

植物生态学报 ›› 2014, Vol. 38 ›› Issue (4): 311-324.DOI: 10.3724/SP.J.1258.2014.00028
• 研究论文 • 下一篇
收稿日期:2013-12-13
接受日期:2014-02-08
出版日期:2014-12-13
发布日期:2014-04-08
通讯作者:
曹坤芳
作者简介:*(E-mail:caokf@xtbg.ac.cn)基金资助:
SUN Shan-Wen1,2, ZHANG Yong-Jiang3, CAO Kun-Fang4,*( )
)
Received:2013-12-13
Accepted:2014-02-08
Online:2014-12-13
Published:2014-04-08
Contact:
CAO Kun-Fang
摘要:
植物的叶片结构和功能性状受到自身、环境和系统发育的影响。该研究选取西双版纳20 hm2热带雨林动态监测大样地内18种分布格局不同的大戟科植物, 测量了幼树叶片的解剖结构、水分关系特征、最大光合能力和暗呼吸, 主要探讨了叶片结构对植物耐旱性和光合能力的影响, 耐旱性和光合能力之间的权衡关系, 以及环境水分条件对植物功能性状相关性的影响。结果表明: 1)生境内植物表现出一定的结构和功能的趋同性, 分布在山脊和山坡的种比沟谷种具有更强的耐失水能力; 2)去除了系统发育的影响后, 一些关键性状(特别是叶片密度和膨压丧失点时的水势、饱和渗透势等)之间存在跨生境尺度上的相关关系, 植物叶片结构同时影响了植物的耐失水能力和光合能力, 植物叶片自身的结构限制导致了植物的耐旱性(高的叶片密度、比叶质量)和光合能力(低的叶片密度、比叶质量)存在反向进化关系; 3)如果研究的植物类群亲缘关系较近, 传统的Pearson相关分析不能很好地揭示其性状间的相关关系, 因而必须采用系统发育独立对照差作相关分析。大戟科植物的结构和功能在水分梯度和光梯度上的生态位分化也从功能性状的角度为热带季雨林能维持高生物多样性, 保持植物物种长期共存提供了一个可能的解释。
孙善文, 章永江, 曹坤芳. 热带季雨林不同小生境大戟科植物幼树的叶片结构、耐旱性和光合能力之间的相关性. 植物生态学报, 2014, 38(4): 311-324. DOI: 10.3724/SP.J.1258.2014.00028
SUN Shan-Wen, ZHANG Yong-Jiang, CAO Kun-Fang. Correlations among leaf structure, drought tolerance and photosynthetic capacity in saplings of Euphorbiaceae from different micro-habitats in a seasonal tropical rainforest. Chinese Journal of Plant Ecology, 2014, 38(4): 311-324. DOI: 10.3724/SP.J.1258.2014.00028
| 种 Species | 分布 Distribution | 丰度 Abundance (Ind.·hm-2) |
|---|---|---|
| 长梗三宝木 Trigonostemon thyrsoideus | 沟谷 Valley | 40.45 |
| 粉绿野桐 Mallotus garrettii | 沟谷 Valley | 34.15 |
| 勐腊核果木 Drypetes hoaensis | 沟谷 Valley | 28.35 |
| 棒柄花 Cleidion brevipetiolatum | 沟谷 Valley | 48.55 |
| 秋枫 Bischofia javanica | 沟谷 Valley | 1.65 |
| 缅桐 Sumbaviopsis albicans | 山坡 Slope | 23.00 |
| 轮叶戟 Lasiococca comberi | 山坡 Slope | 9.20 |
| 风轮桐 Epiprinus siletianus | 山坡 Slope | 6.20 |
| 网脉核果木 Drypetes perreticulata | 山坡 Slope | 1.50 |
| 土蜜树 Bridelia tomentosa | 山坡 Slope | 1.05 |
| 木奶果 Baccaurea ramilflora | 遍及整个样地 Throughout the entire sample plot | 160.60 |
| 山地五月茶 Antidesma montanum | 遍及整个样地 Throughout the entire sample plot | 22.75 |
| 日本五月茶 Antidesma japonicum | 遍及整个样地 Throughout the entire sample plot | 13.75 |
| 尾叶血桐 Macaranga kurzii | 山脊 Ridge | 0.40 |
| 越南巴豆 Croton kongensis | 山脊 Ridge | 7.35 |
| 银背巴豆 Croton argyratus | 山脊 Ridge | 3.00 |
| 椴叶山麻杆 Alchornea tiliifolia | 山脊 Ridge | 18.75 |
| 云南银柴 Aporusa yunnanensis | 山脊 Ridge | 26.40 |
表1 样地内18种大戟科植物物种名、分布及丰度
Table 1 A list of names, distributions, and abundance of the 18 plant species of the Euphobiaceae within study plot
| 种 Species | 分布 Distribution | 丰度 Abundance (Ind.·hm-2) |
|---|---|---|
| 长梗三宝木 Trigonostemon thyrsoideus | 沟谷 Valley | 40.45 |
| 粉绿野桐 Mallotus garrettii | 沟谷 Valley | 34.15 |
| 勐腊核果木 Drypetes hoaensis | 沟谷 Valley | 28.35 |
| 棒柄花 Cleidion brevipetiolatum | 沟谷 Valley | 48.55 |
| 秋枫 Bischofia javanica | 沟谷 Valley | 1.65 |
| 缅桐 Sumbaviopsis albicans | 山坡 Slope | 23.00 |
| 轮叶戟 Lasiococca comberi | 山坡 Slope | 9.20 |
| 风轮桐 Epiprinus siletianus | 山坡 Slope | 6.20 |
| 网脉核果木 Drypetes perreticulata | 山坡 Slope | 1.50 |
| 土蜜树 Bridelia tomentosa | 山坡 Slope | 1.05 |
| 木奶果 Baccaurea ramilflora | 遍及整个样地 Throughout the entire sample plot | 160.60 |
| 山地五月茶 Antidesma montanum | 遍及整个样地 Throughout the entire sample plot | 22.75 |
| 日本五月茶 Antidesma japonicum | 遍及整个样地 Throughout the entire sample plot | 13.75 |
