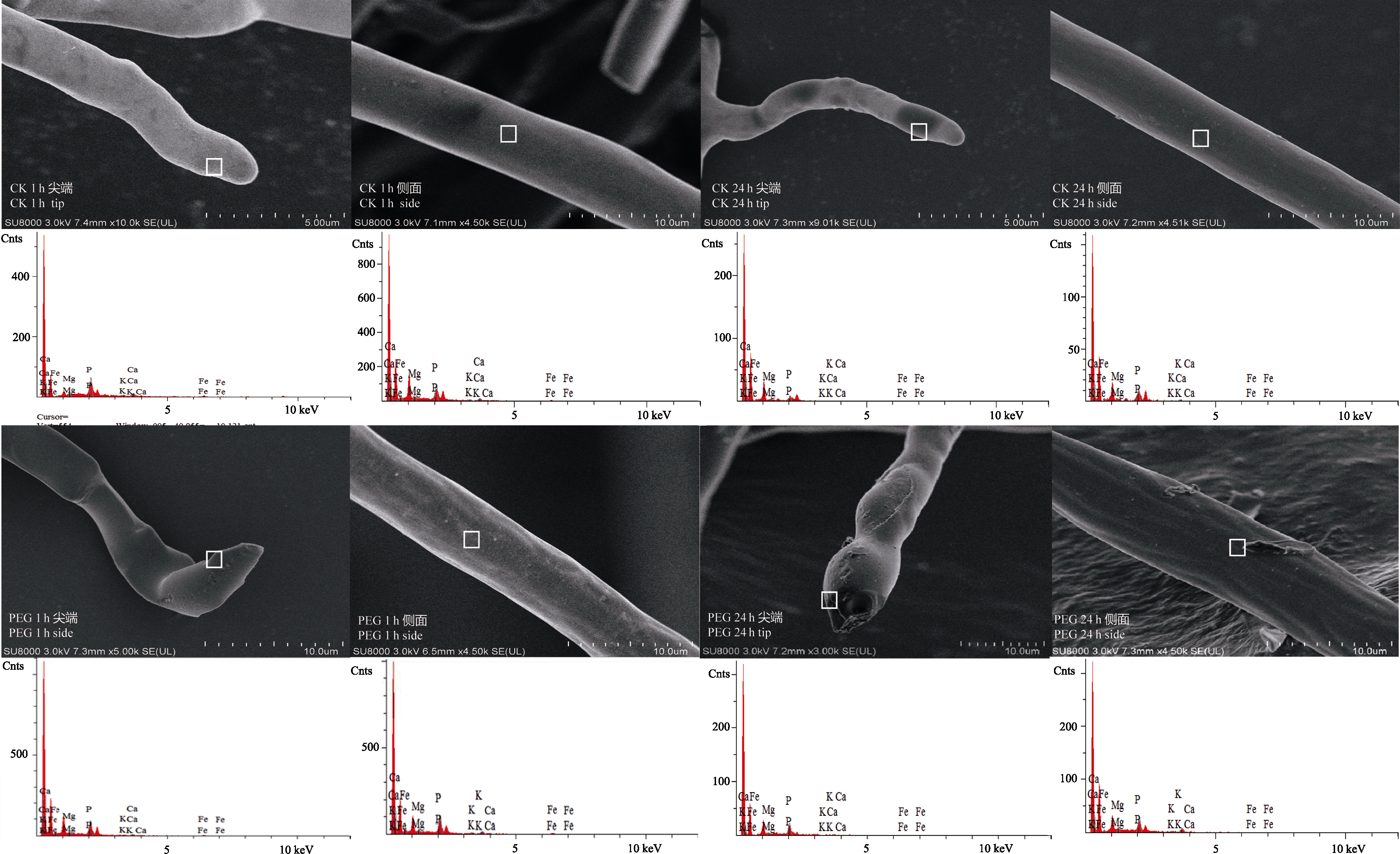
图3 PEG处理1 h (PEG 1 h)和24 h (PEG 24 h)后丛枝菌根真菌根外菌丝FE-SEM图像和能谱分析图。CK 1 h和CK 24 h为空白对照。
Fig. 3 FE-SEM images and selective elemental analysis (by EDS) of arbuscular mycorrhizal fungi hyphae after treatment by PEG for 1 h (PEG 1 h) and 24 h (PEG 24 h). CK 1 h and CK 24 h are corresponding controls.
