

植物生态学报 ›› 2011, Vol. 35 ›› Issue (9): 981-989.DOI: 10.3724/SP.J.1258.2011.00981
• 研究论文 • 上一篇
王芳妹1, 蔡妙珍2,*( ), 张淑娜1, 王宁1, 李华飞1,3, 胡雪娜2, 虞舒航2
), 张淑娜1, 王宁1, 李华飞1,3, 胡雪娜2, 虞舒航2
收稿日期:2011-03-09
接受日期:2011-07-02
出版日期:2011-03-09
发布日期:2011-09-01
通讯作者:
蔡妙珍
作者简介:*(E-mail: mzcai@zjnu.cn)
WANG Fang-Mei1, CAI Miao-Zhen2,*( ), ZHANG Shu-Na1, WANG Ning1, LI Hua-Fei1,3, HU Xue-Na2, YU Shu-Hang2
), ZHANG Shu-Na1, WANG Ning1, LI Hua-Fei1,3, HU Xue-Na2, YU Shu-Hang2
Received:2011-03-09
Accepted:2011-07-02
Online:2011-03-09
Published:2011-09-01
Contact:
CAI Miao-Zhen
摘要:
NO和H2O2是参与植物抗非生物胁迫反应的重要信号分子, 为了确定NO和H2O2在大豆(Glycine max)根尖和根边缘细胞(root border cells, RBCs)耐铝反应中的作用及其相互关系, 以‘浙春3号’大豆为材料, 研究了铝毒胁迫下大豆根尖内源NO和H2O2的变化, 以及外源NO和H2O2诱导大豆根尖和RBCs的耐铝反应。结果表明, 50 μmol·L-1 Al处理48 h显著抑制大豆根的伸长, 提高Al在根尖的积累, 同时显著增加根尖内源NO和H2O2含量。施加0.25 mmol·L-1外源NO供体亚硝基铁氰化钠(Na2[Fe(CN)5NO]·2H2O, sodium nitroprusside, SNP)和0.1 mmol·L-1H2O2, 能有效地缓解Al对大豆根伸长的抑制、根尖Al积累和RBCs的死亡, 该缓解作用可以被0.05 mmol·L-1NO清除剂2-(4-羧基苯)-4,4,5,5-四甲基咪唑-1-氧-3-氧化物,钾盐 (C14H16N2O4·K, carboxy-PTIO, cPTIO)和150 U·mL-1H2O2清除酶(catalase, CAT)逆转。并且外源NO能够显著促进根尖H2O2的积累, 而外源H2O2对根尖NO的含量无显著影响。这表明NO和H2O2是诱导大豆根尖及RBCs耐铝反应的两种信号分子, NO可能通过调控H2O2的形成, 进而诱导大豆根尖及RBCs的耐铝反应。
