

植物生态学报 ›› 2023, Vol. 47 ›› Issue (7): 988-997.DOI: 10.17521/cjpe.2022.0153
所属专题: 光合作用
蒋海港1, 曾云鸿1, 唐华欣1, 刘伟1, 李杰林1, 何国华1, 秦海燕1, 王丽超1, 姚银安1,*( )
)
收稿日期:2022-04-18
接受日期:2022-09-28
出版日期:2023-07-20
发布日期:2023-07-21
通讯作者:
*(姚银安, 基金资助:
JIANG Hai-Gang1, ZENG Yun-Hong1, TANG Hua-Xin1, LIU Wei1, LI Jie-Lin1, HE Guo-Hua1, QIN Hai-Yan1, WANG Li-Chao1, Victor RESCO de DIOS1,2,*( ), YAO Yin-An1,*(
), YAO Yin-An1,*( )
)
Received:2022-04-18
Accepted:2022-09-28
Online:2023-07-20
Published:2023-07-21
Contact:
*(Yao YA, Supported by:摘要:
藓类植物作为缺乏维管组织的非等水植物, 其光合作用和耗水量主要受到光照、温度和水分供应状况的影响; 这些环境条件随昼夜交替改变, 是否使得藓类植物形成了类似维管植物的内在昼夜节律调节机制, 目前还所知甚少; 理解其节律响应特点对于藓类植物的保育与利用具有重要意义。该研究对脆枝青藓(Brachythecium thraustum)、大灰藓(Hypnum plumaeforme)和长叶提灯藓(Mnium lycopodioides)进行12 h/12 h的昼夜光周期驯化后进行24 h/0 h的持续光照处理, 分别测定3种藓类在12 h/12 h和24 h/0 h光周期条件下的净光合速率(Pn)和蒸腾速率(Tr), 结果表明: 排除光照和叶-气水汽压差(VPD)等环境影响之后, 3种藓类的这两个指标均观察到明显的节律变化, 节律调节作用能够解释其Pn和Tr日变化的23.4%和30.2%; 且维管植物中少见的Tr节律作用出现在所有受试的藓类植物上; Pn和Tr的节律响应存在明显的种间差异, 该研究中节律性最强的物种为脆枝青藓。以脆枝青藓为例进一步分析其全光照下非结构性碳水化合物(NSC)的节律响应时序转录组, 结果表明: 35.1%的淀粉、糖类合成代谢相关基因的表达具有节律性, 且与NSC含量的昼夜节律变化相关; 脱落酸信号和气孔调节相关基因的表达变化与Tr的节律调节相关; 光合电子传递和暗反应相关蛋白的转录水平变化与光合作用节律保持一致。藓类植物虽然丢失了生物钟核心反馈环CCA1/LHY基因, 但是核心生物钟功能集中于PRRs家族, 通过级联调节使得光合作用生物学过程和保卫细胞水分/离子运输过程等相关基因表达呈现显著的节律性, 最终导致固碳耗水的节律响应高度保守。
蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用. 植物生态学报, 2023, 47(7): 988-997. DOI: 10.17521/cjpe.2022.0153
JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses. Chinese Journal of Plant Ecology, 2023, 47(7): 988-997. DOI: 10.17521/cjpe.2022.0153