| 尾叶血桐 Macaranga kurzii | 山脊 Ridge | 0.40 |
| 越南巴豆 Croton kongensis | 山脊 Ridge | 7.35 |
| 银背巴豆 Croton argyratus | 山脊 Ridge | 3.00 |
| 椴叶山麻杆 Alchornea tiliifolia | 山脊 Ridge | 18.75 |
| 云南银柴 Aporusa yunnanensis | 山脊 Ridge | 26.40 |
| 性状 Trait | 山脊 Ridge | 山坡 Slope | 沟谷 Valley | 广布种 Cosmopolitan species |
|---|---|---|---|---|
| SWC (%) | 2.660 ± 0.840ab | 1.860 ± 0.330a | 3.280 ± 1.110ab | 4.260 ± 0.770b |
| πo (MPa) | -1.670 ± 0.180a | -1.740 ± 0.250a | -1.190 ± 0.220b | -1.190 ± 0.040b |
| πtlp (MPa) | -1.950 ± 0.180a | -1.990 ± 0.220a | -1.400 ± 0.270b | -1.400 ± 0.020b |
| ε (MPa) | 15.21 ± 6.540a | 22.32 ± 7.480a | 18.70 ± 6.300a | 11.88 ± 2.190a |
| RWCtlp (%) | 84.62 ± 5.280a | 90.70 ± 4.600ab | 92.61 ± 3.560b | 86.99 ± 0.810ab |
| LT (mm) | 0.670 ± 0.380a | 0.540 ± 0.140a | 0.900 ± 0.300a | 1.100 ± 0.460a |
| UET (mm) | 0.069 ± 0.036a | 0.061 ± 0.010a | 0.083 ± 0.017a | 0.164 ± 0.053b |
| PT (mm) | 0.240 ± 0.130a | 0.150 ± 0.060a | 0.190 ± 0.090a | 0.280 ± 0.060a |
| ST (mm) | 0.300 ± 0.230a | 0.260 ± 0.100a | 0.540 ± 0.220a | 0.550 ± 0.340a |
| LET (mm) | 0.063 ± 0.022a | 0.063 ± 0.015a | 0.080 ± 0.018a | 0.101 ± 0.023a |
| P/S (%) | 1.214 ± 0.611a | 0.670 ± 0.345a | 0.432 ± 0.270a | 0.618 ± 0.208a |
| LD (g·cm-3) | 940.4 ± 391.2ab | 1 175.0 ± 385.5a | 514.2 ± 94.2b | 529.5 ± 171.1ab |
| LMA (g·cm-2) | 51.36 ± 12.810a | 59.56 ± 14.080a | 44.91 ± 14.750a | 53.49 ± 6.640a |
| Aa (μmol·m-2·s-1) | 10.380 ± 2.310a | 8.400 ± 3.410a | 7.690 ± 1.520a | 7.710 ± 0.750a |
| Am (nmol·g-1·s-1) | 0.210 ± 0.150a | 0.160 ± 0.110a | 0.190 ± 0.110a | 0.150 ± 0.030a |
| R (μmol·m-2·s-1) | 0.600 ± 0.074a | 0.470 ± 0.074b | 0.500 ± 0.064ab | 0.480 ± 0.046ab |
| Rm (nmol·g-1·s-1) | 0.012 ± 0.003a | 0.008 ± 0.003a | 0.012 ± 0.006a | 0.009 ± 0.001a |
表2 大戟科植物不同小生境分布类群的叶片性状及方差分析结果(平均值±标准误差)
Table 2 Traits values of the Euphobiaceae plants in different habitats and summary of ANOVA analysis (mean ± SD)
| 性状 Trait | 山脊 Ridge | 山坡 Slope | 沟谷 Valley | 广布种 Cosmopolitan species |
|---|---|---|---|---|
| SWC (%) | 2.660 ± 0.840ab | 1.860 ± 0.330a | 3.280 ± 1.110ab | 4.260 ± 0.770b |
| πo (MPa) | -1.670 ± 0.180a | -1.740 ± 0.250a | -1.190 ± 0.220b | -1.190 ± 0.040b |
| πtlp (MPa) | -1.950 ± 0.180a | -1.990 ± 0.220a | -1.400 ± 0.270b | -1.400 ± 0.020b |
| ε (MPa) | 15.21 ± 6.540a | 22.32 ± 7.480a | 18.70 ± 6.300a | 11.88 ± 2.190a |
| RWCtlp (%) | 84.62 ± 5.280a | 90.70 ± 4.600ab | 92.61 ± 3.560b | 86.99 ± 0.810ab |
| LT (mm) | 0.670 ± 0.380a | 0.540 ± 0.140a | 0.900 ± 0.300a | 1.100 ± 0.460a |
| UET (mm) | 0.069 ± 0.036a | 0.061 ± 0.010a | 0.083 ± 0.017a | 0.164 ± 0.053b |
| PT (mm) | 0.240 ± 0.130a | 0.150 ± 0.060a | 0.190 ± 0.090a | 0.280 ± 0.060a |
| ST (mm) | 0.300 ± 0.230a | 0.260 ± 0.100a | 0.540 ± 0.220a | 0.550 ± 0.340a |
| LET (mm) | 0.063 ± 0.022a | 0.063 ± 0.015a | 0.080 ± 0.018a | 0.101 ± 0.023a |
| P/S (%) | 1.214 ± 0.611a | 0.670 ± 0.345a | 0.432 ± 0.270a | 0.618 ± 0.208a |
| LD (g·cm-3) | 940.4 ± 391.2ab | 1 175.0 ± 385.5a | 514.2 ± 94.2b | 529.5 ± 171.1ab |
| LMA (g·cm-2) | 51.36 ± 12.810a | 59.56 ± 14.080a | 44.91 ± 14.750a | 53.49 ± 6.640a |
| Aa (μmol·m-2·s-1) | 10.380 ± 2.310a | 8.400 ± 3.410a | 7.690 ± 1.520a | 7.710 ± 0.750a |
| Am (nmol·g-1·s-1) | 0.210 ± 0.150a | 0.160 ± 0.110a | 0.190 ± 0.110a | 0.150 ± 0.030a |