| P | Ca | Fe | ||||||
|---|---|---|---|---|---|---|---|---|
| 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | |||
| 尖端 Tip | CK | 1 h | 29.53 ± 2.03cd | 30.03 ± 0.52d | 5.97 ± 0.68c | 6.14 ± 0.72c | 15.86 ± 0.26c | 24.64 ± 2.07cd |
| 24 h | 31.34 ± 2.34c | 32.85 ± 0.61d | 4.49 ± 0.86c | 5.39 ± 1.27c | 14.60 ± 1.26c | 26.53 ± 1.91c | ||
| PEG | 1 h | 38.80 ± 1.07b | 39.62 ± 0.41c | 16.15 ± 0.84a | 18.00 ± 0.87a | 37.15 ± 0.69a | 37.44 ± 1.13b | |
| 24 h | 51.49 ± 1.63a | 50.46 ± 0.39a | 5.32 ± 1.45c | 4.91 ± 0.66c | 32.48 ± 1.70ab | 52.43 ± 0.49a | ||
| 侧面 Side | CK | 1 h | 23.51 ± 1.12d | 39.01 ± 0.34c | 7.83 ± 1.3bc | 7.88 ± 1.40bc | 12.03 ± 1.11d | 19.51 ± 1.39d |
| 24 h | 28.00 ± 1.67cd | 43.47 ± 1.07bc | 9.84 ± 0.83b | 10.32 ± 0.92b | 12.07 ± 0.78d | 19.17 ± 0.87d | ||
| PEG | 1 h | 37.69 ± 0.84b | 37.51 ± 1.43c | 10.34 ± 1.12b | 12.03 ± 0.75b | 28.54 ± 0.76b | 33.28 ± 1.13b | |
| 24 h | 56.12 ± 2.21a | 45.04 ± 0.47b | 14.99 ± 0.73a | 16.21 ± 0.40a | 35.09 ± 1.43a | 41.70 ± 0.60ab | ||
表2 PEG处理1 h和24 h后丛枝菌根真菌菌丝尖端和侧面P、Ca和Fe的浓度值(能谱分析结果)(平均值±标准偏差, n = 6)
Table 2 Concentrations of P, Ca and Fe at the tip and side of arbuscular mycorrhizal fungi hyphae after treatment by PEG for 1 h and 24 h (determined by energy-dispersive X-ray spectroscopy) (mean ± SD, n = 6)
| P | Ca | Fe | ||||||
|---|---|---|---|---|---|---|---|---|
| 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | 原子百分比 Atom percentage content (%) | 质量分数 Mass percentage content (%) | |||
| 尖端 Tip | CK | 1 h | 29.53 ± 2.03cd | 30.03 ± 0.52d | 5.97 ± 0.68c | 6.14 ± 0.72c | 15.86 ± 0.26c | 24.64 ± 2.07cd |
| 24 h | 31.34 ± 2.34c | 32.85 ± 0.61d | 4.49 ± 0.86c | 5.39 ± 1.27c | 14.60 ± 1.26c | 26.53 ± 1.91c | ||
| PEG | 1 h | 38.80 ± 1.07b | 39.62 ± 0.41c | 16.15 ± 0.84a | 18.00 ± 0.87a | 37.15 ± 0.69a | 37.44 ± 1.13b | |
| 24 h | 51.49 ± 1.63a | 50.46 ± 0.39a | 5.32 ± 1.45c | 4.91 ± 0.66c | 32.48 ± 1.70ab | 52.43 ± 0.49a | ||
| 侧面 Side | CK | 1 h | 23.51 ± 1.12d | 39.01 ± 0.34c | 7.83 ± 1.3bc | 7.88 ± 1.40bc | 12.03 ± 1.11d | 19.51 ± 1.39d |
| 24 h | 28.00 ± 1.67cd | 43.47 ± 1.07bc | 9.84 ± 0.83b | 10.32 ± 0.92b | 12.07 ± 0.78d | 19.17 ± 0.87d | ||
| PEG | 1 h | 37.69 ± 0.84b | 37.51 ± 1.43c | 10.34 ± 1.12b | 12.03 ± 0.75b | 28.54 ± 0.76b | 33.28 ± 1.13b | |
| 24 h | 56.12 ± 2.21a | 45.04 ± 0.47b | 14.99 ± 0.73a | 16.21 ± 0.40a | 35.09 ± 1.43a | 41.70 ± 0.60ab | ||

图4 PEG处理1 h和24 h对丛枝菌根真菌菌丝尖端和侧面细胞内pH值的影响。荧光图像显示激发波长为488 nm条件下菌丝内pH值变化。
Fig. 4 The effect of PEG treatment for 1 h and 24 h on the pH value at the tip and side of arbuscular mycorrhizal fungi hypha. Fluorescence image at 488 nm excitation shows the pH variation in the hyphal cell.