王芳妹, 蔡妙珍, 张淑娜, 王宁, 李华飞, 胡雪娜, 虞舒航. NO和H2O2诱导大豆根尖和边缘细胞耐铝反应的 作用. 植物生态学报, 2011, 35(9): 981-989. DOI: 10.3724/SP.J.1258.2011.00981
WANG Fang-Mei, CAI Miao-Zhen, ZHANG Shu-Na, WANG Ning, LI Hua-Fei, HU Xue-Na, YU Shu-Hang. Effects of nitric oxide and hydrogen peroxide on induction of a defense response in the root tips and root border cells of soybean plants to Al toxicity. Chinese Journal of Plant Ecology, 2011, 35(9): 981-989. DOI: 10.3724/SP.J.1258.2011.00981
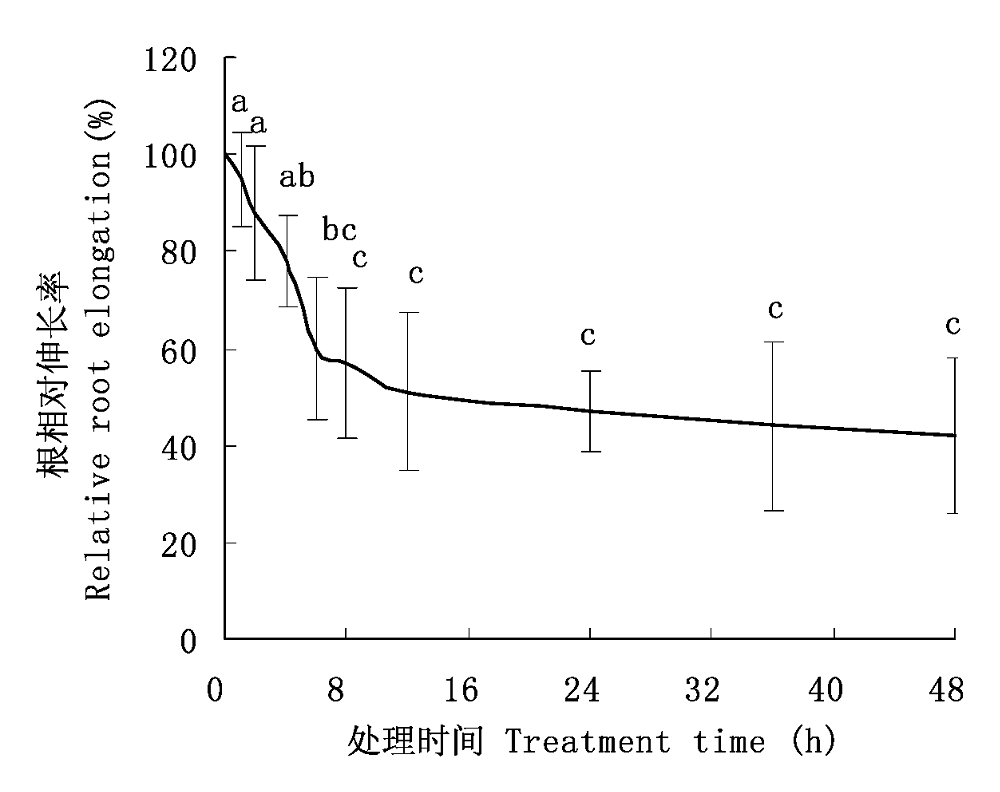
图1 不同铝毒害时间对大豆根长的影响(平均值±标准误差)。 不同小写字母表示差异显著(p < 0.05)。
Fig. 1 Time-dependent effects of Al toxicity on root elongation of soybean (mean ± SE). Different small letters mean significant differences (p < 0.05).
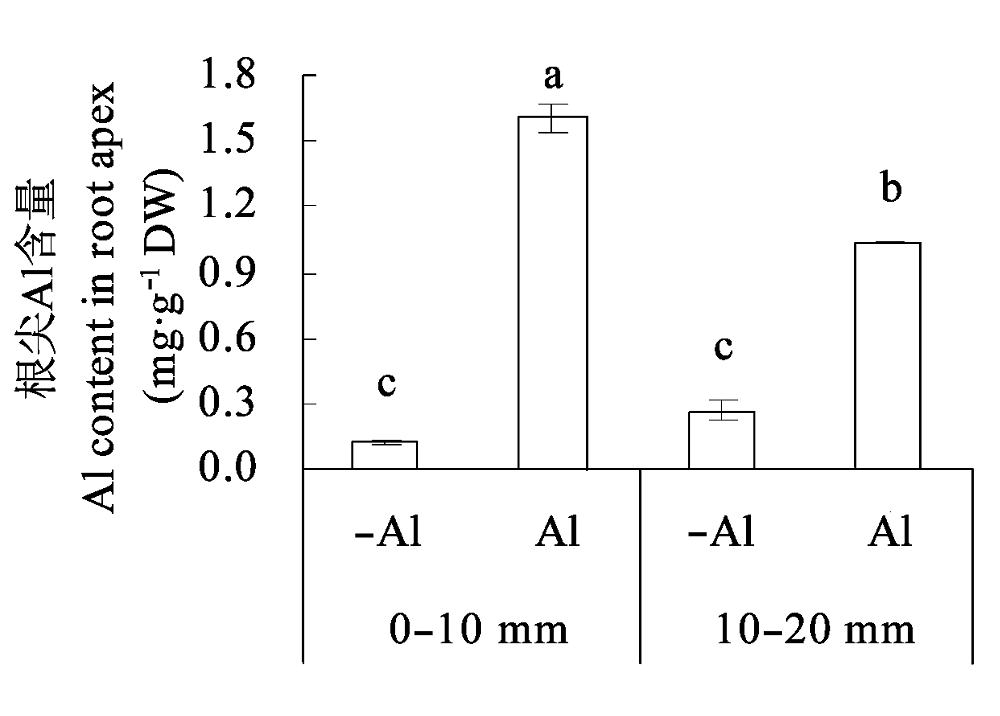
图2 铝毒对大豆根尖Al含量的影响(平均值±标准误差)。 不同小写字母表示差异显著(p < 0.05)。
Fig. 2 Effects of Al toxicity on Al content in soybean root apex (mean ± SE). Different small letters mean significant differences (p < 0.05).