| R (μmol·m-2·s-1) | 0.600 ± 0.074a | 0.470 ± 0.074b | 0.500 ± 0.064ab | 0.480 ± 0.046ab |
| Rm (nmol·g-1·s-1) | 0.012 ± 0.003a | 0.008 ± 0.003a | 0.012 ± 0.006a | 0.009 ± 0.001a |
| SWC | πo | πtlp | ε | RWCtlp | LT | UET | PT | ST | LET | P/S | LD | LMA | Aa | Am | R | Rm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWC | 1 | 0.81*** | 0.82*** | -0.25 | 0.07 | 0.62** | 0.61** | -0.05 | 0.70** | 0.60* | -0.81*** | -0.71*** | -0.44 | 0.25 | 0.51* | 0.19 | 0.57* |
| πo | 0.70** | 1 | 0.98*** | -0.25 | 0.20 | 0.59* | 0.52* | -0.23 | 0.74*** | 0.37 | -0.76*** | -0.71*** | -0.86*** | 0.27 | 0.63** | -0.11 | 0.66** |
| πtlp | 0.77*** | 0.98*** | 1 | -0.20 | 0.28 | 0.55* | 0.53* | -0.24 | 0.69** | 0.41 | -0.82*** | -0.70** | -0.79*** | 0.18 | 0.57** | -0.17 | 0.61** |
| ε | -0.47 | -0.68** | -0.57* | 1 | 0.81*** | -0.65** | -0.45 | 0.01 | -0.74*** | -0.44 | 0.43 | 0.24 | 0.66** | -0.65** | -0.74*** | -0.37 | -0.69** |
| RWCtlp | -0.05 | -0.26 | -0.10 | 0.77*** | 1 | -0.31 | -0.18 | -0.05 | -0.34 | -0.06 | -0.12 | -0.13 | 0.47 | -0.56* | -0.54* | -0.44 | -0.52* |
| LT | 0.64** | 0.60** | 0.60** | -0.13 | 0.25 | 1 | 0.84*** | 0.74*** | 0.95*** | 0.91*** | -0.56* | -0.78*** | 0.33 | 0.01 | -0.25 | -0.03 | -0.33 |
| UET | 0.66** | 0.55* | 0.56* | -0.31 | 0 | 0.71** | 1 | 0.71*** | 0.69** | 0.85*** | -0.45 | -0.62** | 0.23 | -0.06 | -0.23 | -0.10 | -0.28 |
| PT | 0.65** | 0.42 | 0.43 | -0.23 | -0.06 | 0.51* | 0.27 | 1 | 0.49* | 0.69** | 0.20 | -0.61** | 0.23 | 0.40 | 0.05 | 0.36 | -0.08 |
| ST | 0.50* | 0.56* | 0.55* | -0.03 | 0.37 | 0.95*** | 0.66** | 0.23 | 1 | 0.82*** | -0.70** | -0.73*** | 0.32 | -0.13 | -0.33 | -0.17 | -0.37 |
| LET | 0.54* | 0.52* | 0.54* | -0.20 | 0.20 | 0.83*** | 0.63** | 0.60* | 0.71** | 1 | -0.50* | -0.76*** | 0.34 | -0.03 | -0.25 | -0.16 | -0.38 |
| P/S | -0.21 | -0.37 | -0.37 | -0.37 | -0.68** | -0.54* | -0.30 | -0.00 | -0.70** | -0.57* | 1 | 0.69** | 0.44 | -0.21 | -0.45 | 0.06 | -0.41 |
| LD | -0.70** | -0.89*** | -0.87*** | 0.75*** | 0.36 | -0.75** | -0.56* | -0.10 | -0.81*** | -0.60* | 0.48* | 1 | 0.76*** | -0.53* | -0.81*** | -0.23 | -0.82*** |
| LMA | -0.16 | -0.29 | -0.27 | 0.31 | 0.33 | -0.29 | -0.24 | 0.40 | -0.47 | 0 | -0.37 | 0.14 | 1 | -0.22 | -0.77*** | 0.16 | -0.75** |
| Aa | 0.16 | 0 | -0.04 | -0.39 | -0.42 | 0.69** | 0.22 | 0.58* | 0.61** | 0.54* | 0.35 | -0.20 | -0.28 | 1 | 0.77*** | 0.76*** | 0.44 |
| Am | 0.14 | 0.10 | 0.10 | -0.37 | -0.37 | 0.60* | 0.27 | 0.18 | 0.62** | 0.40 | 0.44 | -0.18 | -0.68** | 0.80*** | 1 | 0.49* | 0.87*** |
| R | 0.20 | -0.08 | -0.14 | -0.41 | -0.56* | 0.53* | 0.10 | 0.70** | 0.38 | 0.52* | 0.48* | -0.18 | -0.24 | 0.59** | 0.52* | 1 | -0.62** |
| Rm | 0.16 | 0.12 | 0.12 | -0.36 | -0.40 | 0.50* | 0.25 | 0.04 | 0.56* | 0.30 | 0.49* | -0.15 | -0.86*** | 0.58* | 0.92*** | -0.45 | 1 |
表3 大戟科植物叶片各性状间的相关关系。左下为传统Pearson相关,右上为系统发育独立性比较后的相关
Table 3 Correlations among leaf traits in plant species of the Euphobiaceae. The lower left corner shows conventional Pearson correlation, and the upper right corner shows correlations given by the phylogenetic independent contrasts analysis
| SWC | πo | πtlp | ε | RWCtlp | LT | UET | PT | ST | LET | P/S | LD | LMA | Aa | Am | R | Rm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWC | 1 | 0.81*** | 0.82*** | -0.25 | 0.07 | 0.62** | 0.61** | -0.05 | 0.70** | 0.60* | -0.81*** | -0.71*** | -0.44 | 0.25 | 0.51* | 0.19 | 0.57* |
