

植物生态学报 ›› 2024, Vol. 48 ›› Issue (12): 1692-1707.DOI: 10.17521/cjpe.2023.0221 cstr: 32100.14.cjpe.2023.0221
• 研究论文 • 上一篇
李昀奕1,2, 郑矜1, 严晓艳1,3, 李霜1, 罗林4, 童晋1, 赵春章1,*( )
)
收稿日期:2023-08-01
接受日期:2024-04-08
出版日期:2024-12-20
发布日期:2024-12-20
通讯作者:
*赵春章(zhaochzh04@126.com)基金资助:
LI Yun-Yi1,2, ZHENG Jin1, YAN Xiao-Yan1,3, LI Shuang1, LUO Lin4, TONG Jin1, ZHAO Chun-Zhang1,*( )
)
Received:2023-08-01
Accepted:2024-04-08
Online:2024-12-20
Published:2024-12-20
Contact:
*ZHAO Chun-Zhang(zhaochzh04@126.com)Supported by:摘要: 叶片和根系作为植物对环境变化敏感的重要器官, 二者对气候变暖的响应研究已有很多, 但叶际和根际细菌群落对增温的响应仍不清楚。该研究选择青藏高原东缘亚高山针叶林优势树种云杉(Picea asperata)和优势灌木华西箭竹(Fargesia nitida)为研究对象, 比较两种植物叶际和根际土壤细菌群落特征及其对模拟增温的响应。结果表明, 云杉和华西箭竹根际细菌群落Chao指数和Shannon-Wiener指数都显著高于叶际, 增温降低了云杉叶际和根际细菌多样性, 但提高了华西箭竹叶际和根际细菌多样性。两种植物叶际与根际细菌群落组成和结构均存在显著差异, 其中根瘤菌目(Rhizobiales) (41%-46%)为两种植物叶际优势菌群, Vicinamibacterales为根际优势菌群。增温下云杉叶际菌群伯克氏菌目(Burkholderiales)和棒杆菌目(Corynebacteriales)的相对丰度约增加了2倍, 华西箭竹叶际酸杆菌目(Acidobacteriales)相对丰度有增加趋势, 但增温下两种植物根际细菌群落组成变化较小。两种植物根际细菌群落共现网络复杂性高于叶际, 增温会增加云杉叶际和根际细菌共现网络连接数, 但会减少华西箭竹叶际和根际细菌的连接数。根据FAPROTAX功能预测, 两种植物根际参与碳、氮循环功能的细菌类群相对丰度显著大于叶际, 叶际细菌潜在生态功能对增温更加敏感, 增温提高了两种植物叶际细菌尿素分解功能, 但显著降低了华西箭竹叶际细菌氮呼吸、硝酸盐还原和硝酸盐呼吸功能, 以及云杉叶际细菌潜在固氮作用。因此, 两种植物根际和叶际细菌群落结构及其潜在碳、氮循环功能存在显著差异, 叶际细菌群落对增温的响应较根际敏感, 且存在物种差异。
李昀奕, 郑矜, 严晓艳, 李霜, 罗林, 童晋, 赵春章. 云杉和华西箭竹叶际与根际细菌群落对增温的响应. 植物生态学报, 2024, 48(12): 1692-1707. DOI: 10.17521/cjpe.2023.0221
LI Yun-Yi, ZHENG Jin, YAN Xiao-Yan, LI Shuang, LUO Lin, TONG Jin, ZHAO Chun-Zhang. Effects of warming on phyllosphere and rhizosphere bacterial communities in Picea asperata and Fargesia nitida. Chinese Journal of Plant Ecology, 2024, 48(12): 1692-1707. DOI: 10.17521/cjpe.2023.0221
| 指数 Index | 组间比较 Component comparison | p | ||||||
|---|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | R | W | R × W | ||
| 云杉 Picea asperata | Chao | 781.33 ± 199.63bc | 563.77 ± 121.65c | 1337.98 ± 165.33a | 1167.56 ± 49.55ab | 0.004 | 0.219 | 0.875 |
| Shannon-Wiener | 4.95 ± 0.21b | 4.13 ± 0.32c | 6.69 ± 0.10a | 6.58 ± 0.04a | <0.0 01 | 0.048 | 0.114 | |
| Simpson | 0.07 ± 0.01b | 0.03 ± 0.01b | 0.43 ± 0.04a | 0.41 ± 0.03a | <0.001 | 0.275 | 0.859 | |
| 华西箭竹 Fargesia nitida | Chao | 348.29 ± 79.32b | 480.75 ± 51.24b | 2147.78 ± 45.24a | 2307.66 ± 117.88a | <0.001 | 0.101 | 0.866 |
| Shannon-Wiener | 3.81 ± 0.24b | 4.01 ± 0.10b | 7.12 ± 0.02a | 7.18 ± 0.04a | <0.001 | 0.370 | 0.626 | |
| Simpson | 0.06 ± 0.02b | 0.05 ± 0.01b | 0.47 ± 0.02a | 0.45 ± 0.01a | <0.001 | 0.383 | 0.860 | |
表1 增温对云杉和华西箭竹叶际和根际土壤细菌群落扩增子序列变体α多样性的影响(平均值±标准差, n = 3)
Table 1 Warming effects on amplicon sequence variant (ASV) α diversity of phyllosphere and rhizosphere soil bacterial communities in Picea asperata and Fargesia nitida (mean ± SD, n = 3)
| 指数 Index | 组间比较 Component comparison | p | ||||||
|---|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | R | W | R × W | ||
| 云杉 Picea asperata | Chao | 781.33 ± 199.63bc | 563.77 ± 121.65c | 1337.98 ± 165.33a | 1167.56 ± 49.55ab | 0.004 | 0.219 | 0.875 |
| Shannon-Wiener | 4.95 ± 0.21b | 4.13 ± 0.32c | 6.69 ± 0.10a | 6.58 ± 0.04a | <0.0 01 | 0.048 | 0.114 | |
| Simpson | 0.07 ± 0.01b | 0.03 ± 0.01b | 0.43 ± 0.04a | 0.41 ± 0.03a | <0.001 | 0.275 | 0.859 | |
| 华西箭竹 Fargesia nitida | Chao | 348.29 ± 79.32b | 480.75 ± 51.24b | 2147.78 ± 45.24a | 2307.66 ± 117.88a | <0.001 | 0.101 | 0.866 |
| Shannon-Wiener | 3.81 ± 0.24b | 4.01 ± 0.10b | 7.12 ± 0.02a | 7.18 ± 0.04a | <0.001 | 0.370 | 0.626 | |
| Simpson | 0.06 ± 0.02b | 0.05 ± 0.01b | 0.47 ± 0.02a | 0.45 ± 0.01a | <0.001 | 0.383 | 0.860 | |
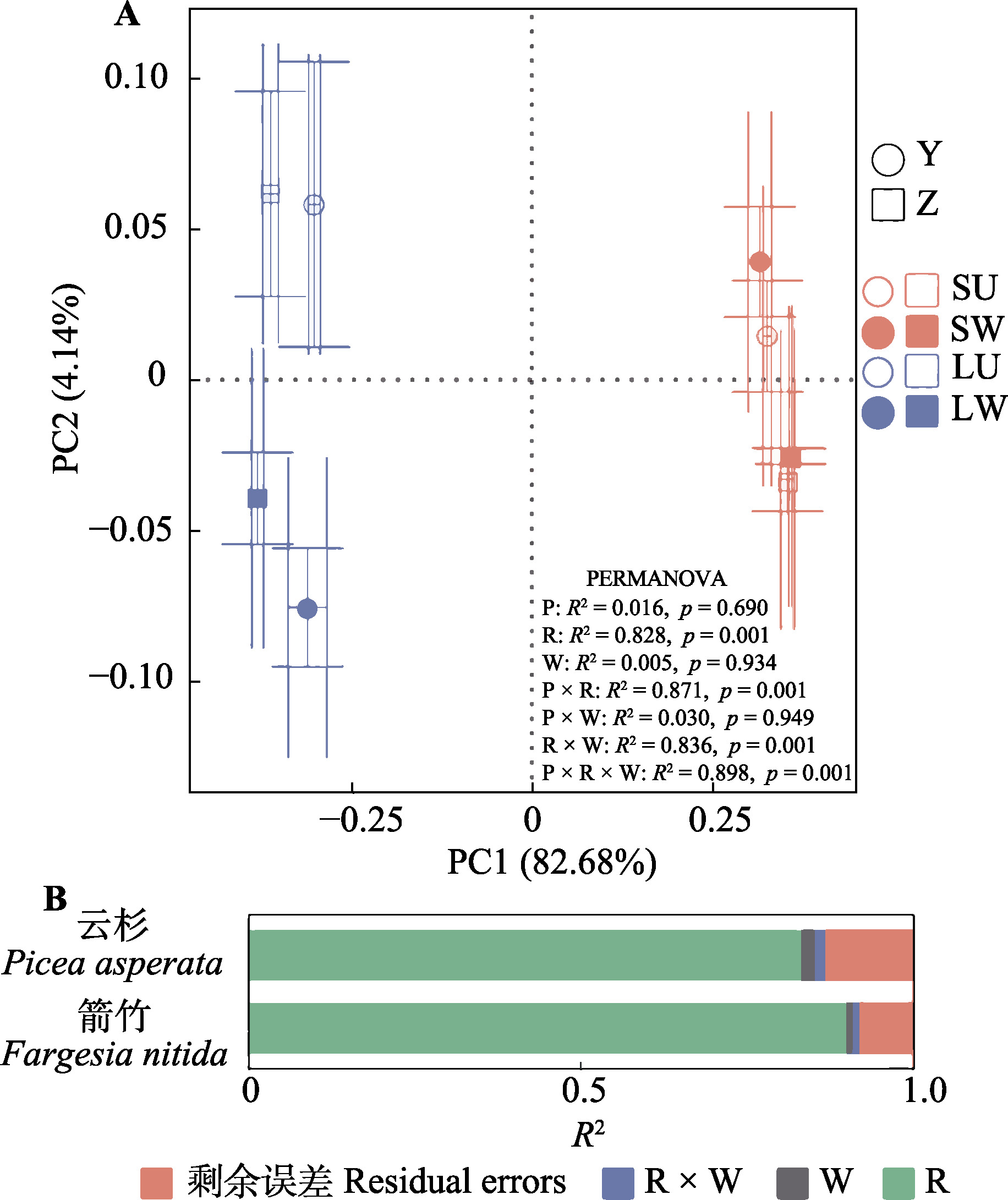
图1 增温对云杉和华西箭竹叶际和根际土壤细菌群落结构的影响(目水平) (A)及贡献率(B)。Y, 云杉; Z, 华西箭竹。 LU, 对照叶际; LW, 增温叶际; SU, 对照根际; SW, 增温根际。P, 宿主效应; R, 附生部位效应; W, 增温效应; P × R, P × W, R × W, P × R × W, 交互作用。
Fig. 1 Effects of warming on phyllosphere and rhizosphere soil bacterial community structure (A) and their contribution (B) in Picea asperata and Fargesia nitida at the order level. Y, P. asperata; Z, F. nitida. LU, unwarmed phyllosphere; LW, warmed phyllosphere; SU, unwarmed rhizosphere; SW, warmed rhizosphere. P, host effect; R, plant compartment effect; W, warming effect; P × R, P × W, R × W, and P × R × W, the interactive effect of P, R and W.