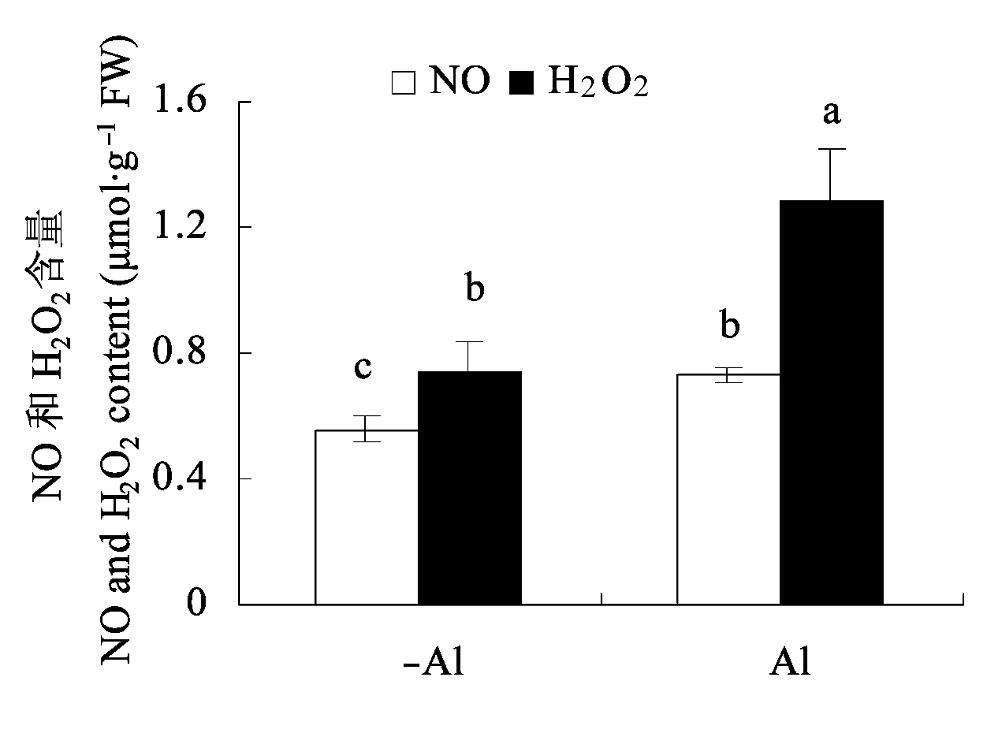
图3 铝毒对大豆根尖内源NO和H2O2含量的影响(平均值±标准误差)。 不同小写字母表示差异显著(p < 0.05)。
Fig. 3 Effects of Al toxicity on endogenous NO and H2O2 contents in soybean root apex (mean ± SE). Different small letters mean significant differences (p < 0.05).