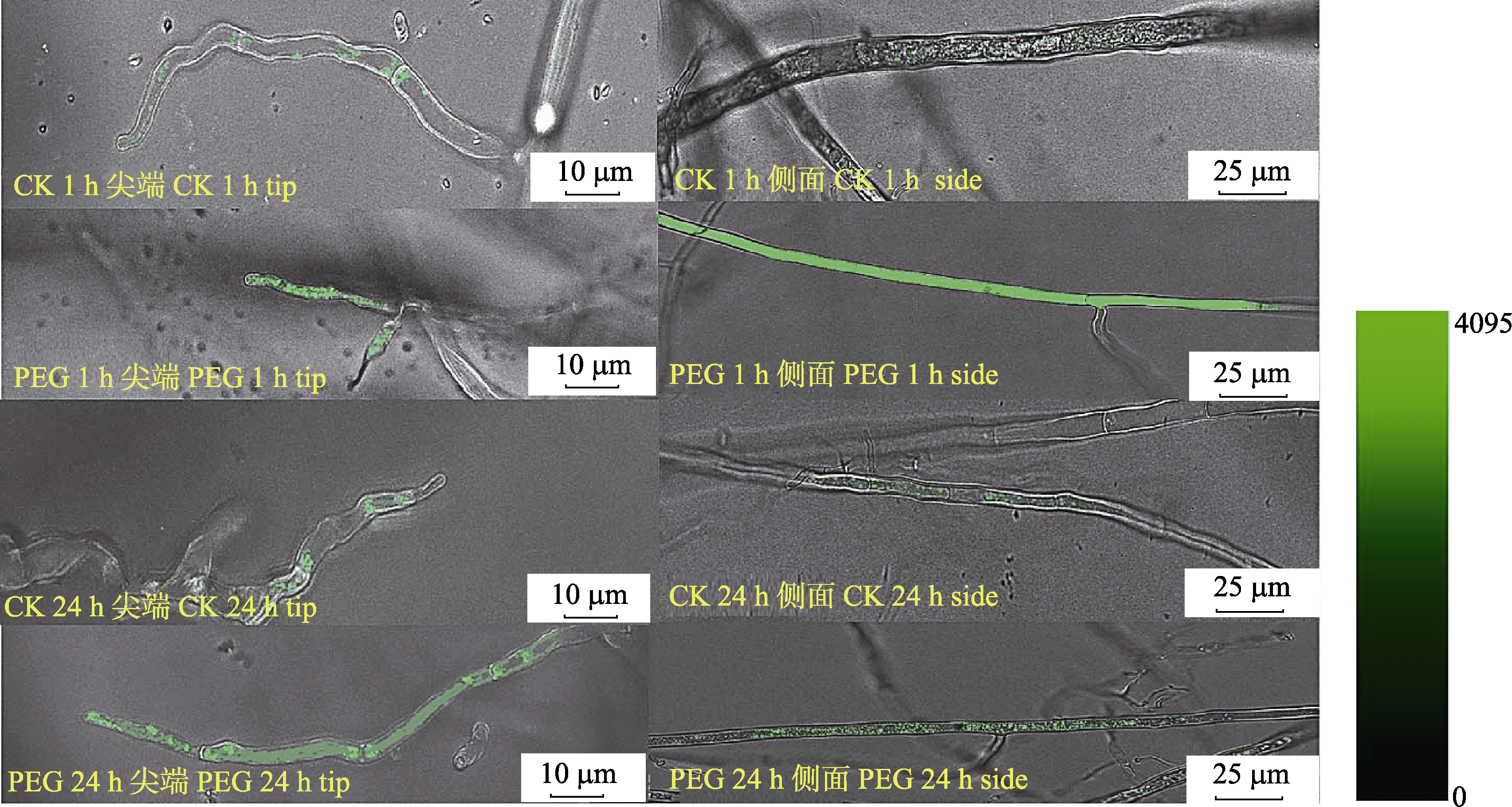
图5 PEG处理1 h和24 h对丛枝菌根真菌菌丝尖端和侧面细胞内Ca2+浓度的影响。荧光图像显示激发波长为488 nm条件下菌丝内Ca2+浓度变化。
Fig. 5 The effect of PEG treatment for 1 h and 24 h on the Ca2+ concentration at the tip and side of arbuscular mycorrhizal fungi hypha. Fluorescence image at 488 nm excitation shows the Ca2+ variation in the hyphal cell.