| 细菌目 Bacterial order | 相对丰度 Relative abundance | p | 细菌目 Bacterial order | 相对丰度 Relative abundance | p | ||
|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | ||||
| Rhizobiales | 46.11 ± 2.00 | 45.71 ± 3.63 | 0.928 | Vicinamibacterales | 16.54 ± 4.11 | 11.31 ± 2.75 | 0.350 |
| Sphingomonadales | 10.80 ± 1.38 | 8.20 ± 2.88 | 0.462 | Rhizobiales | 10.32 ± 0.23 | 10.98 ± 1.37 | 0.658 |
| Burkholderiales | 4.37 ± 1.69 | 11.96 ± 4.45 | 0.192 | Burkholderiales | 5.88 ± 0.62 | 5.98 ± 0.60 | 0.917 |
| Acetobacterales | 8.06 ± 1.15 | 5.76 ± 2.67 | 0.472 | Gaiellales | 4.99 ± 0.56 | 6.16 ± 0.56 | 0.216 |
| Micrococcales | 4.72 ± 0.81 | 4.41 ± 0.88 | 0.807 | Micrococcales | 4.24 ± 0.71 | 4.95 ± 0.36 | 0.419 |
| Cytophagales | 2.22 ± 0.21 | 2.50 ± 0.77 | 0.722 | norank_c__KD4-96 | 2.38 ± 0.35 | 3.28 ± 0.54 | 0.236 |
| Acidobacteriales | 3.37 ± 2.02 | 1.06 ± 0.60 | 0.332 | Microtrichales | 2.32 ± 0.37 | 2.96 ± 0.45 | 0.335 |
| Frankiales | 2.87 ± 0.60 | 1.51 ± 0.18 | 0.096 | IMCC26256 | 2.03 ± 0.23 | 2.58 ± 0.32 | 0.235 |
| Corynebacteriales | 0.94 ± 0.20 | 3.07 ± 2.01 | 0.351 | Bacillales | 1.74 ± 0.16 | 2.83 ± 1.15 | 0.398 |
| Propionibacteriales | 1.63 ± 0.24 | 1.85 ± 0.35 | 0.629 | Solirubrobacterales | 1.93 ± 0.35 | 2.25 ± 0.19 | 0.453 |
表2 增温对云杉叶际和根际优势细菌目相对丰度的影响(平均值±标准差, n = 3)
Table 2 Warming effects on the relative abundance of dominant orders in the phyllosphere and rhizosphere soil bacterial communities of Picea asperata (mean ± SD, n = 3)
| 细菌目 Bacterial order | 相对丰度 Relative abundance | p | 细菌目 Bacterial order | 相对丰度 Relative abundance | p | ||
|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | ||||
| Rhizobiales | 46.11 ± 2.00 | 45.71 ± 3.63 | 0.928 | Vicinamibacterales | 16.54 ± 4.11 | 11.31 ± 2.75 | 0.350 |
| Sphingomonadales | 10.80 ± 1.38 | 8.20 ± 2.88 | 0.462 | Rhizobiales | 10.32 ± 0.23 | 10.98 ± 1.37 | 0.658 |
| Burkholderiales | 4.37 ± 1.69 | 11.96 ± 4.45 | 0.192 | Burkholderiales | 5.88 ± 0.62 | 5.98 ± 0.60 | 0.917 |
| Acetobacterales | 8.06 ± 1.15 | 5.76 ± 2.67 | 0.472 | Gaiellales | 4.99 ± 0.56 | 6.16 ± 0.56 | 0.216 |
| Micrococcales | 4.72 ± 0.81 | 4.41 ± 0.88 | 0.807 | Micrococcales | 4.24 ± 0.71 | 4.95 ± 0.36 | 0.419 |
| Cytophagales | 2.22 ± 0.21 | 2.50 ± 0.77 | 0.722 | norank_c__KD4-96 | 2.38 ± 0.35 | 3.28 ± 0.54 | 0.236 |
| Acidobacteriales | 3.37 ± 2.02 | 1.06 ± 0.60 | 0.332 | Microtrichales | 2.32 ± 0.37 | 2.96 ± 0.45 | 0.335 |
| Frankiales | 2.87 ± 0.60 | 1.51 ± 0.18 | 0.096 | IMCC26256 | 2.03 ± 0.23 | 2.58 ± 0.32 | 0.235 |
| Corynebacteriales | 0.94 ± 0.20 | 3.07 ± 2.01 | 0.351 | Bacillales | 1.74 ± 0.16 | 2.83 ± 1.15 | 0.398 |
| Propionibacteriales | 1.63 ± 0.24 | 1.85 ± 0.35 | 0.629 | Solirubrobacterales | 1.93 ± 0.35 | 2.25 ± 0.19 | 0.453 |
| 细菌目 Bacterial order | 相对丰度 Relative abundance | p | 细菌目 Bacterial order | 相对丰度 Relative abundance | p | ||
|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | ||||
| Rhizobiales | 41.62 ± 5.53 | 45.77 ± 1.49 | 0.508 | Vicinamibacterales | 11.44 ± 2.45 | 10.29 ± 0.27 | 0.664 |
| Acetobacterales | 14.94 ± 4.61 | 16.07 ± 2.00 | 0.833 | Rhizobiales | 8.95 ± 0.63 | 8.69 ± 0.35 | 0.730 |