| πo | 0.70** | 1 | 0.98*** | -0.25 | 0.20 | 0.59* | 0.52* | -0.23 | 0.74*** | 0.37 | -0.76*** | -0.71*** | -0.86*** | 0.27 | 0.63** | -0.11 | 0.66** |
| πtlp | 0.77*** | 0.98*** | 1 | -0.20 | 0.28 | 0.55* | 0.53* | -0.24 | 0.69** | 0.41 | -0.82*** | -0.70** | -0.79*** | 0.18 | 0.57** | -0.17 | 0.61** |
| ε | -0.47 | -0.68** | -0.57* | 1 | 0.81*** | -0.65** | -0.45 | 0.01 | -0.74*** | -0.44 | 0.43 | 0.24 | 0.66** | -0.65** | -0.74*** | -0.37 | -0.69** |
| RWCtlp | -0.05 | -0.26 | -0.10 | 0.77*** | 1 | -0.31 | -0.18 | -0.05 | -0.34 | -0.06 | -0.12 | -0.13 | 0.47 | -0.56* | -0.54* | -0.44 | -0.52* |
| LT | 0.64** | 0.60** | 0.60** | -0.13 | 0.25 | 1 | 0.84*** | 0.74*** | 0.95*** | 0.91*** | -0.56* | -0.78*** | 0.33 | 0.01 | -0.25 | -0.03 | -0.33 |
| UET | 0.66** | 0.55* | 0.56* | -0.31 | 0 | 0.71** | 1 | 0.71*** | 0.69** | 0.85*** | -0.45 | -0.62** | 0.23 | -0.06 | -0.23 | -0.10 | -0.28 |
| PT | 0.65** | 0.42 | 0.43 | -0.23 | -0.06 | 0.51* | 0.27 | 1 | 0.49* | 0.69** | 0.20 | -0.61** | 0.23 | 0.40 | 0.05 | 0.36 | -0.08 |
| ST | 0.50* | 0.56* | 0.55* | -0.03 | 0.37 | 0.95*** | 0.66** | 0.23 | 1 | 0.82*** | -0.70** | -0.73*** | 0.32 | -0.13 | -0.33 | -0.17 | -0.37 |
| LET | 0.54* | 0.52* | 0.54* | -0.20 | 0.20 | 0.83*** | 0.63** | 0.60* | 0.71** | 1 | -0.50* | -0.76*** | 0.34 | -0.03 | -0.25 | -0.16 | -0.38 |
| P/S | -0.21 | -0.37 | -0.37 | -0.37 | -0.68** | -0.54* | -0.30 | -0.00 | -0.70** | -0.57* | 1 | 0.69** | 0.44 | -0.21 | -0.45 | 0.06 | -0.41 |
| LD | -0.70** | -0.89*** | -0.87*** | 0.75*** | 0.36 | -0.75** | -0.56* | -0.10 | -0.81*** | -0.60* | 0.48* | 1 | 0.76*** | -0.53* | -0.81*** | -0.23 | -0.82*** |
| LMA | -0.16 | -0.29 | -0.27 | 0.31 | 0.33 | -0.29 | -0.24 | 0.40 | -0.47 | 0 | -0.37 | 0.14 | 1 | -0.22 | -0.77*** | 0.16 | -0.75** |
| Aa | 0.16 | 0 | -0.04 | -0.39 | -0.42 | 0.69** | 0.22 | 0.58* | 0.61** | 0.54* | 0.35 | -0.20 | -0.28 | 1 | 0.77*** | 0.76*** | 0.44 |
| Am | 0.14 | 0.10 | 0.10 | -0.37 | -0.37 | 0.60* | 0.27 | 0.18 | 0.62** | 0.40 | 0.44 | -0.18 | -0.68** | 0.80*** | 1 | 0.49* | 0.87*** |
| R | 0.20 | -0.08 | -0.14 | -0.41 | -0.56* | 0.53* | 0.10 | 0.70** | 0.38 | 0.52* | 0.48* | -0.18 | -0.24 | 0.59** | 0.52* | 1 | -0.62** |
| Rm | 0.16 | 0.12 | 0.12 | -0.36 | -0.40 | 0.50* | 0.25 | 0.04 | 0.56* | 0.30 | 0.49* | -0.15 | -0.86*** | 0.58* | 0.92*** | -0.45 | 1 |
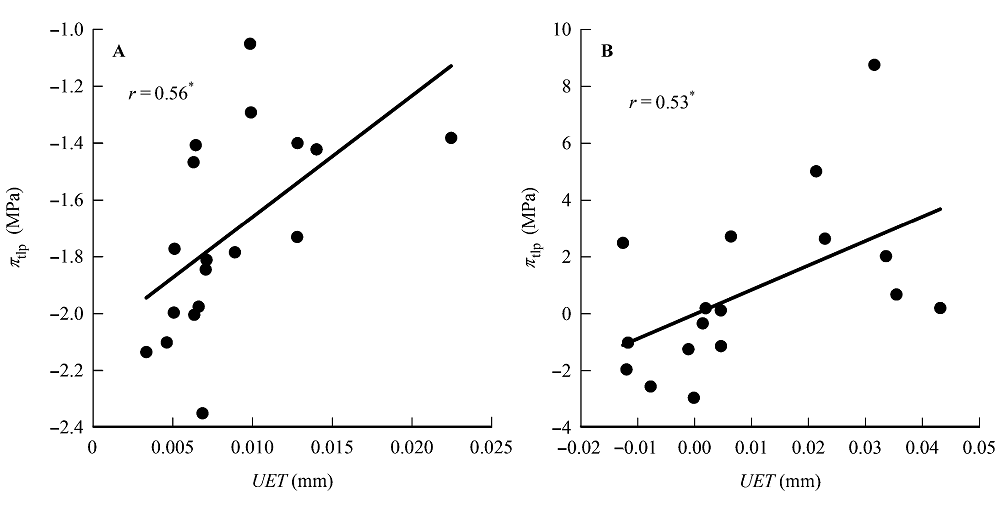
图1 上表皮厚度(UET)与膨压丧失点时的水势(πtlp)的相关关系。 A, 传统Pearson相关。B, 系统发育独立性比较。*, 0.01 < p < 0.05。
Fig. 1 Correlations between upper epidermis thickness (UET) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. *, 0.01 < p < 0.05.
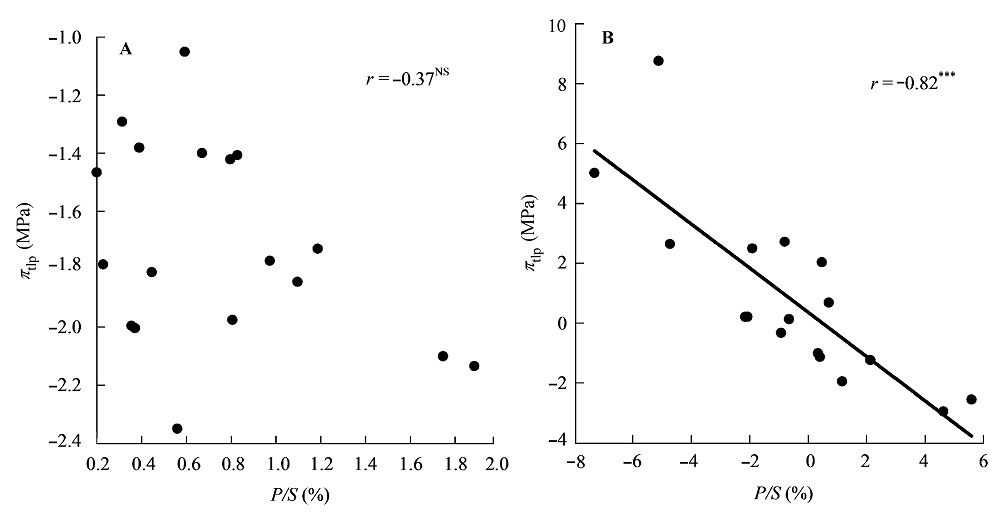
图2 栅栏组织厚度/海绵组织厚度(P/S)与膨压丧失点时的水势(πtlp)的相关关系。 A, 传统Pearson相关。B, 系统发育独立性比较。***, p < 0.001; NS, p > 0.05。
Fig. 2 Correlations between the palisade thickness/spongy thickness (P/S) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. ***, p < 0.001; NS, p > 0.05.
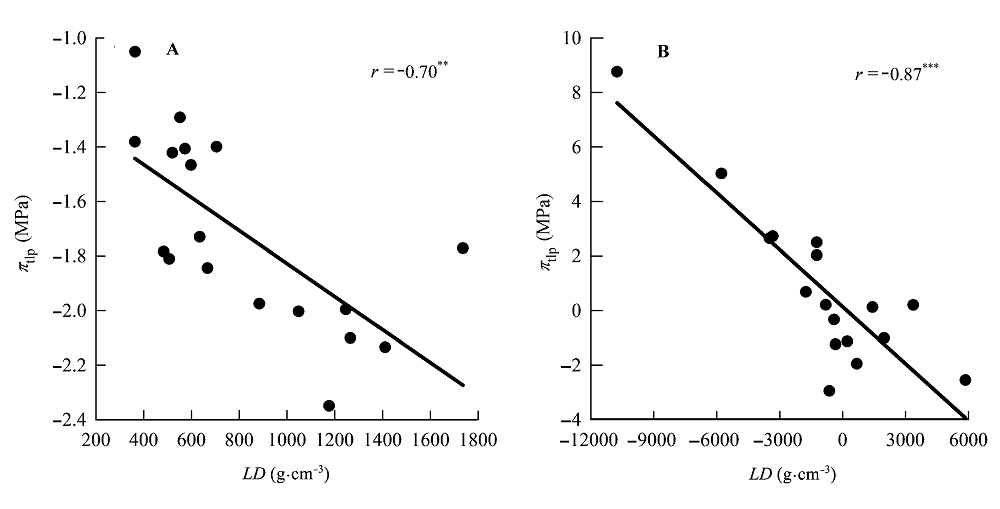
图3 叶片密度(LD)与膨压丧失点时的水势(πtlp)的相关关系。 A, 传统Pearson相关。B, 系统发育独立性比较。**, 0.001 < p < 0.01; ***, p < 0.001。
Fig. 3 Correlations between leaf density (LD) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. **, 0.001 < p < 0.01; ***, p < 0.001.