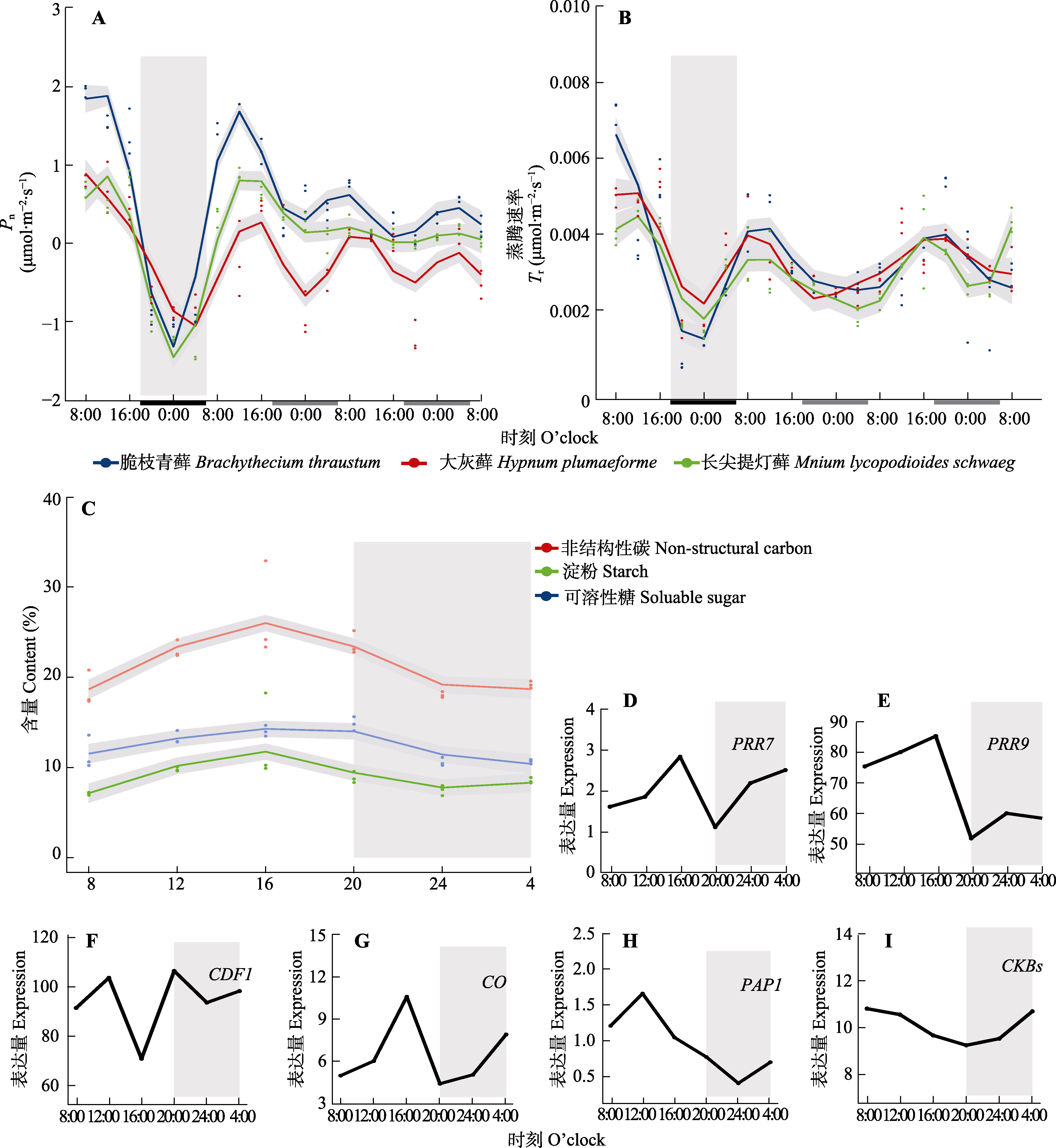
图1 使用广义相加模型(GAM)拟合脆枝青藓、大灰藓和长叶提灯藓的净光合同化速率(Pn)、蒸腾速率(Tr)、非结构性碳水化合物和核心生物钟基因及节律调控相关基因昼夜节律。A、B中灰色部分对应于15 d正常光周期的最后一个夜晚。横坐标上的黑色和白色分别代表植物自然条件下的夜晚和白天, 灰色部分代表植物自然条件下黑暗但给予光照。C-I中灰色部分表示全光照条件下第二天(LL2d)外界条件下的夜晚。D-I分别为脆枝青藓生物钟调控相关基因的表达量。CDF1, CYCLING DOF FACTOR1; CKB, CASEIN KINASE II BETA CHAIN; CO, Constans; PAP1, PRODUCTION OF ANTHOCYANIN PIGMENT 1; PRR7, PSEUDO RESPONSE REGULATOR 7; PRR9, PSEUDO RESPONSE REGULATOR。
Fig. 1 Circadian rhythms of core circadian clock genes and rhythm regulation-related genes fitted to photosynthetic assimilation rate (Pn), transpiration rate (Tr), and non-structural carbon (NSC) in Brachythecium thraustum、Hypnum plumaeforme and Mnium lycopodioides using generalized additive model (GAM). The gray part in A and B corresponds to the last night of the 15 d normal photoperiod. The black and white parts on the horizontal coordinates represent the night and daytime of the plant under natural conditions, respectively, while the gray part represents the darkness of the plant under natural conditions but given light. The gray part in C-I represents the night under full light conditions on the second day (LL2d) under external conditions. D-I represent expression of clock-regulated genes in the moss Ceratodon purpureus. CDF1, CYCLING DOF FACTOR1; CKB, CASEIN KINASE II BETA CHAIN; CO, Constans; PAP1, PRODUCTION OF ANTHOCYANIN PIGMENT 1; PRR7, PSEUDO RESPONSE REGULATOR 7; PRR9, PSEUDO RESPONSE REGULATOR.