| Sphingomonadales | 6.31 ± 0.71 | 7.60 ± 1.40 | 0.457 | Gaiellales | 5.35 ± 0.08 | 5.44 ± 0.20 | 0.692 |
| Cytophagales | 5.71 ± 1.24 | 6.93 ± 1.17 | 0.514 | Burkholderiales | 4.90 ± 0.23 | 5.22 ± 0.34 | 0.469 |
| Burkholderiales | 8.12 ± 2.61 | 4.41 ± 1.40 | 0.279 | Bacillales | 4.12 ± 0.63 | 4.19 ± 0.27 | 0.917 |
| Micrococcales | 2.83 ± 0.45 | 3.57 ± 0.85 | 0.480 | Solirubrobacterales | 3.81 ± 0.55 | 3.23 ± 0.11 | 0.356 |
| Acidobacteriales | 2.77 ± 0.44 | 2.93 ± 1.16 | 0.903 | Micrococcales | 3.12 ± 0.64 | 3.57 ± 0.60 | 0.635 |
| Pseudomonadales | 3.05 ± 0.64 | 1.20 ± 0.32 | 0.062 | norank_c__KD4-96 | 2.80 ± 0.15 | 3.85 ± 0.21 | 0.015 |
| Enterobacterales | 1.15 ± 0.91 | 1.60 ± 0.63 | 0.708 | Microtrichales | 2.59 ± 0.26 | 2.94 ± 0.50 | 0.568 |
| Corynebacteriales | 2.02 ± 0.46 | 0.72 ± 0.34 | 0.086 | Propionibacteriales | 2.11 ± 0.26 | 2.37 ± 0.54 | 0.688 |
表3 增温对华西箭竹叶际和根际优势细菌目相对丰度的影响(平均值±标准差, n = 3)
Table 3 Warming effects on the relative abundance of dominant orders in the phyllosphere and rhizosphere soil bacterial communities of Fargesia nitida (mean ± SD, n = 3)
| 细菌目 Bacterial order | 相对丰度 Relative abundance | p | 细菌目 Bacterial order | 相对丰度 Relative abundance | p | ||
|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | ||||
| Rhizobiales | 41.62 ± 5.53 | 45.77 ± 1.49 | 0.508 | Vicinamibacterales | 11.44 ± 2.45 | 10.29 ± 0.27 | 0.664 |
| Acetobacterales | 14.94 ± 4.61 | 16.07 ± 2.00 | 0.833 | Rhizobiales | 8.95 ± 0.63 | 8.69 ± 0.35 | 0.730 |
| Sphingomonadales | 6.31 ± 0.71 | 7.60 ± 1.40 | 0.457 | Gaiellales | 5.35 ± 0.08 | 5.44 ± 0.20 | 0.692 |
| Cytophagales | 5.71 ± 1.24 | 6.93 ± 1.17 | 0.514 | Burkholderiales | 4.90 ± 0.23 | 5.22 ± 0.34 | 0.469 |
| Burkholderiales | 8.12 ± 2.61 | 4.41 ± 1.40 | 0.279 | Bacillales | 4.12 ± 0.63 | 4.19 ± 0.27 | 0.917 |
| Micrococcales | 2.83 ± 0.45 | 3.57 ± 0.85 | 0.480 | Solirubrobacterales | 3.81 ± 0.55 | 3.23 ± 0.11 | 0.356 |
| Acidobacteriales | 2.77 ± 0.44 | 2.93 ± 1.16 | 0.903 | Micrococcales | 3.12 ± 0.64 | 3.57 ± 0.60 | 0.635 |
| Pseudomonadales | 3.05 ± 0.64 | 1.20 ± 0.32 | 0.062 | norank_c__KD4-96 | 2.80 ± 0.15 | 3.85 ± 0.21 | 0.015 |
| Enterobacterales | 1.15 ± 0.91 | 1.60 ± 0.63 | 0.708 | Microtrichales | 2.59 ± 0.26 | 2.94 ± 0.50 | 0.568 |
| Corynebacteriales | 2.02 ± 0.46 | 0.72 ± 0.34 | 0.086 | Propionibacteriales | 2.11 ± 0.26 | 2.37 ± 0.54 | 0.688 |
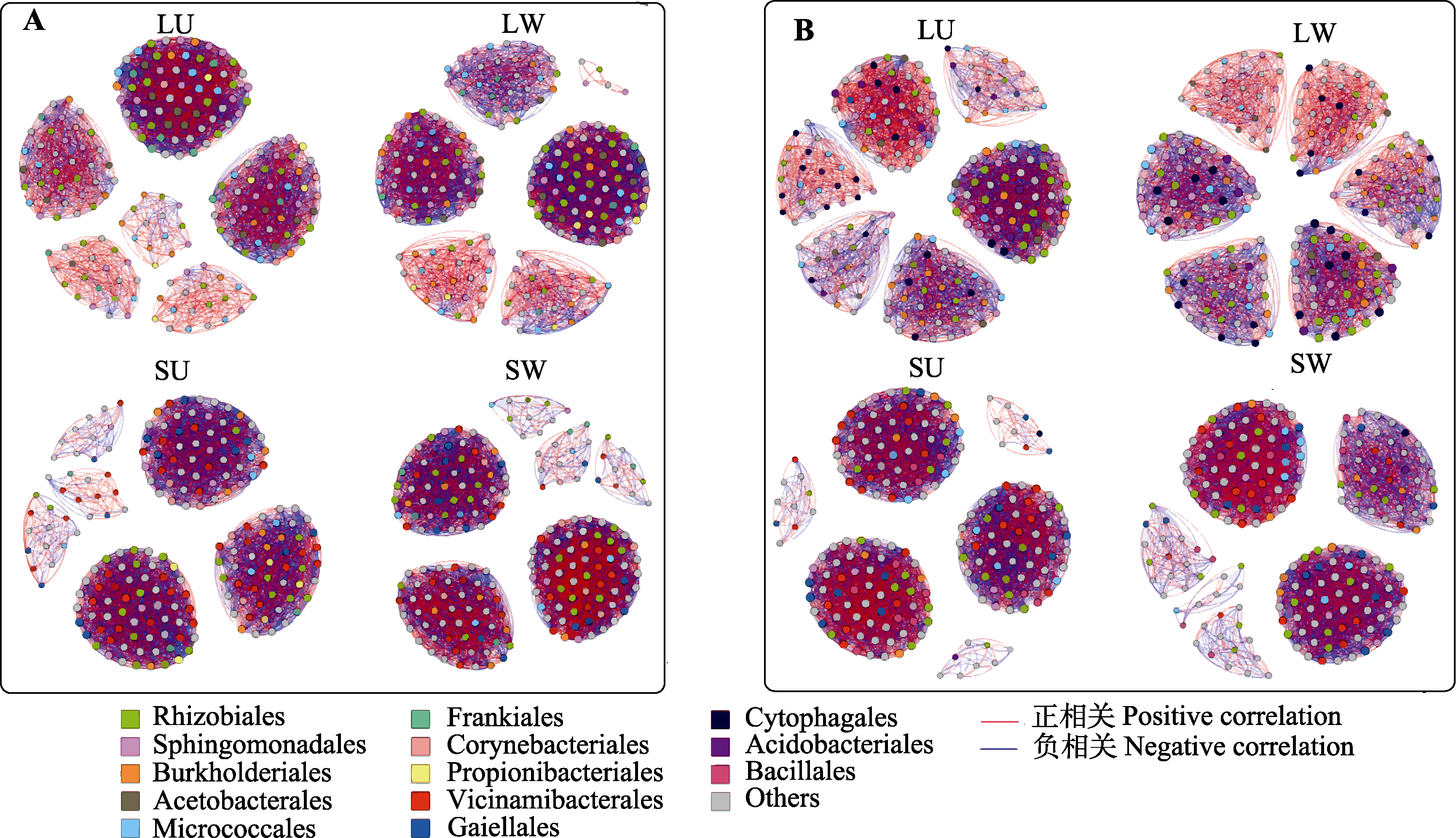
图2 增温对云杉(A)和华西箭竹(B)叶际与根际土壤细菌扩增子序列变体(ASV) (丰度前200)共现性网络的影响(基于斯皮尔曼相关性)。图中一个节点代表一个ASV, 一个连接代表一个稳定的(相关系数|ρ| > 0.6)和显著(p < 0.01)的相关性。每个节点的大小与连接数成比例, 按目水平着色。LU, 对照叶际; LW, 增温叶际; SU, 对照根际; SW, 增温根际。
Fig. 2 Effects of warming on co-occurrence of phyllosphere and rhizosphere soil bacterial amplicon sequence variant (ASV) (top 200 in abundance) in Picea asperata (A) and Fargesia nitida (B) based on Spearman correlation analysis. A node represented an ASV and a link represented a stable (correlation coefficient |ρ| > 0.6) and significant (p < 0.01) correlation. The size of each node is proportional to the number of links, colored at the order level. LU, unwarmed phyllosphere; LW, warmed phyllosphere; SU, unwarmed rhizosphere; SW, warmed rhizosphere.