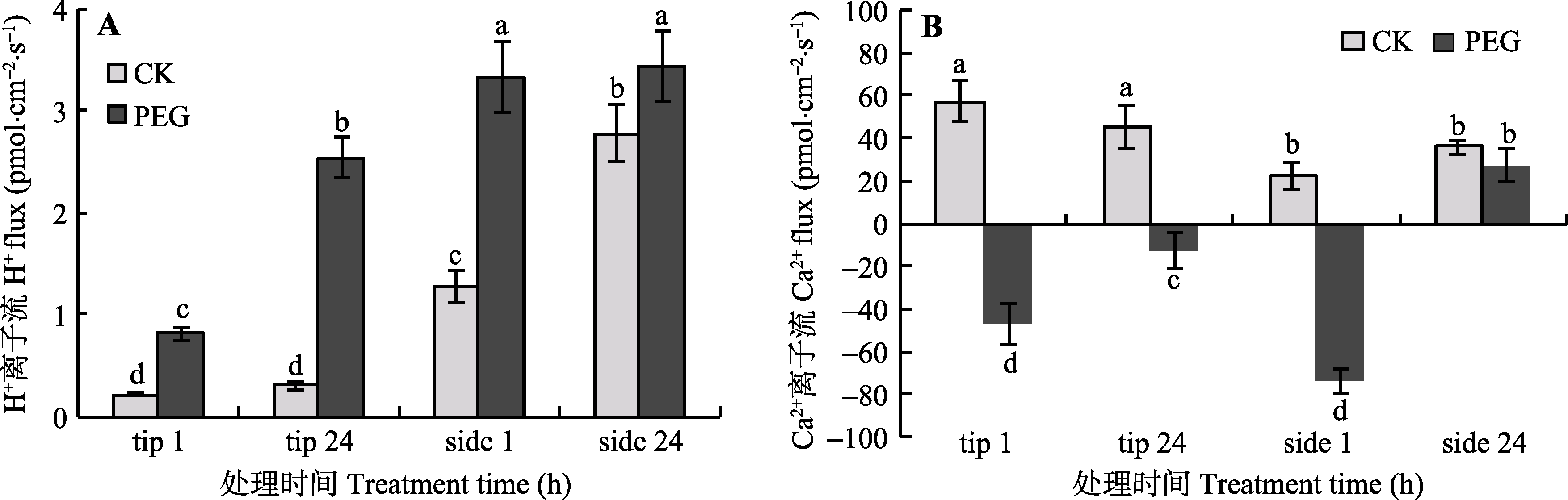
图6 PEG处理1 h和24 h对菌丝尖端和侧面H+ (A), 和Ca2+ (B)离子流的影响(平均值±标准偏差, n = 6)。tip表示菌丝尖端, side表示菌丝侧面。柱形上方标示不同字母代表相应处理之间有显著性差异(Duncan’s多重比较, p < 0.05)。
Fig. 6 The effects of PEG treatment on H+ (A) and Ca2+ (B) flux at the tip and side of arbuscular mycorrhizal hyphae (mean ± SD, n = 6). tip stands for hyphal tip, and side stands for lateral hypha. Columns marked by different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
| [1] |
Augé RM, Duan X ( 1991). Mycorrhizal fungi and nonhydraulic root signals of soil drying. Plant Physiology, 97, 821-824.