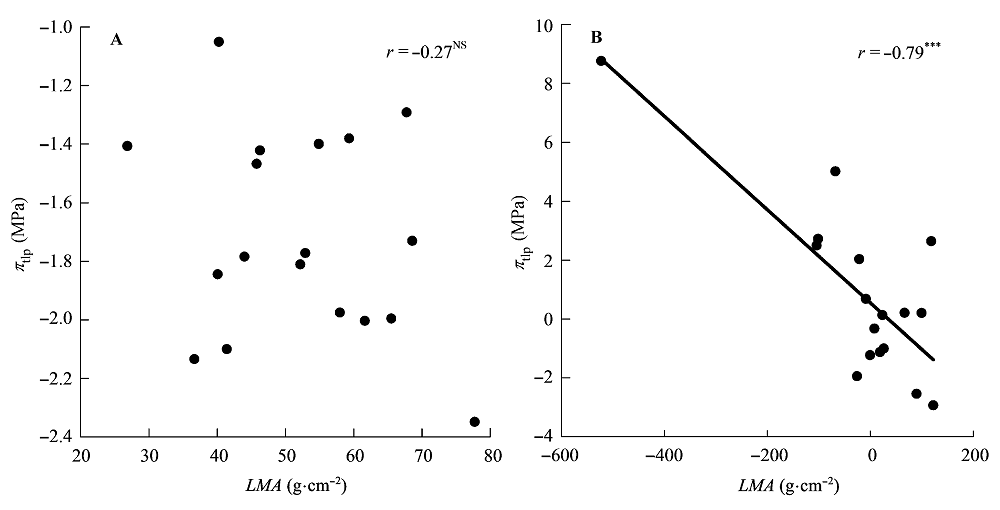
图4 比叶质量(LMA)与膨压丧失点时的水势(πtlp)的相关关系。 A, 传统Pearson相关。B, 系统发育独立性比较。***, p < 0.001; NS, p > 0.05。
Fig. 4 Correlations between leaf mass per area (LMA) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. ***, p < 0.001; NS, p > 0.05.
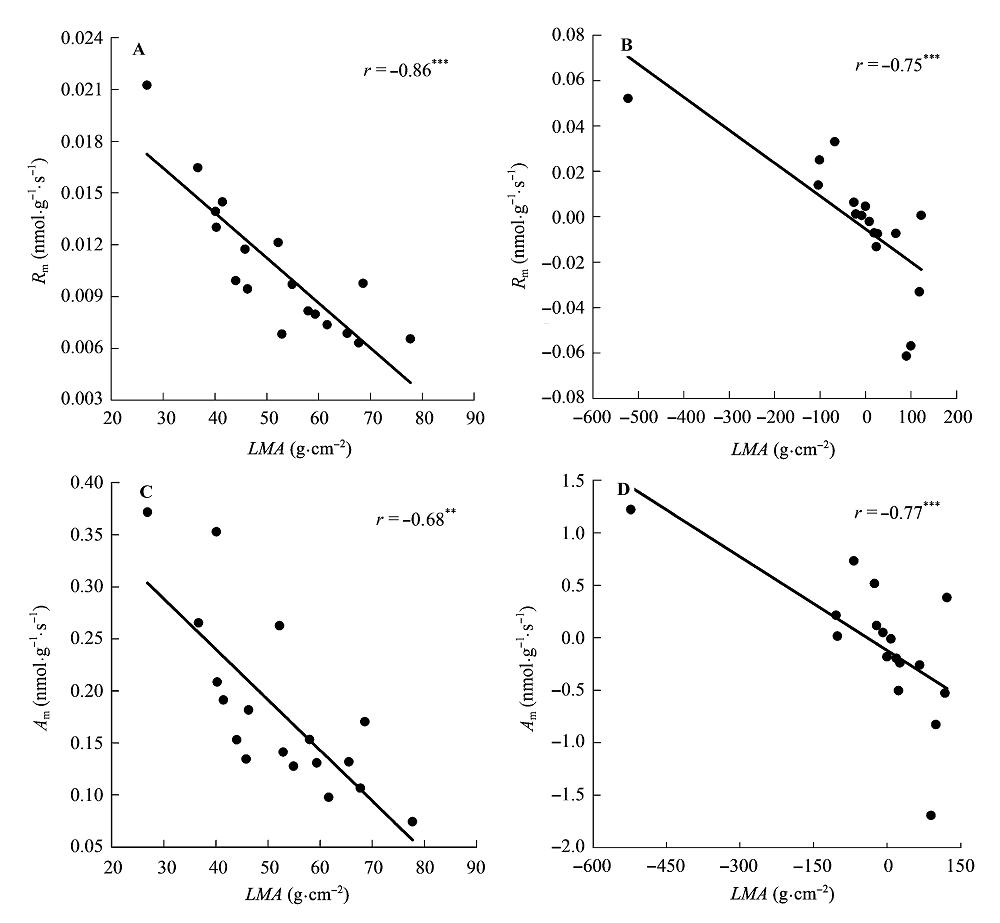
图5 比叶质量(LMA)与单位叶干质量最大光合速率(Am)和单位叶干质量暗呼吸速率(Rm)的相关关系。 A, B, 传统Pearson相关。C, D, 系统发育独立性比较。**, 0.001 < p < 0.01; ***, p < 0.001。
Fig. 5 Correlations of leaf mass per area (LMA) with maximum photosynthesis per leaf dry mass (Am) and dark respiration per leaf dry mass (Rm). A, B, Traditional Pearson correlation. C, D, Correlation given by the phylogenetic independent contrasts analysis. **, 0.001 < p < 0.01; ***, p < 0.001.