| 网络参数 Network parameter | 云杉 Picea asperata | 华西箭竹 Fargesia nitida | ||||||
|---|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | LU | LW | SU | SW | |
| 点数量 Number of nodes | 200 | 200 | 200 | 199 | 200 | 200 | 200 | 199 |
| 连接数量 Number of links | 4 153 | 4 348 | 4 679 | 4 775 | 3 673 | 3 307 | 5 015 | 4 700 |
| 正相关连接 Positive correlation (%) | 55.33 | 58.10 | 51.14 | 50.97 | 61.04 | 59.99 | 50.63 | 50.55 |
| 负相关连接 Negative correlation (%) | 44.67 | 41.90 | 48.86 | 49.03 | 38.96 | 40.01 | 49.37 | 49.45 |
| 模块化程度 Modularity | 0.675 | 0.668 | 0.683 | 0.672 | 0.749 | 0.819 | 0.680 | 0.665 |
| 平均度 Average degree | 41.53 | 43.48 | 46.79 | 47.99 | 36.73 | 33.07 | 50.15 | 47.24 |
| 图密度 Density | 0.209 | 0.218 | 0.235 | 0.242 | 0.185 | 0.166 | 0.252 | 0.239 |
表4 云杉和华西箭竹叶际及根际细菌共现性网络拓扑参数
Table 4 Topological parameters of co-occurrence network in the phyllosphere and rhizosphere soil bacterial community of Picea asperata and Fargesia nitida
| 网络参数 Network parameter | 云杉 Picea asperata | 华西箭竹 Fargesia nitida | ||||||
|---|---|---|---|---|---|---|---|---|
| LU | LW | SU | SW | LU | LW | SU | SW | |
| 点数量 Number of nodes | 200 | 200 | 200 | 199 | 200 | 200 | 200 | 199 |
| 连接数量 Number of links | 4 153 | 4 348 | 4 679 | 4 775 | 3 673 | 3 307 | 5 015 | 4 700 |
| 正相关连接 Positive correlation (%) | 55.33 | 58.10 | 51.14 | 50.97 | 61.04 | 59.99 | 50.63 | 50.55 |
| 负相关连接 Negative correlation (%) | 44.67 | 41.90 | 48.86 | 49.03 | 38.96 | 40.01 | 49.37 | 49.45 |
| 模块化程度 Modularity | 0.675 | 0.668 | 0.683 | 0.672 | 0.749 | 0.819 | 0.680 | 0.665 |
| 平均度 Average degree | 41.53 | 43.48 | 46.79 | 47.99 | 36.73 | 33.07 | 50.15 | 47.24 |
| 图密度 Density | 0.209 | 0.218 | 0.235 | 0.242 | 0.185 | 0.166 | 0.252 | 0.239 |
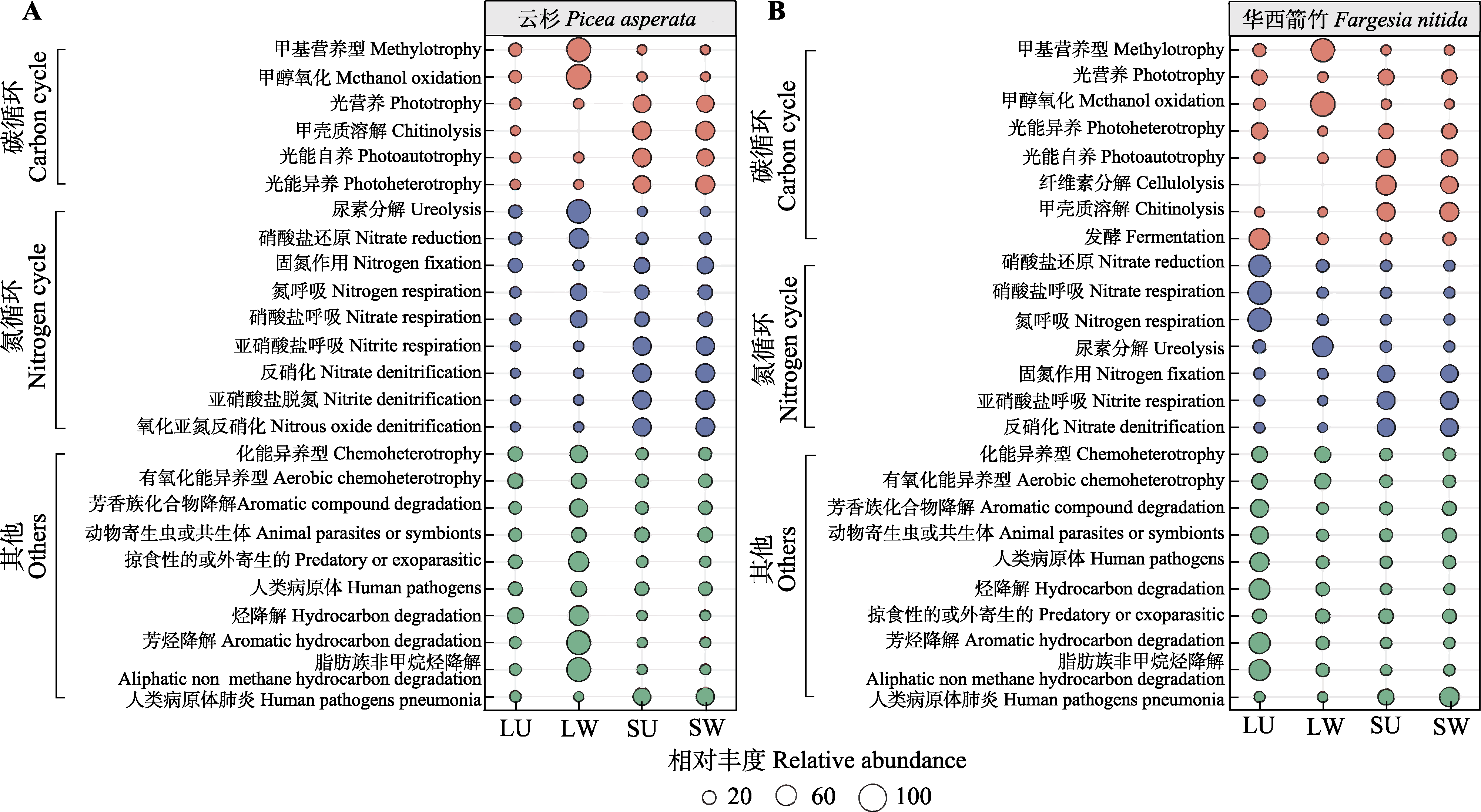
图3 云杉(A)和华西箭竹(B)细菌群落(扩增子序列变体丰度前25)的FAPROTAX生态功能预测。LU, 对照叶际; LW, 增温叶际; SU, 对照根际; SW, 增温根际。
Fig. 3 FAPROTAX function prediction (top 25 abundance) of Picea asperata (A) and Fargesia nitida (B) phyllosphere and rhizosphere soil bacteria at the amplicon sequence variant level. LU, unwarmed phyllosphere; LW, warmed phyllosphere; SU, unwarmed rhizosphere; SW, warmed rhizosphere.
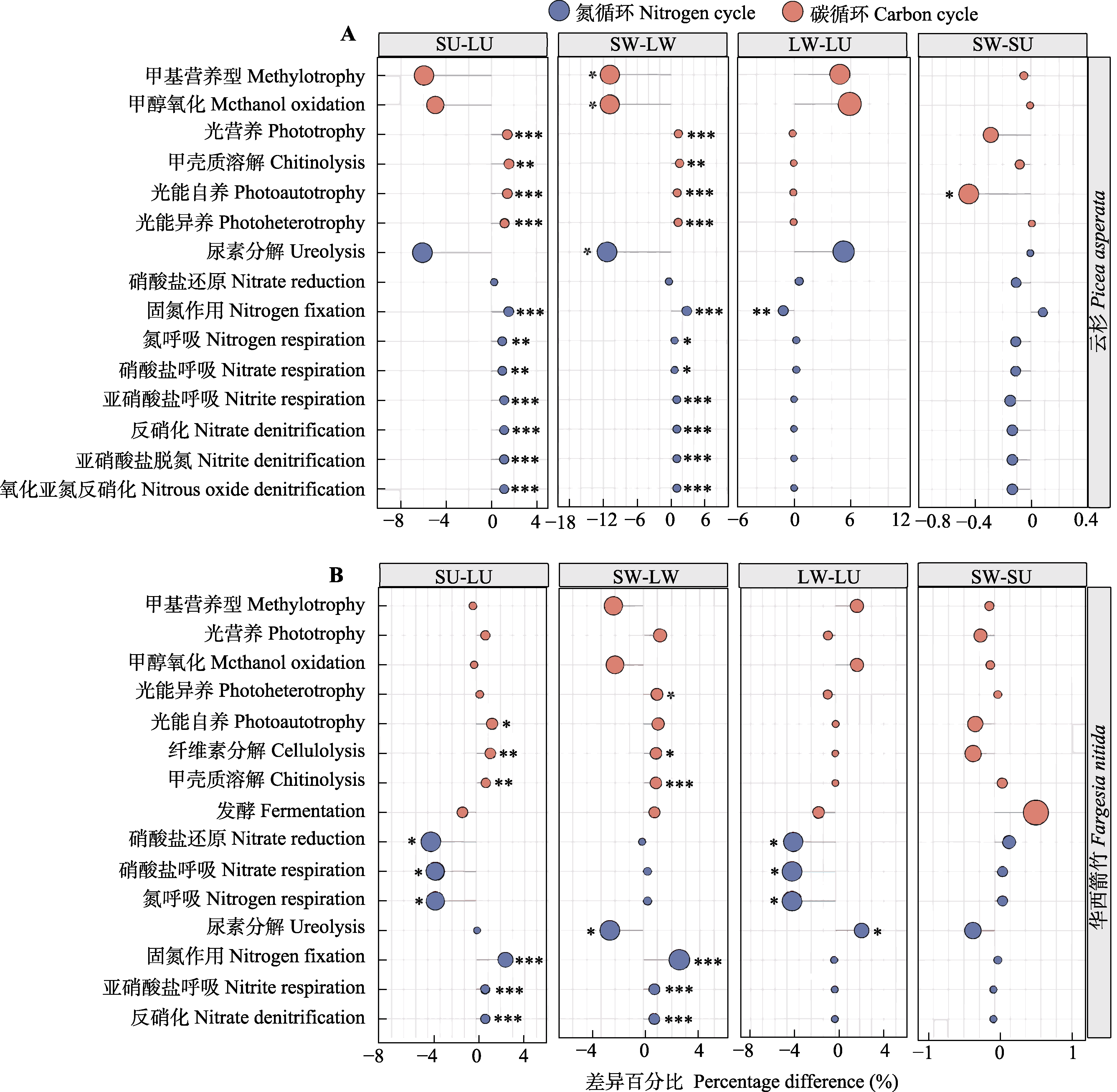
图4 云杉(A)和华西箭竹(B)叶际及根际土壤细菌群落扩增子序列变体水平生态功能的事后多重比较分析。LU, 对照叶际; LW, 增温叶际; SU, 对照根际; SW, 增温根际。*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001。圆的大小代表相对丰度。
Fig. 4 Post-hoc test of the predictive ecological functions in Picea asperata (A) and Fargesia nitida (B) phyllosphere and rhizosphere soil bacteria at the amplicon sequence variant level. LU, unwarmed phyllosphere; LW, warmed phyllosphere; SU, unwarmed rhizosphere; SW, warmed rhizosphere. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. The size of each node indicate the relative abundance.