DOI URL |
| [2] |
Augé RM, Toler HD, Saxton AM ( 2015). Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza, 25, 13-24.
DOI URL PMID |
| [3] |
Ayling SM, Smith SE, Smith FA ( 2000). Transmembrane electric potential difference of germ tubes of arbuscular mycorrhizal fungi responds to external stimuli. New Phytologist, 147, 631-639.
DOI URL |
| [4] |
Azad AK, Sawa Y, Ishikawa T, Shibata H ( 2004). Phosphorylation of plasma membrane aquaporin regulates temperature-?dependent opening of tulip petals. Plant and Cell Physiology, 45, 608-617.
DOI URL PMID |
| [5] |
Bárzana G, Aroca R, Paz JA, Chaumont F, Martinez-Ballesta MC, Carvajal M, Ruiz-Lozano JM ( 2012). Arbuscular mycorrhizal symbiosis increases relative apoplastic water flow in roots of the host plant under both well-watered and drought stress conditions. Annals of Botany, 109, 1009-1017.
DOI URL PMID |
| [6] |
Bartnicki-Garcia S, Bracker CE, Gierz G, López-Franco R, Lu H ( 2000). Mapping the growth of fungal hyphae: Orthogonal cell wall expansion during tip growth and the role of turgor. Biophysical Journal, 79, 2382-2390.
DOI URL PMID |
| [7] |
Bécard G, Fortin JA ( 1988). Early events of vesicular?-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytologist, 108, 211-218.
DOI URL |
| [8] |
Bunney TD, Shaw PJ, Watkins PA, Taylor JP, Beven AF, Wells B, Calder GM, Dr?bak BK ( 2000). ATP-dependent regulation of nuclear Ca 2+ levels in plant cells . FEBS Letters, 476, 145-149.
DOI URL PMID |
| [9] |
Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, San Segundo B ( 2014). Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiology, 165, 688-704.
DOI URL PMID |
| [10] |
Chitarra W, Pagliarani C, Maserti B, Lumini E, Siciliano I, Cascone P, Schubert A, Gambino G, Balestrini R, Guerrieri E ( 2016). Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiology, 171, 1009-1023.
DOI URL PMID |
| [11] | Duncan DB ( 1955). Multiple range and multiple F tests. Biometrics, 11, 1-42. |
| [12] | Ferrol N, Barea JM, Azcón-Aguilar C ( 2000). The plasma membrane H +-ATPase gene family in the arbuscular mycorrhizal fungus Glomus mosseae. Current Genetics, 37, 112-118. |
| [13] |
Fromm J, Lautner S ( 2007). Electrical signals and their physiological significance in plants. Plant, Cell & Environment, 30, 249-257.
DOI URL PMID |
| [14] |
Gaxiola RA, Palmgren MG, Schumacher K ( 2007). Plant proton pumps. FEBS Letters, 581, 2204-2214.
DOI URL |
| [15] |
Gévaudant F, Duby G, von Stedingk E, Zhao R, Morsomme P, Boutry M ( 2007). Expression of a constitutively activated plasma membrane H+-ATPase alters plant development and increases salt tolerance . Plant Physiology, 144, 1763-1776.
DOI URL |
| [16] |
Gianinazzi-Pearson V, Smith SE, Gianinazzi S, Smith FA ( 1991). Enzymatic studies on the metabolism of vesicular-?arbuscular mycorrhizas. New Phytologist, 117, 61-74.
DOI URL |
| [17] | Gong DS, Xiong YC, Ma BL, Wang TM, Ge JP, Qin XL, Li PF, Kong HY, Li ZZ, Li FM ( 2010). Early activation of plasma membrane H +-ATPase and its relation to drought adaptation in two contrasting oat ( Avena sativa L.) genotypes. Environmental and Experimental Botany, 69, 1-8. |
| [18] |
Harrison MJ ( 2005). Signaling in the arbuscular mycorrhizal symbiosis. Annual Review Microbiology, 59, 19-42.