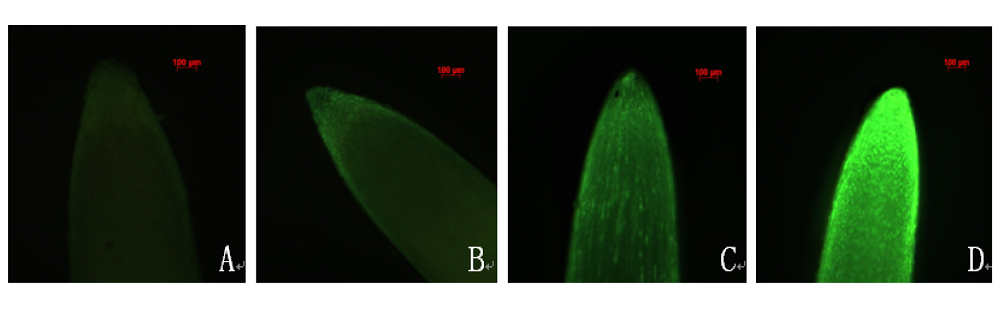
图4 铝毒害下大豆根尖NO和H2O2积累的原位观察。 A, B, NO积累的原位观察(A, -Al; B, Al)。C, D, H2O2积累的原位观察(C, -Al; D, Al)。
Fig. 4 The in situ observation of NO and H2O2 accumulation in root apex of soybean under Al toxicity. A, B, the in situ observation of NO accumulation (A, -Al; B, Al). C, D, the in situ observation of H2O2 accumulation (C, -Al; D, Al).
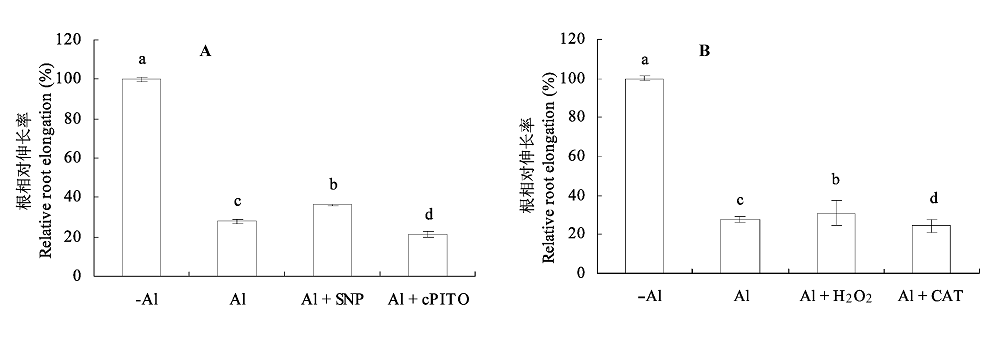
图5 NO和H2O2对铝胁迫下大豆根相对伸长率的影响(平均值±标准误差)。 A, NO。B, H2O2。CAT, 过氧化氢酶; cPTIO, 2-(4-羧基苯)-4,4,5,5-四甲基咪唑-1-O-3-氧化物, 钾盐; SNP, 亚硝基铁氰化钠。不同小写字母表示差异显著(p < 0.05)。
Fig. 5 Effects of NO and H2O2 on relative root elongation in soybean under Al toxicity (mean ± SE). A, NO. B, H2O2. CAT, catalase; cPTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyllimidazoline-1-oxyl-3-oxyde, potassium salt; SNP, sodium nitroprusside. Different small letters mean significant differences (p < 0.05).
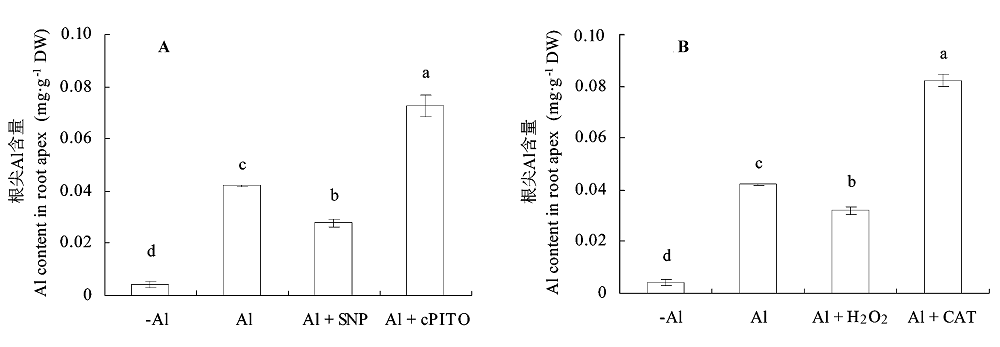
图6 NO和H2O2对铝胁迫下大豆根尖Al含量的影响(平均值±标准误差)。 A, NO。B, H2O2。CAT, 过氧化氢酶; cPTIO, 2-(4-羧基苯)-4,4,5,5-四甲基咪唑-1-O-3-氧化物, 钾盐; SNP, 亚硝基铁氰化钠。不同小写字母表示差异显著(p < 0.05)。
Fig. 6 Effects of NO and H2O2 on Al content in root apex of soybean under Al toxicity (mean ± SE). A, NO. B, H2O2. CAT, catalase; cPTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyllimidazoline-1-oxyl-3-oxyde, potassium salt; SNP, sodium nitroprusside. Different small letters mean significant differences (p < 0.05).