| [1] | Abadi VAJM, Sepehri M, Rahmani HA, Zarei M, Ronaghi A, Taghavi SM, Shamshiripour M (2020). Role of dominant phyllosphere bacteria with plant growth-promoting characteristics on growth and nutrition of maize (Zea mays L.). Journal of Soil Science and Plant Nutrition, 20, 2348-2363. |
| [2] | Allard SM, Walsh CS, Wallis AE, Ottesen AR, Brown EW, Micallef SA (2016). Solanum lycopersicum (tomato) hosts robust phyllosphere and rhizosphere bacterial communities when grown in soil amended with various organic and synthetic fertilizers. Science of the Total Environment, 573, 555-563. |
| [3] | Allison SD, Treseder KK (2011). Climate change feedbacks to microbial decomposition in boreal soils. Fungal Ecology, 4, 362-374. |
| [4] | Ayangbenro AS, Babalola OO (2021). Reclamation of arid and semi-arid soils: the role of plant growth-promoting archaea and bacteria. Current Plant Biology, 25, 100173. DOI: 10.1016/j.cpb.2020.100173. |
| [5] | Aydogan EL, Moser G, Müller C, Kämpfer P, Glaeser SP (2018). Long-term warming shifts the composition of bacterial communities in the phyllosphere of Galium album in a permanent grassland field-experiment. Frontiers in Microbiology, 9, 144. DOI: 10.3389/fmicb.2018.00144. |
| [6] |
Badri DV, Weir TL, van der Lelie D, Vivanco JM (2009). Rhizosphere chemical dialogues: plant-microbe interactions. Current Opinion in Biotechnology, 20, 642-650.
DOI PMID |
| [7] |
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology, 57, 233-266.
PMID |
| [8] | Bao L, Cai W, Cao J, Zhang X, Liu J, Chen H, Wei Y, Zhuang X, Zhuang G, Bai Z (2020). Microbial community overlap between the phyllosphere and rhizosphere of three plants from Yongxing Island, South China Sea. Microbiology Open, 9, e1048. DOI: 10.1002/mbo3.1048. |
| [9] | Barberán A, Bates ST, Casamayor EO, Fierer N (2012). Using network analysis to explore co-occurrence patterns in soil microbial communities. The ISME Journal, 6, 343-351. |
| [10] | Bashir I, War AF, Rafiq I, Reshi ZA, Rashid I, Shouche YS (2022). Phyllosphere microbiome: diversity and functions. Microbiological Research, 254, 126888. DOI: 10.1016/j.micres.2021.126888. |
| [11] | Bastian M, Heymann S, Jacomy M (2009). Gephi: an open source software for exploring and manipulating networks// ICWSM. Proceedings of the International AAAI Conference on Web and Social Media. Vol. 3 No. 1. San Jose, USA. 361-362. |
| [12] | Bodenhausen N, Horton MW, Bergelson J (2013). Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS ONE, 8, e56329. DOI: 10.1371/journal.pone.0056329. |
| [13] | Brin LD, Giblin AE, Rich JJ (2015). Effects of experimental warming and carbon addition on nitrate reduction and respiration in coastal sediments. Biogeochemistry, 125, 81-95. |
| [14] | Bruto M, Prigent-Combaret C, Muller D, Moënne-Loccoz Y (2014). Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Scientific Reports, 4, 6261. DOI: 10.1038/srep06261. |
| [15] |
Bulgarelli D, Schlaeppi K, Spaepen S, Ver Loren van Themaat E, Schulze-Lefert P (2013). Structure and functions of the bacterial microbiota of plants. Annual Review of Plant Biology, 64, 807-838.
DOI PMID |
| [16] |
Chaffron S, Rehrauer H, Pernthaler J,von Mering C (2010). A global network of coexisting microbes from environmental and whole-genome sequence data. Genome Research, 20, 947-959.
DOI PMID |
| [17] | Chen WJ, Zhou HK, Wu Y, Li YZ, Qiao LL, Wang J, Zhai JY, Song YH, Zhao ZW, Zhang ZH, Liu GB, Zhao XQ, You QM, Xue S (2021). Plant-mediated effects of long-term warming on soil microorganisms on the Qinghai-Tibet Plateau. Catena, 204, 105391. DOI: 10.1016/j.catena. 2021.105391. |
| [18] | Cid FP, Inostroza NG, Graether SP, Bravo LA, Jorquera MA (2017). Bacterial community structures and ice recrystallization inhibition activity of bacteria isolated from the phyllosphere of the Antarctic vascular plant Deschampsia antarctica. Polar Biology, 40, 1319-1331. |
| [19] | Csardi G, Tamas N (2006). The igraph software package for complex network research. [2023-07-31]. . |
| [20] | Dautin N, de Sousa-d’Auria C, Constantinesco-Becker F, Labarre C, Oberto J, de la Sierra-Gallay IL, Dietrich C, Issa H, Houssin C, Bayan N (2017). Mycoloyltransferases: a large and major family of enzymes shaping the cell envelope of Corynebacteriales. Biochimica et Biophysica Acta, 1861, 3581-3592. |
| [21] | Dong CJ, Wang LL, Li Q, Shang QM (2019). Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE, 14, e0223847. DOI: 10.1371/journal.pone.0223847. |
| [22] | Espenshade J, Thijs S, Gawronski S, Bové H, Weyens N, Vangronsveld J (2019). Influence of urbanization on epiphytic bacterial communities of the Platanus × hispanica tree leaves in a biennial study. Frontiers in Microbiology, 10, 675. DOI: 10.3389/fmicb.2019.00675. |
| [23] |
Faticov M, Abdelfattah A, Roslin T, Vacher C, Hambäck P, Blanchet FG, Lindahl BD, Tack AJM (2021). Climate warming dominates over plant genotype in shaping the seasonal trajectory of foliar fungal communities on oak. New Phytologist, 231, 1770-1783.
DOI PMID |
| [24] |
Faust K, Raes J (2012). Microbial interactions: from networks to models. Nature Reviews Microbiology, 10, 538-550.
DOI PMID |
| [25] | Flores-Núñez VM, Fonseca-García C, Desgarennes D, Eloe- Fadrosh E, Woyke T, Partida-Martínez LP (2020). Functional signatures of the epiphytic prokaryotic microbiome of agaves and cacti. Frontiers in Microbiology, 10, 3044. DOI: 10.3389/fmicb.2019.03044. |
| [26] | Fuhrman JA, Steele JA (2008). Community structure of marine bacterioplankton: patterns, networks, and relationships to function. Aquatic Microbial Ecology, 53, 69-81. |
| [27] | Genitsaris S, Stefanidou N, Leontidou K, Matsi T, Karamanoli K, Mellidou I (2020). Bacterial communities in the rhizosphere and phyllosphere of halophytes and drought- tolerant plants in mediterranean ecosystems. Microorganisms, 8, 1708. DOI: 10.3390/microorganisms8111708. |
| [28] | Guo LY, Miao LF, Li DD, Xiang LS, Yang F (2022). Effects of nitrogen addition and warming on growth, development, and physiological characteristics of Dalbergia odorifera T. Chen seedlings. Plant Science Journal, 40, 259-268. |
| [ 郭璐瑶, 苗灵凤, 李大东, 向丽珊, 杨帆 (2022). 施氮和增温对降香黄檀幼苗生长发育和生理特征的影响. 植物科学学报, 40, 259-268.] | |
| [29] |
Hardoim PR, van Overbeek LS, Elsas JD (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends in Microbiology, 16, 463-471.