DOI URL |
| [19] |
Hijikata N, Murase M, Tani C, Ohtomo R, Osaki M, Ezawa T ( 2010). Polyphosphate has a central role in the rapid and massive accumulation of phosphorus in extraradical mycelium of an arbuscular mycorrhizal fungus. New Phytologist, 186, 285-289.
DOI URL PMID |
| [20] | Isfort RJ, Cody DB, Asquith TN, Ridder GM, Stuard SB, Leboeuf RA ( 1993). Induction of protein phosphorylation, protein synthesis, immediate-early-gene expression and cellular proliferation by intracellular pH modulation. FEBS Journal, 213, 349-357. |
| [21] |
Kühtreiber WM, Jaffe LF ( 1990). Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. The Journal of Cell Biology, 110, 1565-1573.
DOI URL |
| [22] |
Li T, Du J, Hao ZP, Zhang X, Chen BD ( 2012). Molecular basis for enhancement of plant drought tolerance by arbuscular mycorrhizal symbiosis: A mini-review. Acta Ecologica Sinica, 32, 7169-7176.
DOI URL |
|
[ 李涛, 杜娟, 郝志鹏, 张莘, 陈保冬 ( 2012). 丛枝菌根提高宿主植物抗旱性分子机制研究进展. 生态学报, 32, 7169-7176.]
DOI URL |
|
| [23] |
Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD ( 2013). First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist, 197, 617-630.
DOI URL PMID |
| [24] | Liu T, Li Z, Hui C, Tang M, Zhang H ( 2016). Effect of Rhizophagus irregularis on osmotic adjustment, antioxidation and aquaporin PIP genes expression of Populus × canadensis ‘Neva’ under drought stress. Acta Physiologiae Plantarum, 38, 191. DOI: 10.1007/s11738-016-2207-6. |
| [25] | Liu RJ, Chen YL ( 2007). Mycorrhizology. Science Press, Beijing. 447-448. |
| [26] |
Ludwig AA, Romeis T, Jones JD ( 2004). CDPK-mediated signalling pathways: Specificity and cross-talk. Journal of Experimental Botany, 55, 181-188.
DOI URL PMID |
| [27] |
Ma R, Zhang M, Li B, Du G, Wang J, Chen J ( 2005). The effects of exogenous Ca 2+ on endogenous polyamine levels and drought-resistant traits of spring wheat grown under arid conditions . Journal of Arid Environments, 63, 177-190.
DOI URL |
| [28] |
Mak M, Babla M, Xu SC, O’Carrigan A, Liu XH, Gong YM, Holford P, Chen ZH ( 2014). Leaf mesophyll K+, H+and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean . Environmental and Experimental Botany, 98, 1-12.
DOI URL |
| [29] |
Porcel R, Ruiz-Lozano JM ( 2004). Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. Journal of Experimental Botany, 55, 1743-1750.
DOI URL PMID |
| [30] |
Querejeta J, Egerton-Warburton LM, Allen MF ( 2003). Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia, 134, 55-64.
DOI URL |
| [31] |
Ramos AC, Fa?anha AR, Feijó JA ( 2008a). Ion dynamics during the polarized growth of arbuscular mycorrhizal fungi: From presymbiosis to symbiosis. In: Varma A ed. Mycorrhiza. Springer, Berlin, Heidelberg.
DOI URL |
| [32] |
Ramos AC, Fa?anha AR, Feijó JA ( 2008b). Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi . New Phytologist, 178, 177-188.
DOI URL |
| [33] |
Requena N, Breuninger M, Franken P, Ocón A ( 2003). Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiology, 132, 1540-1549.
DOI URL PMID |
| [34] | Riquelme M, Bartnicki-Garcia S ( 2004). Key differences between lateral and apical branching in hyphae of Neurospora crassa. Fungal Genetics and Biology, 41, 842-851. |
| [35] |
Rudd JJ, Franklin-Tong VE ( 2001). Unravelling response-?specificity in Ca 2+ signalling pathways in plant cells . New Phytologist, 151, 7-33.
DOI URL |
| [36] |
Sánchez-Romera B, Ruiz-Lozano JM, Zamarre?o áM, García-?Mina JM, Aroca R ( 2016). Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza, 26, 111-122.