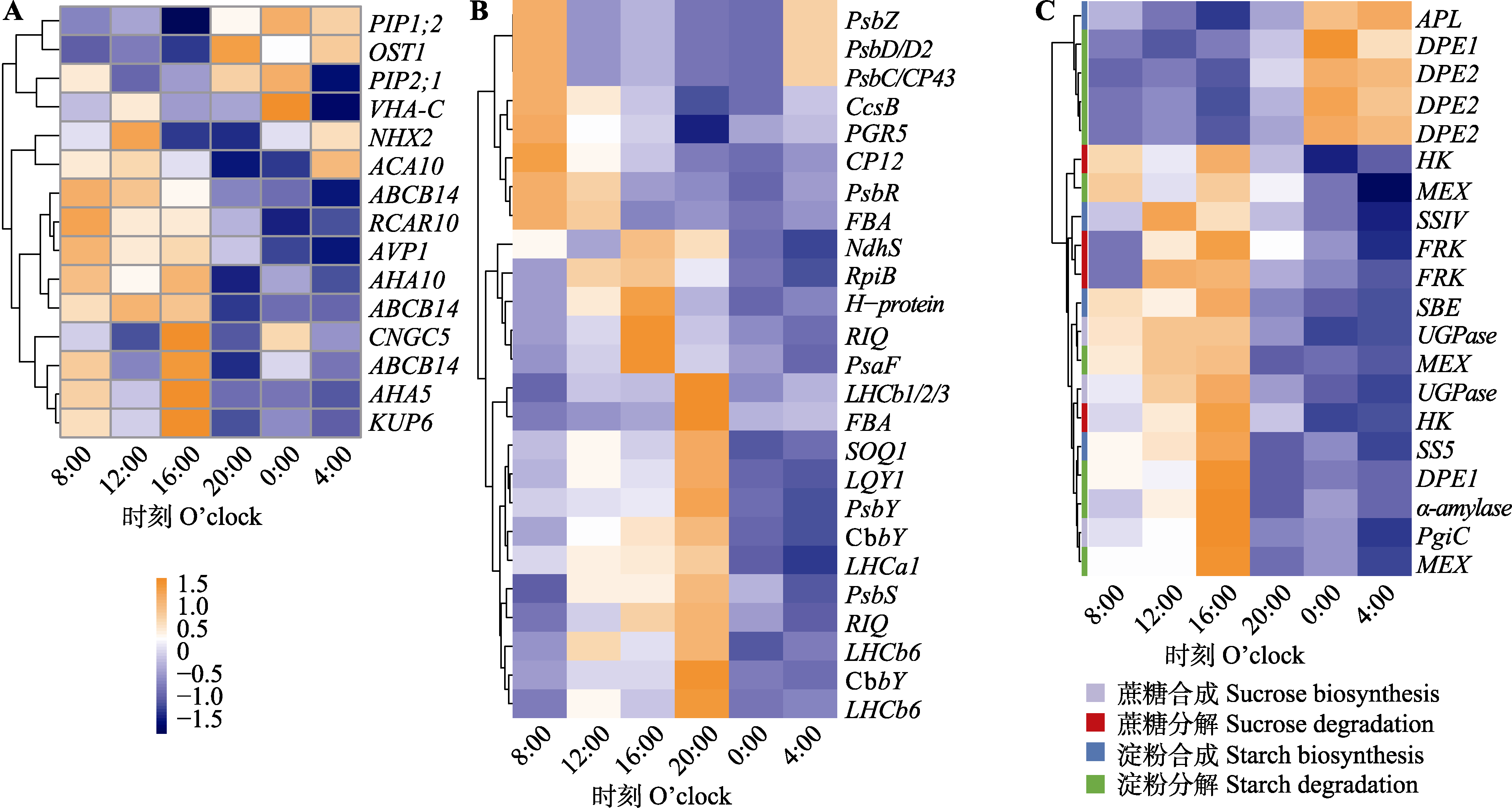
图2 在恒定光照下脆枝青藓相关基因的表达。A, 15个显著的气孔相关节律性基因热图。B, 25个显著的光合相关节律性基因热图。C, 20个显著的非结构性碳水化合物相关节律性基因热图。热图采用了归一化表达量, 范围为-1.5到1.5。
Fig. 2 Expression of significant rhythmic genes related to stomata (A), photosynthesis(B) and non-structural carbohydrates (C) for Brachythecium thraustum under continuous illumination and constant environmental conditions. The heatmap used normalized expression levels, ranging from -1.5 to 1.5.