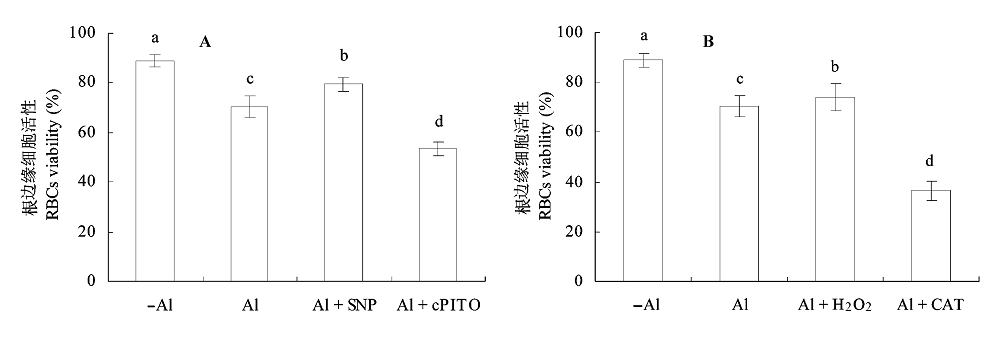
图7 Al毒胁迫下NO和H2O2对大豆根尖根边缘细胞活性的影响(平均值±标准误差)。 A, NO。B, H2O2。CAT, 过氧化氢酶; cPTIO, 2-(4-羧基苯)-4,4,5,5-四甲基咪唑-1-O-3-氧化物, 钾盐; SNP, 亚硝基铁氰化钠。不同小写字母表示差异显著(p < 0.05)。
Fig. 7 Effects of NO and H2O2 on root border cells (RBCs) viability in root apex of soybean under Al toxicity(mean ± SE). A, NO. B, H2O2. CAT, catalase; cPTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyllimidazoline-1-oxyl-3-oxyde, potassium salt; SNP, sodium nitroprusside. Different small letters mean significant differences (p < 0.05).
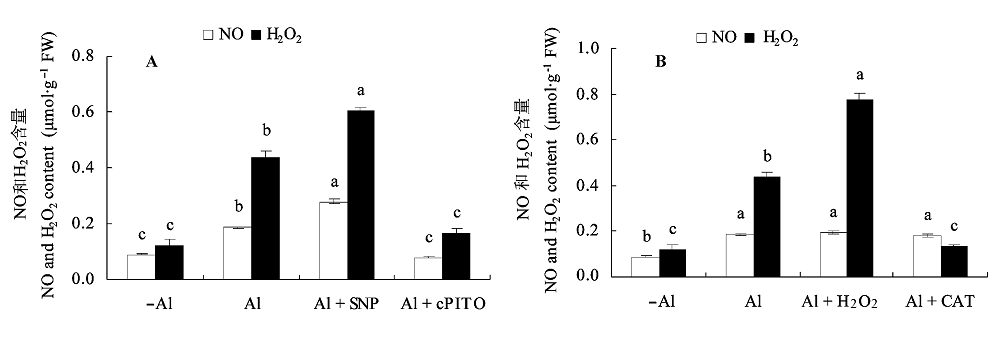
图8 Al毒胁迫下外源NO和H2O2对大豆根尖NO和H2O2含量的影响(平均值±标准误差)。 A, NO。B, H2O2。CAT, 过氧化氢酶; cPTIO, 2-(4-羧基苯)-4,4,5,5-四甲基咪唑-1-O-3-氧化物, 钾盐; SNP, 亚硝基铁氰化钠。不同小写字母表示差异显著(p < 0.05)。
Fig. 8 Effects of exogenous NO and H2O2 contents in root apex of soybean under Al toxicity (mean ± SE). A, NO. B, H2O2. CAT, catalase; cPTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyllimidazoline-1-oxyl-3-oxyde, potassium salt; SNP, sodium nitroprusside. Different small letters mean significant differences (p < 0.05).
| [1] |
Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ (2000). NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. The Plant Journal, 24, 667-677.