DOI URL PMID |
| [37] |
Santi S, Locci G, Monte R, Pinton R, Varanini Z ( 2003). Induction of nitrate uptake in maize roots: Expression of a putative high-affinity nitrate transporter and plasma membrane H+- ATPase isoforms . Journal of Experimental Botany, 54, 1851-1864.
DOI URL PMID |
| [38] | Shi Y, Zhang Y, Han W, Feng R, Hu Y, Guo J, Gong H ( 2016). Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Frontiers in Plant Science, 7, 196. DOI: 10.3389/fpls.?2016.?00196. |
| [39] | Smith S, Read D ( 2008). Mycorrhizal Symbiosis. Academic Press, New York. |
| [40] | St-Arnaud M, Hamel C, Vimard B, Caron M, Fortin JA ( 1996). Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycological Research, 100, 328-332. |
| [41] |
Sun YP, Unestam T, Lucas SD, Johanson KJ, Kenne L, Finlay R ( 1999). Exudation-reabsorption in a mycorrhizal fungus, the dynamic interface for interaction with soil and soil microorganisms. Mycorrhiza, 9, 137-144.
DOI URL |
| [42] |
Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frey NF, Gianinazzi-?Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, Kawaguchi M, Krajinski F, Lammers PJ, Masclaux FG, Murat C, Morin E, Ndikumana S, Pagni M, Petitpierre D, Requena N, Rosikiewicz P, Riley R, Saito K, Clemente HS, Shapiro H, van Tuinen D, Bécard G, Bonfante P, Paszkowski U, Shachar-Hill YY, Tuskan GA, Young JW, Sanders IR, Henrissat B, Rensing SA, Grigoriev IV, Corradi N, Roux C, Martin F ( 2013). Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proceedings of the National Academy of Sciences of the United States of America, 110, 20117-20122.
DOI URL PMID |
| [43] | van Hees PA, Rosling A, Essén S, Godbold DL, Jones DL, Finlay RD ( 2006). Oxalate and ferricrocin exudation by the extramatrical mycelium of an ectomycorrhizal fungus in symbiosis withPinus sylvestris. New Phytologist, 169, 367-378. |
| [44] | Wu S, Zhang X, Sun Y, Wu Z, Li T, Hu Y, Su D, Lü J, Li G, Zhang Z, Zheng L, Zhang J, Chen B ( 2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environmental Science & Technology, 49, 14036-14047. |
| [45] |
Xiong YC, Li FM, Zhang T, Xia C ( 2007). Evolution mechanism of non-hydraulic root-to-shoot signal during the anti-drought genetic breeding of spring wheat. Environmental and Experimental Botany, 59, 193-205.
DOI URL |
| [46] |
Xu L, Li T, Wu Z, Feng H, Yu M, Zhang X, Chen B ( 2018). Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Applied Soil Ecology, 125, 213-221.
DOI URL |
| [47] |
Yan F, Zhu Y, Müller C, Z?rb C, Schubert S ( 2002). Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency . Plant Physiology, 129, 50-63.
DOI URL |
| [48] | Yang PZ ( 2012). Mechanism Involved in Drought/Salt Tolerance Improvement in Alfalfa Due to Symbiotic Interaction with Rhizobium. PhD dissertation, Northwest A&F University,Yangling, Shaanxi. 4-6. |
| [ 杨培志 ( 2012). 紫花苜蓿根瘤菌共生对干旱及盐胁迫的响应机制研究. 博士学位论文, 西北农林科技大学, 陕西杨凌. 4-6.] | |
| [49] |
Yoshida S ( 1991). Chilling-induced inactivation and its recovery of tonoplast H+-ATPase in mung bean cell suspension cultures . Plant Physiology, 95, 456-460.
DOI URL |
| [50] |
Zhao R, Guo W, Bi N, Guo J, Wang L, Zhao J, Zhang J ( 2015a). Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Applied Soil Ecology, 88, 41-49.
DOI URL |
| [51] |
Zhao X, Xu M, Wei R, Liu Y ( 2015b). Expression OsCAS (calcium-sensing receptor) in an Arabidopsis mutant increases drought tolerance. PLOS ONE, 10, e0131272. DOI: 10.1371/journal.pone.0131272.