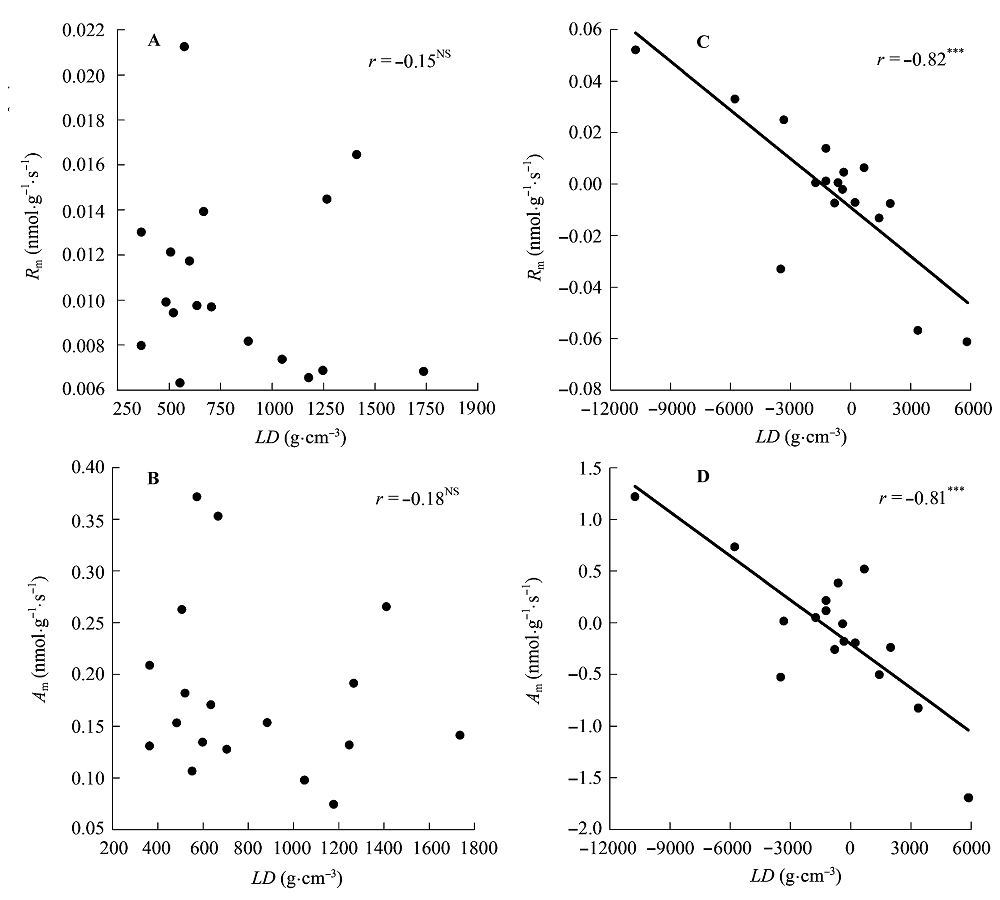
图6 叶片密度(LD)与单位叶干质量最大光合速率(Am)和单位叶干质量暗呼吸速率(Rm)的相关关系。 A, B, 传统Pearson相关。C, D, 系统发育独立性比较。***, p < 0.001; NS, p > 0.05。
Fig. 6 Correlations of leaf density (LD) with maximum photosynthesis per leaf dry mass (Am) and dark respiration per leaf dry mass (Rm). A, B, Traditional Pearson correlation. C, D, Correlation given by the phylogenetic independent contrasts analysis. ***, p < 0.001; NS, p > 0.05.
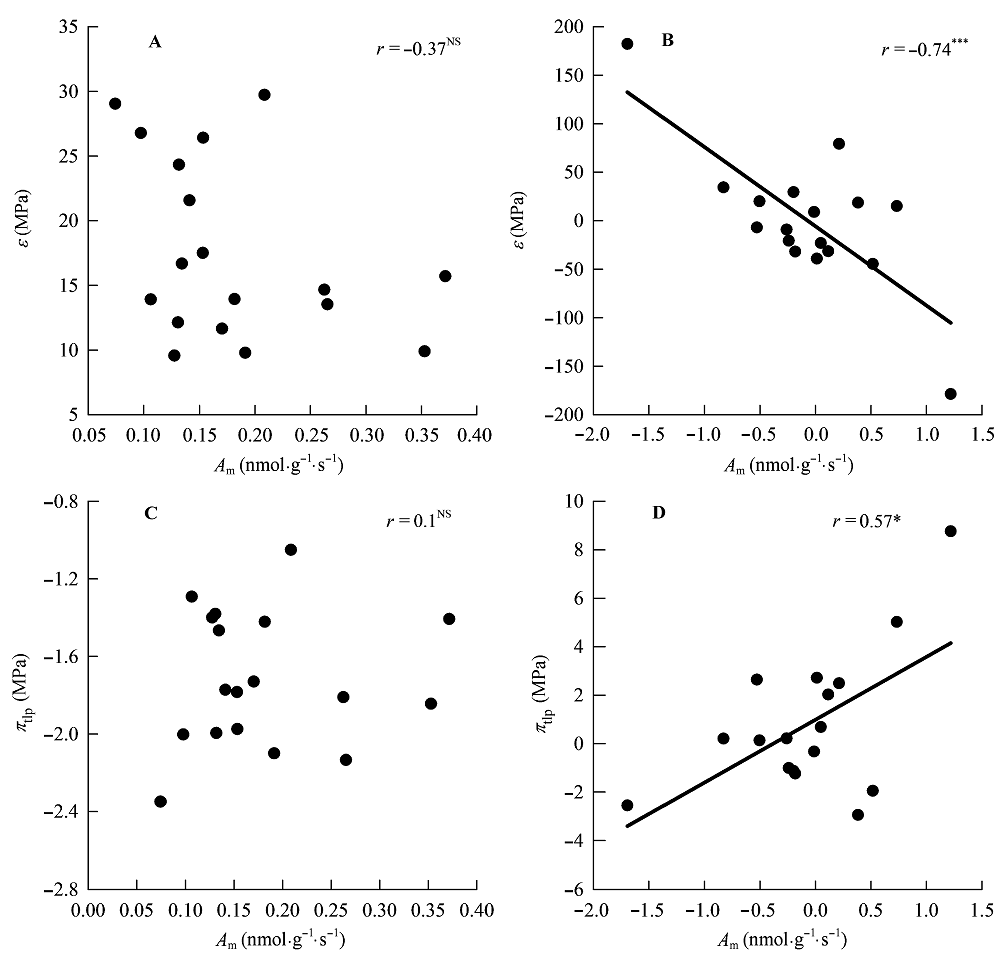
图7 单位叶干质量最大光合速率(Am)与膨压丧失点时的水势(πtlp)和叶片弹性模数(ε)的相关关系。 A、B, 传统Pearson相关。C、D, 系统发育独立性比较。*, 0.01 < p < 0.05; ***, p < 0.001; NS, p > 0.05。
Fig. 7 Correlations of maximum photosynthesis per leaf dry mass (Am) with water potential at turgor loss point (πtlp) and modulus of elasticity at full turgor (ε). A, B, Traditional Pearson correlation. C, D, Correlation given by the phylogenetic independent contrasts analysis. *, 0.01 < p < 0.05; ***, p < 0.001; NS, p > 0.05.
| [1] | Aranda I, Castro L, Pardos M, Gil L, Pardos JA (2005). Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. Forest Ecology and Management, 210, 117-129. |
| [2] |
Bacelar EA, Correia CM, Moutinho-Pereira JM, Goncalves BC, Lopes JI, Torres-Pereira JM (2004). Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiology, 24, 233-239.
URL PMID |
| [3] | Bartlett MK, Scoffoni C, Ardy R, Zhang Y, Sun SW, Cao KF, Sack L (2012a). Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods in Ecology and Evolution, 3, 880-888. |
| [4] |
Bartlett MK, Scoffoni C, Sack L (2012b). The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters, 15, 393-405.