URL PMID |
| [2] |
Delhaize E, Ryan PR (1995). Aluminum toxicity and tolerance in plants. Plant Physiology, 107, 315-321.
URL PMID |
| [3] |
Delledonne M, Zeier J, Marocco A, Lamb C (2001). Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences of the United States of America, 98, 13454-13459.
DOI URL PMID |
| [4] |
Fan B, Shen L, Liu KL, Zhao DY, Yu MM, Sheng JP (2008). Interaction between nitric oxide and hydrogen peroxide in postharvest tomato resistance response to Rhizopus nigricans. Journal of the Science of Food and Agriculture, 88, 1238-1244.
DOI URL |
| [5] |
Hawes MC, Gunawardena U, Miyasaka S, Zhao XW (2000). The role of root border cells in plant defense. Trends in Plant Science, 5, 128-133.
DOI URL PMID |
| [6] |
Horst WJ, Wang YX, Eticha D (2010). The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Annals of Botany, 106, 185-197.
URL PMID |
| [7] | Jia ZQ ( 贾芝琪), Yuan HY ( 袁海永), Li YZ ( 李颖章) (2007). Effects of Verticillium dahliae toxin on the NO and H2O2 production and the GST gene expression in cotton suspension cells. Chinese Science Bulletin (科学通报), 52, 911-917. (in Chinese) |
| [8] |
Kawai K (1980). The relationship of phosphorus adsorption to amorphous aluminum for characterizing andosols. Soil Science, 129, 186-190.
DOI URL |
| [9] |
Kovtun Y, Chiu WL, Tnea G, Sheen J (2000). Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proceedings of the National Academy of Sciences of the United States of America, 97, 2940-2945.
URL PMID |
| [10] |
Li G ( 李刚), Xu FJ ( 徐芳杰), Jiang SS ( 蒋思丝), Zhang YS ( 章永松), Lin XY ( 林咸永) (2010). Effects of aluminum on hydrogen peroxide content and cell wall-bound peroxidase activity in wheat root tips. Plant Nutrition and Fertilizer Science (植物营养与肥料学报), 16, 887-892. (in Chinese with English abstract)
DOI URL |
| [11] | Li RF ( 李荣峰), Cai MZ ( 蔡妙珍), Liu P ( 刘鹏), Xu GD ( 徐根娣), Chen MY ( 陈敏燕), Liang H ( 梁和) (2008). Phytoecological effect of Al 3+ on the inductivity of programmed cell death of border cells in soybean root. Journal of Plant Ecology (Chinese Version)(植物生态学报), 32, 690-697. (in Chinese with English abstract) |
| [12] | Li ZG ( 李忠光), Song YQ ( 宋玉泉), Long M ( 垄明) (2007). Xylenol orange method used for the measurement of hydrogen peroxide in plant tissue. Journal of Yunnan Normal University (Natural Sciences Edition) (云南师范大学学报(自然科学版)), 27, 50-54. (in Chinese with English abstract) |
| [13] |
Ma JF (2007). Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. International Review of Cytology, 264, 225-252.
URL PMID |
| [14] |
Mittle R, Vanderauwera S, Gollery M, Breusegem FV (2004). Reactive oxygen gene network in plants. Trends in Plant Science, 9, 490-498.
DOI URL PMID |
| [15] |
Miyasaka SC, Hawes MC (2001). Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiology, 125, 1978-1987.
URL PMID |
| [16] |
Murphy ME, Noack E (1994). Nitric oxide assay using hemoglobin method. Methods in Enzymology, 233, 240-250.
URL PMID |
| [17] |
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002). Hydrogen peroxide and nitric oxide as signaling molecules in plants. Journal of Experimental Botany, 53, 1237-1247.
URL PMID |
| [18] |
Orozco-Cárdenas ML, Ryan CA (2002). Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiology, 130, 487-493.