DOI PMID |
| [30] | Hassani MA, Durán P, Hacquard S (2018). Microbial interactions within the plant holobiont. Microbiome, 6, 58. DOI: 10.1186/s40168-018-0445-0. |
| [31] | He YZ, Huang WD, Wang HH, Zhu YZ (2022). Leaf photosynthetic responses to warming and precipitation reduction of three dominant species in Horqin sandy land. Acta Botanica Boreali-Occidentalia Sinica, 42, 684-693. |
| [ 何远政, 黄文达, 王怀海, 朱远忠 (2022). 沙质草地3种优势植物叶片光合生理对增温和降水减少的响应. 西北植物学报, 42, 684-693.] | |
| [32] | Hu JH, Li M, Cao X, Chen JY, Jiang W, Zhang SM, Li J, Wu HQ (2022). Effects of microbial fertilizer on bacterial community and yield of soybean rhizosphere soil in cold region. Journal of Microbiology, 42(4), 12-20. |
| [ 胡基华, 李萌, 曹旭, 陈静宇, 姜威, 张淑梅, 李晶, 吴皓琼 (2022). 微生物菌肥对寒地大豆根围土壤细菌群落和产量的影响. 微生物学杂志, 42(4), 12-20.] | |
| [33] | Ibekwe AM, Ors S, Ferreira JFS, Liu X, Suarez DL, Ma J, Ghasemimianaei A, Yang C (2020). Functional relationships between aboveground and belowground spinach (Spinacia oleracea L., cv. Racoon) microbiomes impacted by salinity and drought. Science of the Total Environment, 717, 137207. DOI: 10.1016/j.scitotenv.2020.137207. |
| [34] | IPCC(Intergovernmental Panel on Climate Change) (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland. 151. |
| [35] |
Jacobs JL, Carroll TL, Sundin GW (2005). The role of pigmentation, ultraviolet radiation tolerance, and leaf colonization strategies in the epiphytic survival of phyllosphere bacteria. Microbial Ecology, 49, 104-113.
PMID |
| [36] | Jia T, Yao YS, Wang RH (2020). Characteristics of phyllosphere and rhizosphere bacterial communities in Bothriochloa ischaemum in copper tailings. Environmental Science, 12, 5628-5635. |
| [ 贾彤, 姚玉珊, 王瑞宏 (2020). 铜尾矿白羊草叶际和根际细菌群落特征. 环境科学, 12, 5628-5635.] | |
| [37] | Kazenel MR, Kivlin SN, Taylor DL, Lynn JS, Rudgers JA (2019). Altitudinal gradients fail to predict fungal symbiont responses to warming. Ecology, 100, e02740. DOI: 10.1002/ecy.2740. |
| [38] | Knief C, Delmotte N, Chaffron S, Stark M, Innerebner G, Wassmann R, Mering C, Vorholt JA (2012). Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. The ISME Journal, 6, 1378-1390. |
| [39] |
Kuffner M, Hai B, Rattei T, Melodelima C, Schloter M, Zechmeister-Boltenstern S, Jandl R, Schindlbacher A, Sessitsch A (2012). Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. FEMS Microbiology Ecology, 82, 551-562.
DOI PMID |
| [40] |
Lambais MR, Barrera SE, Santos EC, Crowley DE, Jumpponen A (2017). Phyllosphere metaproteomes of trees from the Brazilian Atlantic forest show high levels of functional redundancy. Microbial Ecology, 73, 123-134.
DOI PMID |
| [41] | Lavergne C, Bovio-Winkler P, Etchebehere C, García-Gen S (2020). Towards centralized biogas plants: co-digestion of sewage sludge and pig manure maintains process performance and active microbiome diversity. Bioresource Technology, 297, 122442. DOI: 10.1016/j.biortech.2019.122442. |
| [42] | Lemanceau P, Barret M, Mazurier S, Mondy S, Pivato B, Fort T, Vacher C (2017). Plant communication with associated microbiota in the spermosphere, rhizosphere and phyllosphere. Advances in Botanical Research, 82, 101-133. |
| [43] | Li D, Ni HW, Jiao S, Lu YH, Zhou JZ, Sun B, Liang YT (2021). Coexistence patterns of soil methanogens are closely tied to methane generation and community assembly in rice paddies. Microbiome, 9, 20. DOI: 10.1186/s40168-020-00978-8. |
| [44] | Li M, Cherubini P, Dobbertin M, Arend M, Xiao W, Rigling A (2013). Responses of leaf nitrogen and mobile carbohydrates in different Quercus species/provenances to moderate climate changes. Plant Biology, 15, 177-184. |
| [45] | Li WT, Liu QH, Xie LL, Yin CY (2023). Interspecific plant-plant interactions increase the soil microbial network stability, shift keystone microbial taxa, and enhance their functions in mixed stands. Forest Ecology and Management, 533, 120851. DOI: 10.1016/j.foreco.2023.120851. |
| [46] |
Li YS, Wu XK, Chen T, Wang WF, Liu GX, Zhang W, Li SW, Wang MH, Zhao CM, Zhou HZ, Zhang GS (2018). Plant phenotypic traits eventually shape its microbiota: a common garden test. Frontiers in Microbiology, 9, 2479.
DOI PMID |
| [47] |
Lindow SE, Leveau JHJ (2002). Phyllosphere microbiology. Current Opinion in Biotechnology, 13, 238-243.
DOI PMID |
| [48] | Liu JP, Tang YJ, Bao JS, Wang HK, Peng FR, Chen MY, Tan PP (2022a). Pecan plantation age influences the structures, ecological networks, and functions of soil microbial communities. Land Degradation & Development, 33, 3294-3309. |
| [49] | Liu JW, Li XZ, Yao MJ (2021). Research progress on assembly of plant rhizosphere microbial community. Acta Microbiologica Sinica, 61, 231-248. |
| [ 刘京伟, 李香真, 姚敏杰 (2021). 植物根际微生物群落构建的研究进展. 微生物学报, 61, 231-248.] | |
| [50] | Liu J, Zou H, Bachelot B, Dong T, Zhu Z, Liao Y, Plenković-Moraj A, Wu Y (2021). Predicting the responses of subalpine forest landscape dynamics to climate change on the eastern Tibetan Plateau. Global Change Biology, 27, 4352-4366. |
| [51] | Liu PK, Bai FY, Huang JZ, Lu YS, Wu YH, He CQ, Liu XY, Yang TY, Chen XP (2022b). Stratification of microbial communities and their functions in mossy biofilms colonizing the giant monolithic statue of Buddha. International Biodeterioration & Biodegradation, 173, 105456. DOI: 10.1016/j.ibiod.2022.105456. |
| [52] | Liu WW (2019). Soil Microbial Composition and Response of Nitrogen and Phosphorus Soil Enzymes to Habitat in Different Stages of Karst Vegetation Restoration. Master degree dissertation, Guizhou University, Guiyang. |
| [ 刘雯雯 (2019). 喀斯特植被恢复不同阶段土壤微生物组成及氮磷土壤酶对生境响应. 硕士学位论文, 贵州大学, 贵阳.] | |
| [53] |
Louca S, Parfrey LW, Doebeli M (2016). Decoupling function and taxonomy in the global ocean microbiome. Science, 353, 1272-1277.
DOI PMID |
| [54] | Luo L, Guo M, Wang ET, Yin CY, Wang YJ, He HL, Zhao CZ (2022). Effects of mycorrhiza and hyphae on the response of soil microbial community to warming in eastern Tibetan Plateau. Science of the Total Environment, 837, 155498. DOI: 10.1016/j.scitotenv.2022.155498. |
| [55] | Ma B, Wang H, Dsouza M, Lou J, He Y, Dai Z, Brookes PC, Xu J, Gilbert JA (2016). Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in Eastern China. The ISME Journal, 10, 1891-1901. |
| [56] | Ma B, Wang Y, Ye S, Liu S, Stirling E, Gilbert JA, Faust K, Knight R, Jansson JK, Cardona C, Röttjers L, Xu J (2020). Earth microbial co-occurrence network reveals interconnection pattern across microbiomes. Microbiome, 8, 82. DOI: 10.1186/s40168-020-00857-2. |
| [57] | Ma ZY, Liu HY, Mi ZR, Zhang ZH, Wang YH, Xu W, Jiang L, He JS (2017). Climate warming reduces the temporal stability of plant community biomass production. Nature Communications, 8, 15378. DOI: 10.1038/ncomms15378. |
| [58] | Mathur P, Mukherjee S (2021). Dynamic pool of nitric oxide (NO) in rhizosphere modulates root architecture, nutrient acquisition and stress tolerance in plants//Mukherjee S, Baluška F. Rhizobiology: Molecular Physiology of Plant Roots. Springer, Cham, Switzerland. 149-166. |
| [59] | Mendes LW, Kuramae EE, Navarrete AA, van Veen JA, Tsai SM (2014). Taxonomical and functional microbial community selection in soybean rhizosphere. The ISME Journal, 8, 1577-1587. |
| [60] | Moreira JCF (2019). Microbiomes of the Amazon Forest: Bacterial Diversity and Community Structure in the Phyllosphere, Litter and Soil. PhD dissertation, Universidade de São Paulo, São Paulo. |
| [61] |
Papen H, Gessler A, Zumbusch E, Rennenberg H (2002). Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Current Microbiology, 44, 56-60.