DOI URL PMID |
| [52] | Zou JJ, Li XD, Ratnasekera D, Wang C, Liu WX, Song LF, Zhang WZ, Wu WH ( 2015). Arabidopsis calcium-dependent protein kinase 8 and catalase 3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. The Plant Cell, 27, 1445-1460. |
| [53] |
Zhou S, Han YY, Chen Y, Kong X, Wang W ( 2015). The involvement of expansins in response to water stress during leaf development in wheat. Journal of Plant Physiology, 183, 64-74.
DOI URL PMID |
| [1] | 陈科宇 邢森 唐玉 孙佳慧 任世杰 张静 纪宝明. 不同草地型土壤丛枝菌根真菌群落特征及其驱动因素[J]. 植物生态学报, 2024, 48(5): 660-674. |
| [2] | 胡蝶 蒋欣琪 戴志聪 陈戴一 张雨 祁珊珊 杜道林. 丛枝菌根真菌提高入侵杂草南美蟛蜞菊对除草剂的耐受性[J]. 植物生态学报, 2024, 48(5): 651-659. |
| [3] | 陈保冬, 付伟, 伍松林, 朱永官. 菌根真菌在陆地生态系统碳循环中的作用[J]. 植物生态学报, 2024, 48(1): 1-20. |
| [4] | 杨佳绒, 戴冬, 陈俊芳, 吴宪, 刘啸林, 刘宇. 丛枝菌根真菌多样性对植物群落构建和稀有种维持的研究进展[J]. 植物生态学报, 2023, 47(6): 745-755. |
| [5] | 何斐, 李川, Faisal SHAH, 卢谢敏, 王莹, 王梦, 阮佳, 魏梦琳, 马星光, 王卓, 姜浩. 丛枝菌根菌丝桥介导刺槐-魔芋间碳转运和磷吸收[J]. 植物生态学报, 2023, 47(6): 782-791. |
| [6] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [7] | 周洁, 杨晓东, 王雅芸, 隆彦昕, 王妍, 李浡睿, 孙启兴, 孙楠. 梭梭和骆驼刺对干旱的适应策略差异[J]. 植物生态学报, 2022, 46(9): 1064-1076. |
| [8] | 谢伟, 郝志鹏, 张莘, 陈保冬. 丛枝菌根网络介导的植物间信号交流研究进展及展望[J]. 植物生态学报, 2022, 46(5): 493-515. |
| [9] | 马炬峰, 辛敏, 徐陈超, 祝琬莹, 毛传澡, 陈欣, 程磊. 丛枝菌根真菌与氮添加对不同根形态基因型水稻氮吸收的影响[J]. 植物生态学报, 2021, 45(7): 728-737. |
| [10] | 庞芳, 夏维康, 何敏, 祁珊珊, 戴志聪, 杜道林. 固氮菌缓解氮限制环境中丛枝菌根真菌对加拿大一枝黄花的营养竞争[J]. 植物生态学报, 2020, 44(7): 782-790. |
| [11] | 刘丽燕, 冯锦霞, 刘文鑫, 万贤崇. 干旱胁迫对转PtPIP2;8基因84K杨苗木光合、生长和根系结构的影响[J]. 植物生态学报, 2020, 44(6): 677-686. |
| [12] | 崔利, 郭峰, 张佳蕾, 杨莎, 王建国, 孟静静, 耿耘, 李新国, 万书波. 摩西斗管囊霉改善连作花生根际土壤的微环境[J]. 植物生态学报, 2019, 43(8): 718-728. |
| [13] | 高文童, 张春艳, 董廷发, 胥晓. 丛枝菌根真菌对不同性别组合模式下青杨雌雄植株根系生长的影响[J]. 植物生态学报, 2019, 43(1): 37-45. |
| [14] | 刘海跃, 李欣玫, 张琳琳, 王姣姣, 贺学礼. 西北荒漠带花棒根际丛枝菌根真菌生态地理分布[J]. 植物生态学报, 2018, 42(2): 252-260. |
| [15] | 王曦,胡红玲,胡庭兴,张城浩,王鑫,刘丹. 干旱胁迫对桢楠幼树渗透调节与活性氧代谢的影响及施氮的缓解效应[J]. 植物生态学报, 2018, 42(2): 240-251. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19