URL PMID |
| [5] | Becker P, Wong M (1994). Drought induced mortality in tropical heath forest. Journal of Tropical Sciences, 5, 416-417. |
| [6] | Brodersen CR, Vogelmann TC (2007). Do epidermal lens cells facilitate the absorptance of diffuse light? American Journal of Botang, 94, 1061-1066. |
| [7] |
Brown C, Burslem DFRP, Illian JB, Bao L, Brockelman W, Cao M, Chang LW, Dattaraja HS, Davies S, Gunatilleke CVS, Gunatilleke IAUN, Huang J, Kassim AR, LaFrankie JV, Lian J, Lin L, Ma K, Mi X, Nathalang A, Noor S, Ong P, Sukumar R, Su SH, Sun IF, Suresh HS, Tan S, Thompson J, Uriarte M, Valencia R, Yap SL, Ye W, Law R (2013). Multispecies coexistence of trees in tropical forests: spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proceedings of the Royal Society B: Biological Sciences, 280, 20130502.
DOI URL PMID |
| [8] |
Bucci S, Goldstein G, Meinzer F, Scholz F, Franco A, Bust- amante M (2004). Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiology, 24, 891-899.
URL PMID |
| [9] |
Cao KF (2000). Leaf anatomy and chlorophyll content of 12 woody species in contrasting light conditions in a bornean heath forest. Canadian Journal of Botany, 78, 1245-1253.
DOI URL |
| [10] |
Cao M, Zou XM, Warren M, Zhu H (2006). Tropical forests of Xishuangbanna, China. Biotropica, 38, 306-309.
DOI URL |
| [11] |
Chartzoulakis K, Patakas A, Kofidis G, Bosabalidis A, Nastou A (2002). Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Scientia Horticulturae, 95, 39-50.
DOI URL |
| [12] | Choat B, Medek DE, Stuart SA, Pasquet-Kok J, Egerton JJG, Salari H, Sack L, Ball MC (2011). Xylem traits mediate a trade-off between resistance to freeze-thaw-induced embolism and photosynthetic capacity in overwintering evergreens. New Phytologist, 191, 996-1005. |
| [13] | Cutler J, Rains D, Loomis R (1977). The importance of cell size in the water relations of plants. Physiologia Plantarum, 40, 255-260. |
| [14] | DeLucia EH, Nelson K, Vogelmann TC, Smith WK (1996). Contribution of intercellular reflectance to photosynthesis in shade leaves. Plant, Cell & Environment, 19, 159-170. |
| [15] | Evans J, Vogelmann T, Williams W, Gorton H (2004). Chloroplast to leaf. In: Smith W, Vogelmann T, Critchley C eds. Photosynthetic Adaptation. Springer, New York. 178. 15-41. |
| [16] | Felsenstein J (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1-15. |
| [17] |
Feng YL, Lei YB, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit , Li YP, Zheng YL (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106, 1853-1856.
URL PMID |
| [18] |
Fu PL, Jiang YJ, Wang AY, Brodribb TJ, Zhang JL, Zhu SD, Cao KF (2012). Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an asian tropical dry karst forest. Annals of Botany, 110, 189-199.
DOI URL PMID |
| [19] | Garland T, Harvey PH, Ives AR (1992). Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology, 41, 18-32. |
| [20] |
Green DS, Kruger EL (2001). Light-mediated constraints on leaf function correlate with leaf structure among deciduous and evergreen tree species. Tree Physiology, 21, 1341-1346.
DOI URL PMID |
| [21] | Hanba YT, Miyazawa SI, Terashima I (1999). The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Functional Ecology, 13, 632-639. |
| [22] |
Hassiotou F, Renton M, Ludwig M, Evans JR, Veneklaas EJ (2010). Photosynthesis at an extreme end of the leaf trait spectrum: How does it relate to high leaf dry mass per area and associated structural parameters? Journal of Experimental Botany, 61, 3015-3028.
DOI URL PMID |
| [23] | Hessini K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly C (2009). Effect of water stress on growth, osmo- tic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environmental and Experimental Botany, 67, 312-319. |
| [24] |
Hu YH, Sheng DY, Xiang YZ, Yang ZJ, Xu DP, Zhang NN, Shi LL (2013). The environment, not space, dominantly structures the landscape patterns of the richness and composition of the tropical understory vegetation. PLoS ONE, 8, e81308.
URL PMID |
| [25] | Karabourniotis G (1998). Light-guiding function of foliar sclereids in the evergreen sclerophyll phillyrea latifolia: a quantitative approach. Journal of Experimental Botany, 49, 739-746. |
| [26] | Karabourniotis G, Bornman JF (1999). Penetration of UV-A, UV-B and blue light through the leaf trichome layers of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiologia Plantarum, 105, 655-661. |
| [27] | Karabourniotis G, Papastergiou N, Kabanopoulou E, Fasseas C (1994). Foliar sclereids of Olea europaea may function as optical fibres. Canadian Journal of Botany, 72, 330-336. |
| [28] | Kubiske ME, Abrams MD (1990). Pressure-volume relation- ships in non-rehydrated tissue at various water deficits. Plant, Cell & Environment, 13, 995-1000. |
| [29] | Kursar TA, Engelbrecht BMJ, Burke A, Tyree MT, Omari BE, Giraldo JP (2009). Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Functional Ecology, 23, 93-102. |
| [30] |
Lan GY, Getzin S, Wiegand T, Hu YH, Xie GS, Zhu H, Cao M (2012). Spatial distribution and interspecific associations of tree species in a tropical seasonal rain forest of China. PLoS ONE, 7, e46074.
DOI URL PMID |
| [31] | Lan YG, Hu YH, Cao M, Zhu H, Wang H, Zhou SS, Deng SS, Deng XB, Cui JY, Huang JG, Liu LY, Xu HL, Song JP, He YC (2008). Establishment of Xishuangbanna tropical forest dynamics plot: species compositions and spatial distribution patterns. Journal of Plant Ecology (Chinese Version), 32, 287-298. (in Chinese with English abstract) |
| [ 兰国玉, 胡跃华, 曹敏, 朱华, 王洪, 周仕顺, 邓晓保, 崔景云, 黄建国, 刘林云, 许海龙, 宋军平, 何有才 (2008). 西双版纳热带森林动态监测样地——树种组成与空间分布格局. 植物生态学报, 32, 287-298.] | |
| [32] | Lin LX, Comita LS, Zheng Z, Cao M (2012). Seasonal differentiation in density-dependent seedling survival in a tropical rain forest. Journal of Ecology, 100, 905-914. |
| [33] | Markesteijn L, Poorter L, Paz H, Sack L, Bongers F (2011). Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell & Environment, 34, 137-148. |
| [34] |
Mediavilla S, Escudero A, Heilmeier H (2001). Internal leaf anatomy and photosynthetic resource-use efficiency: inter- specific and intraspecific comparisons. Tree Physiology, 21, 251-259.
DOI URL PMID |
| [35] |
Meinzer FC (2003). Functional convergence in plant responses to the environment. Oecologia, 134, 1-11.
DOI URL PMID |
| [36] |
Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A (2008). Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiologia Plantarum, 134, 237-245.