PMID |
| [62] |
Peñuelas J, Rico L, Ogaya R, Jump AS, Terradas J (2012). Summer season and long-term drought increase the richness of bacteria and fungi in the foliar phyllosphere of Quercus ilex in a mixed Mediterranean forest. Plant Biology, 14, 565-575.
DOI PMID |
| [63] | Pold G, Melillo JM, DeAngelis KM (2015). Two decades of warming increases diversity of a potentially lignolytic bacterial community. Frontiers in Microbiology, 6, 480. DOI: 10.3389/fmicb.2015.00480. |
| [64] | Qi CR, Yin RR, Cheng JW, Xu ZC, Chen J, Gao XZ, Li GX, Nghiem L, Luo WH (2022a). Bacterial dynamics for gaseous emission and humification during bio-augmented composting of kitchen waste with lime addition for acidity regulation. Science of the Total Environment, 848, 157653. DOI: 10.1016/j.scitotenv.2022.157653. |
| [65] | Qi YQ, Liu HL, Zhang BP, Geng MX, Cai XX, Wang JH, Wang YP (2022b). Investigating the effect of microbial inoculants Frankia F1 on growth-promotion, rhizosphere soil physicochemical properties, and bacterial community of ginseng. Applied Soil Ecology, 172, 104369. DOI: 10.1016/j.apsoil.2021.104369. |
| [66] |
Randriamanana TR, Lavola A, Julkunen-Tiitto R (2015). Interactive effects of supplemental UV-B and temperature in European aspen seedlings: implications for growth, leaf traits, phenolic defense and associated organisms. Plant Physiology and Biochemistry, 93, 84-93.
DOI PMID |
| [67] |
Remus-Emsermann MNP, Schlechter RO (2018). Phyllosphere microbiology: at the interface between microbial individuals and the plant host. New Phytologist, 218, 1327-1333.
DOI PMID |
| [68] | Renaudin M, Blasi C, Bradley RL, Bellenger JP (2022). New insights into the drivers of moss-associated nitrogen fixation and cyanobacterial biomass in the eastern Canadian boreal forest. Journal of Ecology, 110, 1403-1418. |
| [69] |
Rico L, Ogaya R, Terradas J, Peñuelas J (2014). Community structures of N2-fixing bacteria associated with the phyllosphere of a Holm oak forest and their response to drought. Plant Biology, 16, 586-593.
DOI PMID |
| [70] |
Röttjers L, Faust K (2018). From hairballs to hypotheses- biological insights from microbial networks. FEMS Microbiology Reviews, 42, 761-780.
DOI PMID |
| [71] | Santana JO, Gramacho KP, de Souza Eduvirgens Ferreira KT, Rezende RP, Mangabeira PAO, Dias RPM, Couto FM, Pirovani CP (2018). Witches’ broom resistant genotype CCN51 shows greater diversity of symbiont bacteria in its phylloplane than susceptible genotype catongo. BMC Microbiology, 18, 194. DOI: 10.1186/s12866-018-1339-9. |
| [72] | Schindlbacher A, Rodler A, Kuffner M, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S (2011). Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biology & Biochemistry, 43, 1417-1425. |
| [73] |
Schlechter RO, Miebach M, Remus-Emsermann MNP (2019). Driving factors of epiphytic bacterial communities: a review. Journal of Advanced Research, 19, 57-65.
DOI PMID |
| [74] | Smith GW, Hayasaka SS (1982). Nitrogenase activity associated with Zostera marina from a North Carolina estuary. Canadian Journal of Microbiology, 28, 448-451. |
| [75] |
Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao I, Lata JC, Kishii M, Braun HJ (2015). Suppression of soil nitrification by plants. Plant Science, 233, 155-164.
DOI PMID |
| [76] | Sun S, Wu Y, Zhang J, Wang G, DeLuca TH, Zhu W, Li A, Duan M, He L (2019). Soil warming and nitrogen deposition alter soil respiration, microbial community structure and organic carbon composition in a coniferous forest on eastern Tibetan Plateau. Geoderma, 353, 283-292. |
| [77] | Sun XG, Zheng Y, Xu G, Guo QQ, Tan JH, Ding GJ (2021). Fungal diversity within the phyllosphere of Pinus massoniana and the possible involvement of phyllospheric fungi in litter decomposition. Fungal Biology, 125, 785-795. |
| [78] | Thrall PH, Hochberg ME, Burdon JJ, Bever JD (2007). Coevolution of symbiotic mutualists and parasites in a community context. Trends in Ecology & Evolution, 22, 120-126. |
| [79] | Trombetta T, Vidussi F, Roques C, Mas S, Scotti M, Mostajir B (2021). Co-occurrence networks reveal the central role of temperature in structuring the plankton community of the Thau Lagoon. Scientific Reports, 11, 17675. DOI: 10.1038/s41598-021-97173-y. |
| [80] |
Truchado P, Gil MI, Moreno-Candel M, Allende A (2019). Impact of weather conditions, leaf age and irrigation water disinfection on the major epiphytic bacterial genera of baby spinach grown in an open field. Food Microbiology, 78, 46-52. DOI: 10.1016/j.fm.2018.09.015.
PMID |
| [81] | Truyens S, Weyens N, Cuypers A, Vangronsveld J (2015). Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environmental Microbiology Reports, 7, 40-50. |
| [82] | Vacher C, Hampe A, Porté AJ, Sauer U, Compant S, Morris CE (2016). The phyllosphere: microbial jungle at the plant-climate interface. Annual Review of Ecology, Evolution, and Systematics, 47, 1-24. |
| [83] |
van Loon LC, Bakker PA, Pieterse CM (1998). Systemic resistance induced by rhizosphere bacteria. Annual Review of Phytopathology, 36, 453-483.
PMID |
| [84] |
Vorholt JA (2012). Microbial life in the phyllosphere. Nature Reviews Microbiology, 10, 828-840.
DOI PMID |
| [85] | Voronina OL, Kunda MS, Ryzhova NN, Aksenova EI, Sharapova NE, Semenov AN, Amelina EL, Chuchalin AG, Gintsburg AL (2018). On Burkholderiales order microorganisms and cystic fibrosis in Russia. BMC Genomics, 19, 74. DOI: 10.1186/s12864-018-4472-9. |
| [86] | Wallace J, Laforest-Lapointe I, Kembel SW (2018). Variation in the leaf and root microbiome of sugar maple (Acer saccharum) at an elevational range limit. PeerJ, 6, e5293. DOI: 10.7717/peerj.5293. |
| [87] | Wang WJ, Lü H, Zhong YM, Chen LJ, Li JW, Ma Q (2019). Relationship between heteromorphic leaf traits of Populus euphratica and its individual development. Journal of Beijing Forestry University, 41(2), 62-69. |
| [ 王文娟, 吕慧, 钟悦鸣, 陈利俊, 李景文, 马青 (2019). 胡杨异形叶性状与其个体发育的关系. 北京林业大学学报, 41(2), 62-69.] | |
| [88] | Wei SR, Liu BH, Ni K, Ma LF, Shi YZ, Leng Y, Zheng SH, Gao SL, Yang XD, Ruan JY (2023). Rhizosphere microbial community shows a greater response than soil properties to tea (Camellia sinensis L.) cultivars. Agronomy, 13, 221. DOI: 10.3390/agronomy13010221. |
| [89] | Wu SY (2019). Seasonal Dynamics of Leaf Functional Traits in Artemisia ordosica and Their Influencing Factors. Master degree dissertation, Beijing Forestry University, Beijing. |
| [ 吴水瑛 (2019). 油蒿叶功能性状季节动态及其影响因素. 硕士学位论文, 北京林业大学, 北京.] | |
| [90] | Wu X, Peng J, Liu P, Bei Q, Rensing C, Li Y, Yuan H, Liesack W, Zhang F, Cui Z (2021). Metagenomic insights into nitrogen and phosphorus cycling at the soil aggregate scale driven by organic material amendments. Science of the Total Environment, 785, 147329. DOI: 10.1016/j.scitotenv.2021.147329. |
| [91] | Xiong C, Zhu Y, Wang J, Singh B, Han L, Shen J, Li P, Wang G, Wu C, Ge A, Zhang L, He J (2021). Host selection shapes crop microbiome assembly and network complexity. New Phytologist, 229, 1091-1104. |
| [92] | Xu N, Tan GC, Wang HY, Gai XP (2016). Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. European Journal of Soil Biology, 74, 1-8. |
| [93] |
Xu P, Stirling E, Xie H, Li W, Lv X, Matsumoto H, Cheng HY, Xu A, Lai W, Wang Y, Zheng Z, Wang M, Liu X, Ma B, Xu J (2023). Continental scale deciphering of microbiome networks untangles the phyllosphere homeostasis in tea plant. Journal of Advanced Research, 44, 13-22.