URL PMID |
| [19] |
Pan JW, Zhu MY, Chen H (2001). Aluminium-induced cell death in root-tip cells of barley. Environmental and Experimental Botany, 46, 71-79.
DOI URL PMID |
| [20] | Pan JW, Zhu MY, Chen H, Han N (2002). Inhibition of cell growth caused by aluminum toxicity results from aluminum-induced cell death in barley suspension cells. Journal of Plant Nutrition, 25, 1063-1073. |
| [21] | Polverari A, Molesini B, Pezzotti M, Buonaurio R, Marte M, Delledonne M (2003). Nitric oxide-mediated transcrip- tional changes in Arabidopsis thaliana. Molecular Plantmicrobe Interactions, 16, 1094-1105. |
| [22] |
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2006). Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant, Cell & Environment, 29, 1532-1544.
URL PMID |
| [23] |
Tada Y, Mori T, Shinogi T, Yao N, Takahashi S, Betsuyaku S, Sakamoto M, Park P, Nakayashiki H, Tosa Y, Mayama S (2004). Nitric oxide and reactive oxygen species do not elicit hypersensitive cell death but induce apoptosis in the adjacent cells during the defense response of oat. Molecular Plant-Microbe Interactions, 17, 245-253.
DOI URL PMID |
| [24] |
Tamás L, Budíková S, Huttová J, Mistrík I, Šimomovičová M, Široká B (2005). Aluminum-induced cell death of barley- root border cells is correlated with peroxidase- and oxalate oxidase-mediated hydrogen peroxide production. Plant Cell Reports, 24, 189-194.
URL PMID |
| [25] | Tian QY, Sun DH, Zhao MG, Zhang WH (2007). Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytologist, 174, 322-331. |
| [26] |
Wang HH, Huang JJ, Bi YR (2010). Nitrate reductase- dependent nitric oxide production is involved in aluminum tolerance in red kidney bean roots. Plant Science, 179, 281-288.
DOI URL |
| [27] | Wang J, Higgins VJ (2006). Nitric oxide modulates H2O2- mediated defenses in the Colletotrichum coccodes-tomato interaction. Physiological and Molecular Plant Pathology, 67, 131-137. |
| [28] |
Wang YS, Yang ZM (2005). Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant and Cell Physiology, 46, 1915-1923.
DOI URL PMID |
| [29] | Xu MJ ( 徐茂军), Dong JF ( 董菊芳), Zhang XB ( 张新波) (2008). The signal interactions of NO and H2O2 in mediating heat shock induced Hypericum monogynum cell synthesis hypericin. Science China (中国科学), 38, 643-653. (in Chinese) |
| [30] |
Xue YJ, Tao L, Yang ZM (2008). Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. Journal of Agricultural and Food Chemistry, 56, 9676-9684.
URL PMID |
| [31] |
Yang JL ( 杨建立), He YF ( 何云峰), Zheng SJ ( 郑绍建) (2005). Research progresses in aluminum tolerance mechanisms in plants. Plant Nutrition and Fertilizer Science (植物营养与肥料学报), 11, 836-845. (in Chinese with English abstract)
DOI URL |
| [32] | Yu M, Shen RF, Liu JY, Chen RF, Xu MM, Yang Y, Xiao HD, Wang HZ, Wang HY, Wang CQ (2009). The role of root border cells in aluminum resistance of pea (Pisum sativum) grown in mist culture. Journal of Plant Nutrition and Soil Science, 172, 528-534. |
| [33] | Yu YH ( 禹艳红), Bin JH ( 宾金华) (2002). The occurrence and biological function of root border cells. Chinese Bulletin of Botany (植物学通报), 19, 756-762. (in Chinese with English abstract) |
| [34] | Zhang F, Wang YP, Wang D (2007). Role of nitric oxide and hydrogen peroxide during the salt resistance response. Plant Signalling & Behavior, 2, 473-474. |
| [35] | Zhang WL ( 张文利), Shen WB ( 沈文飚), Ye MB ( 叶茂炳), Xu LC ( 徐朗菜) (2002). Sensitivity of wheat leaf aconitase to nitric oxide and hydrogen peroxide. Journal of Plant Physiology and Molecular Biology (植物生理与分子生物学学报), 28, 99-104. (in Chinese Chinese with English abstract) |
| [36] |
Zhao MG, Tian QY, Zhang WH (2007). Nitric oxide synthase- dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiology, 144, 206-217.