URL PMID |
| [37] | Morgan JM (1984). Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology, 35, 299-319. |
| [38] | Niinemets Ü (1999). Research review. Components of leaf dry mass per area-thickness and density-alter leaf photo- synthetic capacity in reverse directions in woody plants. New Phytologist, 144, 35-47. |
| [39] | Niinemets Ü (2001). Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology, 82, 453-469. |
| [40] |
Niinemets Ü, Diaz-Espejo A, Flexas J, Galmes J, Warren CR (2009). Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. Journal of Experimental Botany, 60, 2249-2270.
DOI URL PMID |
| [41] |
Nikolopoulos D, Liakopoulos G, Drossopoulos I, Karabour- niotis G (2002). The relationship between anatomy and photosynthetic performance of heterobaric leaves. Plant Physiology, 129, 235-243.
DOI URL PMID |
| [42] | Onoda Y, Hikosaka K, Hirose T (2004). Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Functional Ecology, 18, 419-425. |
| [43] | Parkhurst DF (1994). Diffusion of CO2 and other gases inside leaves. New Phytologist, 126, 449-479. |
| [44] | Patakas A, Nikolaou N, Zioziou E, Radoglou K, Noitsakis B (2002). The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Science, 163, 361-367. |
| [45] |
Poorter H, Evans JR (1998). Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia, 116, 26-37.
URL PMID |
| [46] | Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist, 182, 565-588. |
| [47] | Poulson ME, Vogelmann TC (1990). Epidermal focussing and effects upon photosynthetic light-harvesting in leaves of oxalis. Plant, Cell & Environment, 13, 803-811. |
| [48] | Reich P, Wright I, Cavender-Bares J, Craine J, Oleksyn J, Westoby M, Walters M (2003). The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences, 164, S143-S164. |
| [49] |
Richardson AD, Berlyn GP (2002). Changes in foliar spectral reflectance and chlorophyll fluorescence of four temperate species following branch cutting. Tree Physiology, 22, 499-506.
DOI URL PMID |
| [50] |
Sack L, Frole K (2006). Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology, 87, 483-491.
DOI URL PMID |
| [51] | Sack L, Pasquet-Kok J (2011). Leaf pressure-volume curve parameters. http://prometheuswiki.publish.csiro.au/tiki- index.php?page=Pressure-volume+curves. Cited: 23 Dec. 2013. |
| [52] |
Savé R, Biel C, de Herralde F (2000). Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus creticus L. Biologia Plantarum, 43, 239-244.
DOI URL |
| [53] | Scafaro AP, von Caemmerer S, Evans JR, Atwell BJ (2011). Temperature response of mesophyll conductance in cultiv- ated and wild oryza species with contrasting mesophyll cell wall thickness. Plant, Cell & Environment, 34, 1999-2008. |
| [54] | Smith T, Huston M (1989). A theory of the spatial and temporal dynamics of plant communities. Plant Ecology, 83, 49-69. |
| [55] | Smith WK, Vogelmann TC, DeLucia EH, Bell DT, Shepherd KA (1997). Leaf form and photosynthesis. BioScience, 47, 785-793. |
| [56] |
Swenson NG, Enquist BJ (2007). Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. American Journal of Botany, 94, 451-459.
DOI URL PMID |
| [57] |
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731-2739.
URL PMID |
| [58] |
Terashima I, Hanba YT, Tholen D, Niinemets U (2011). Leaf functional anatomy in relation to photosynthesis. Plant Physiology (Rockville), 155, 108-116.
DOI URL PMID |
| [59] |
Tomas M, Flexas J, Copolovici L, Galmes J, Hallik L, Medrano H, Ribas-Carbo M, Tosens T, Vislap V, Niinemets Ü (2013). Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany, 64, 2269-2281.
DOI URL PMID |
| [60] | Vogelmann TC (1993). Plant tissue optics. Annual Review of Plant Physiology and Plant Molecular Biology, 44, 231-251. |
| [61] | Vogelmann TC, Bornman JF, Yates DJ (1996a). Focusing of light by leaf epidermal cells. Physiologia Plantarum, 98, 43-56. |
| [62] | Vogelmann TC, Martin G (1993). The functional-significance of palisade tissue—Penetration of directional versus diffuse light. Plant, Cell & Environment, 16, 65-72. |
| [63] | Vogelmann TC, Nishio JN, Smith WK (1996b). Leaves and light capture: light propagation and gradients of carbon fixation within leaves. Trends in Plant Science, 1, 65-70. |
| [64] | Walters M, Reich P (2000). Trade-offs in low-light CO2 exchange: a component of variation in shade tolerance among cold temperate tree seedlings. Functional Ecology, 14, 155-165. |
| [65] | Winter H, Robinson DG, Heldt HW (1993). Subcellular volumes and metabolite concentrations in barley leaves. Planta, 191, 180-190. |
| [66] |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M (2004). The worldwide leaf economics spectrum. Nature, 428, 821-827.
DOI URL PMID |
| [1] | 文佳 张新娜 王娟 赵秀海 张春雨. 性状调节幼苗存活率对邻体竞争和环境的响应 [J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 萨其拉, 张霞, 朱琳, 康萨如拉. 长期不同放牧强度下荒漠草原优势种无芒隐子草叶片解剖结构变化[J]. 植物生态学报, 2024, 48(3): 331-340. |
| [3] | 马常钦, 黄海龙, 彭政淋, 吴纯泽, 韦庆钰, 贾红涛, 卫星. 水曲柳雌雄株复叶类型及光合功能对不同生境的响应[J]. 植物生态学报, 2023, 47(9): 1287-1297. |
| [4] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [5] | 冯珊珊, 黄春晖, 唐梦云, 蒋维昕, 白天道. 细叶云南松针叶形态和显微性状地理变异及其环境解释[J]. 植物生态学报, 2023, 47(8): 1116-1130. |
| [6] | 蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用[J]. 植物生态学报, 2023, 47(7): 988-997. |
| [7] | 冯可, 刘冬梅, 张琦, 安菁, 何双辉. 旅游干扰对松山油松林土壤微生物多样性及群落结构的影响[J]. 植物生态学报, 2023, 47(4): 584-596. |
| [8] | 石荡, 郭传超, 蒋南林, 唐莹莹, 郑凤, 王瑾, 廖康, 刘立强. 新疆野杏天然更新幼株的个体特征及空间分布格局[J]. 植物生态学报, 2023, 47(4): 515-529. |
| [9] | 汪晶晶, 王嘉浩, 黄致云, Vanessa Chiamaka OKECHUKW, 胡蝶, 祁珊珊, 戴志聪, 杜道林. 不同氮水平下内生固氮菌对入侵植物南美蟛蜞菊生长策略的影响[J]. 植物生态学报, 2023, 47(2): 195-205. |
| [10] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [11] | 余秋伍, 杨菁, 沈国春. 浙江天童常绿阔叶林林冠结构与群落物种组成的关系[J]. 植物生态学报, 2022, 46(5): 529-538. |
| [12] | 孟庆静, 樊卫国. 刺梨的适钙类型及对高钙生境的适应性[J]. 植物生态学报, 2022, 46(12): 1562-1572. |
| [13] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [14] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [15] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19