DOI PMID |
| [94] | Xue K, Xie J, Zhou A, Liu F, Li D, Wu L, Deng Y, He Z, van Nostrand JD, Luo Y, Zhou J (2016). Warming alters expressions of microbial functional genes important to ecosystem functioning. Frontiers in Microbiology, 7, 668. DOI: 10.3389/fmicb.2016.00668. |
| [95] | Yadav RKP, Karamanoli K, Vokou D (2011). Bacterial populations on the phyllosphere of Mediterranean plants: influence of leaf age and leaf surface. Frontiers of Agriculture in China, 5, 60-63. |
| [96] | Yang JW, Yu QL, Su WH, Wang SJ, Wang XC, Han Q, Qu JP, Li H (2023). Metagenomics reveals elevated temperature causes nitrogen accumulation mainly by inhibiting nitrate reduction process in polluted water. Science of the Total Environment, 882, 163631. DOI: 10.1016/j.scitotenv.2023.163631. |
| [97] | Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M, Peñuelas J, Richter A, Sardans J, Wanek W (2015). The application of ecological stoichiometry to plant-microbial- soil organic matter transformations. Ecological Monographs, 85, 133-155. |
| [98] |
Zhang D, Wang YY, Yang HL, Lan SH, Chen C, Dai BY, Wang C, Li XD, Xie YF (2023). Using intermittent moving aeration to repair hypereutrophic pond: nutrient removal efficiency and microbial diversity analysis. Environmental Science and Pollution Research International, 30, 46697-46710.
DOI PMID |
| [99] |
Zhang HL, Carnevale G, Polese B, Simard M, Thurairajah B, Khan N, Gentile ME, Fontes G, Vinh DC, Pouliot R, King IL (2019). CD109 restrains activation of cutaneous IL-17-producing γδ T cells by commensal microbiota. Cell Reports, 29, 391-405.
DOI PMID |
| [100] | Zhang JW, Ge ZH, Ma ZH, Huang DY, Zhang JB (2022a). Seasonal changes driving shifts of aquatic rhizosphere microbial community structure and the functional properties. Journal of Environmental Management, 322, 116124. DOI: 10.1016/j.jenvman.2022.116124. |
| [101] | Zhang Q, Acuña JJ, Inostroza NG, Duran PL, Mora ML, Sadowsky MJ, Jorquera MA (2020). Niche differentiation in the composition, predicted function, and co-occurrence networks in bacterial communities associated with Antarctic vascular plants. Frontiers in Microbiology, 11, 1036. DOI: 10.3389/fmicb.2020.01036. |
| [102] |
Zhang X, Johnston ER, Barberán A, Ren Y, Lü X, Han X (2017). Decreased plant productivity resulting from plant group removal experiment constrains soil microbial functional diversity. Global Change Biology, 23, 4318-4332.
DOI PMID |
| [103] | Zhang XY, Miao YY, Gu ZQ, Liu FX (2017). Comparison of bacterial diversity based on typical water samples from Xiaotianchi lake and Changbai waterfall in Changbai Mountain. Global Geology, 36, 632-642. |
| [ 张修源, 苗雨雁, 顾政权, 刘风香 (2017). 长白山小天池和长白瀑布水体典型样本的细菌多样性比较. 世界地质, 36, 632-642.] | |
| [104] | Zhang Y, Zhang YJ, Xu WJ, Hu J, Zhang ZJ (2022b). Possible effects of temperature on bacterial communities in the rhizosphere of rice under different climatic regions. Archives of Microbiology, 204, 212. DOI: 10.1007/s00203-022-02812-1. |
| [105] | Zhao CZ, Zhu LY, Liang J, Yin HJ, Yin CY, Li DD, Zhang NN, Liu Q (2014). Effects of experimental warming and nitrogen fertilization on soil microbial communities and processes of two subalpine coniferous species in Eastern Tibetan Plateau, China. Plant and Soil, 382, 189-201. |
| [106] | Zhou S, Li H, Giles M, Neilson R, Yang X, Su J (2021). Microbial flow within an air-phyllosphere-soil continuum. Frontiers in Microbiology, 11, 615481. DOI: 10.3389/fmicb.2020.615481. |
| [107] | Zhou S, Lie Z, Liu X, Zhu Y, Peñuelas J, Neilson R, Su X, Liu Z, Chu G, Meng Z, Yan J, Liu J (2023). Distinct patterns of soil bacterial and fungal community assemblages in subtropical forest ecosystems under warming. Global Change Biology, 29, 1501-1513. |
| [108] | Zhu Y, Xiong C, Wei Z, Chen Q, Ma B, Zhou S, Tan J, Zhang L, Cui H, Duan G (2022). Impacts of global change on the phyllosphere microbiome. New Phytologist, 234, 1977-1986. |
| [1] | 陈龙 郭柯 勾晓华 赵秀海 马泓若. 祁连圆柏林群落组成及特征[J]. 植物生态学报, 2025, 49(植被): 0-0. |
| [2] | 王娟 张登山 肖元明 裴全帮 王博 樊博 周国英. 长期围封后高寒草原植物根系分泌物特征与环境因子关系[J]. 植物生态学报, 2025, 49(预发表): 1-0. |
| [3] | 梁天豪 熊德成 刘源豪 杜旭龙 杨智杰 黄锦学. 不同菌根类型树种的根系分泌物特征及其根际效应研究进展[J]. 植物生态学报, 2025, 49(预发表): 1-0. |
| [4] | 王麟 李雪 王愉 王新 胡小文 杨梅 朱剑霄. 不同配方种衣剂对高寒草地乡土草种种子生长与建植的影响[J]. 植物生态学报, 2025, 49(1): 118-128. |
| [5] | 王雯莹 肖元明 王小赟 徐嘉昕 马玉花 李强峰 周国英. 多功能群物种配置模式下退化高寒草甸植物多样性与生态系统多功能性的关联[J]. 植物生态学报, 2025, 49(1): 103-117. |
| [6] | 冉佳鑫, 张宇辉, 王云, 杨智杰, 毛超. 增温和氮磷添加对亚热带森林凋落物溶解有机碳生物可降解性的影响[J]. 植物生态学报, 2024, 48(9): 1232-1242. |
| [7] | 龙吉兰, 蒋铮, 刘定琴, 缪宇轩, 周灵燕, 冯颖, 裴佳宁, 刘瑞强, 周旭辉, 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(7): 817-827. |
| [8] | 蔡慧颖, 梁亚涛, 娄虎, 杨光, 孙龙. 白桦细根功能性状和根际细菌群落随火后时间的变化[J]. 植物生态学报, 2024, 48(7): 828-843. |
| [9] | 刘瑶, 钟全林, 徐朝斌, 程栋梁, 郑跃芳, 邹宇星, 张雪, 郑新杰, 周云若. 不同大小刨花楠细根功能性状与根际微环境关系[J]. 植物生态学报, 2024, 48(6): 744-759. |
| [10] | 付粱晨, 丁宗巨, 唐茂, 曾辉, 朱彪. 北京东灵山白桦和蒙古栎的根际效应及其季节动态[J]. 植物生态学报, 2024, 48(4): 508-522. |
| [11] | 程可心, 杜尧, 李凯航, 王浩臣, 杨艳, 金一, 何晓青. 玉米与叶际微生物组的互作遗传机制[J]. 植物生态学报, 2024, 48(2): 215-228. |
| [12] | 索南吉, 李博文, 吕汪汪, 王文颖, 拉本, 陆徐伟, 宋扎磋, 陈程浩, 苗琪, 孙芳慧, 汪诗平. 增温增水情景下钉柱委陵菜物候序列的变化及其抗冻性[J]. 植物生态学报, 2024, 48(2): 158-170. |
| [13] | 张英, 张常洪, 汪其同, 朱晓敏, 尹华军. 氮沉降下西南山地针叶林根际和非根际土壤固碳贡献差异[J]. 植物生态学报, 2023, 47(9): 1234-1244. |
| [14] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [15] | 赵艳超, 陈立同. 土壤养分对青藏高原高寒草地生物量响应增温的调节作用[J]. 植物生态学报, 2023, 47(8): 1071-1081. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19