URL PMID |
| [1] | 姚萌, 康荣华, 王盎, 马方园, 李靳, 台子晗, 方运霆. 利用15N示踪技术研究木荷与马尾松幼苗叶片对NO2的吸收与分配[J]. 植物生态学报, 2023, 47(1): 114-122. |
| [2] | 叶子飘, 段世华, 安婷, 康华靖. 最大电子传递速率的确定及其对电子流分配的影响[J]. 植物生态学报, 2018, 42(4): 498-507. |
| [3] | 王丹, 乔匀周, 董宝娣, 葛静, 杨萍果, 刘孟雨. 昼夜不对称性与对称性升温对大豆产量和水分利用的影响[J]. 植物生态学报, 2016, 40(8): 827-833. |
| [4] | 彭东海,杨建波,李健,邢永秀,覃刘东,杨丽涛,李杨瑞. 间作大豆对甘蔗根际土壤细菌及固氮菌多样性的影响[J]. 植物生态学报, 2014, 38(9): 959-969. |
| [5] | 郭数进,李玮瑜,马艳芸,赵恒,乔玲,李贵全. 山西不同生态型大豆品种苗期耐低温性综合评价[J]. 植物生态学报, 2014, 38(9): 990-1000. |
| [6] | 刘俊, 陈贵青, 徐卫红, 韩桂琪, 张海波, 王慧先, 张明中. 锌对不同油菜品种的生理特性、光合作用、根尖细胞超微结构及籽粒锌积累的影响[J]. 植物生态学报, 2012, 36(10): 1082-1094. |
| [7] | 张杰琦, 李奇, 任正炜, 杨雪, 王刚. 氮素添加对青藏高原高寒草甸植物群落物种丰富度及其与地上生产力关系的影响[J]. 植物生态学报, 2010, 34(10): 1125-1131. |
| [8] | 魏国平, 朱月林, 刘正鲁, 张古文, 杨立飞. 硝酸钙胁迫对营养液栽培嫁接茄子叶片抗坏血酸-谷胱甘肽循环的影响[J]. 植物生态学报, 2008, 32(5): 1023-1030. |
| [9] | 蔡妙珍, 邢承华, 刘鹏, 徐根娣, 吴韶辉, 何璠. 大豆根尖边缘细胞和粘液分泌对铝胁迫解除的响应[J]. 植物生态学报, 2008, 32(5): 1007-1014. |
| [10] | 严茂粉, 李向华, 王克晶. 北京地区野生大豆种群SSR标记的遗传多样性评价[J]. 植物生态学报, 2008, 32(4): 938-950. |
| [11] | 李荣峰, 蔡妙珍, 刘鹏, 徐根娣, 陈敏燕, 梁和. Al3+对大豆根边缘细胞程序性死亡诱导的生理生态作用[J]. 植物生态学报, 2008, 32(3): 690-697. |
| [12] | 苗保河, 李向东, 刘波, 何启平, 朱陶, 刘兴坦, 朱启玉, 乔广法, 樊廷安, 陈成君, 董庆裕, 余松烈. 波浪冠层栽培模式对高油大豆叶片活性氧代谢和膜脂过氧化的影响[J]. 植物生态学报, 2008, 32(3): 673-680. |
| [13] | 宋开山, 张柏, 王宗明, 刘殿伟, 刘焕军. 基于小波分析的大豆叶绿素a含量高光谱反演模型[J]. 植物生态学报, 2008, 32(1): 152-160. |
| [14] | Tripathi OP, Pandey HN, Tripathi RS. EFFECTS OF HUMAN ACTIVITIES ON STRUCTURE AND COMPOSITION OF WOODY SPECIES OF THE NOKREK BIOSPHERE RESERVE OF MEGHALAYA, NORTHEAST INDIA[J]. 植物生态学报, 2008, 32(1): 73-79. |
| [15] | 金森. 基于Huffman编码的群落结构复杂性[J]. 植物生态学报, 2007, 31(6): 1154-1160. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19