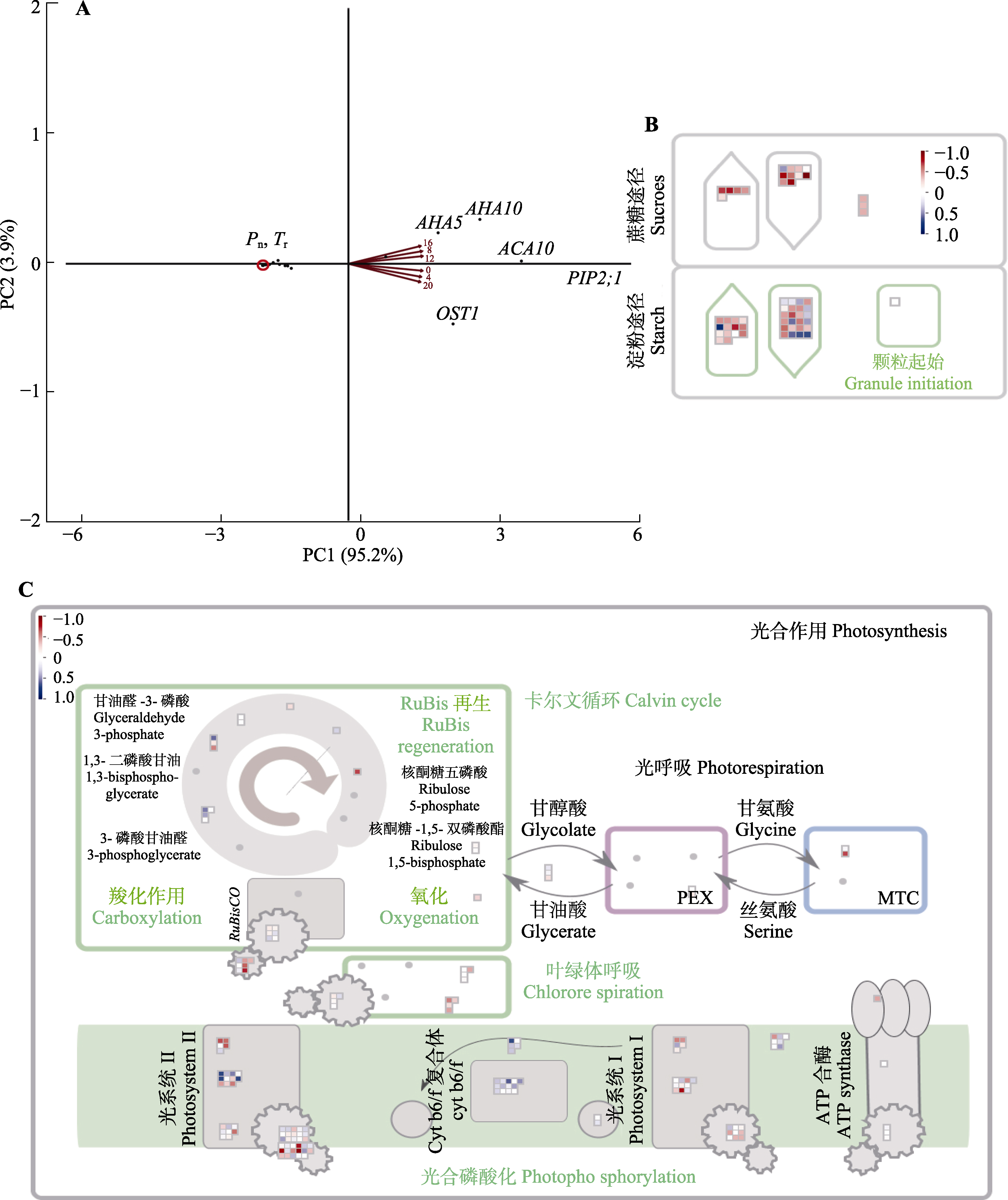
图3 脆枝青藓相关基因的相关性分析。A, 15个显著的气孔相关节律性基因主成分(PC)分析图。B, 57个显著的碳固定相关节律性基因相关性图, 红色表示正相关, 蓝色表示负相关。C, 159个显著的光合相关节律性基因相关性图。热图采用了归一化表达量, 范围为-1到1。蓝色区域代表高表达量, 而红色区域则代表低表达量。ACA10, Ca2 +-ATP酶10; AHA5, 质膜H+-ATP酶; AHA10, H+-ATP酶10; MTC, PSII反应中心相关的膜内捕光复合物; OST1, 气孔开放因子1; PEX, 光系统II (PSII)外围蛋白质; PIP2;1, 水通道蛋白PIP2-1; Pn, 净光合速率; Tr, 蒸腾速率。
Fig. 3 Correlation analysis of genes related to Brachythecium thraustum. Principal Component (PC) analysis of 15 significant stomatal-related rhythmicity genes (A), 57 significant carbon fixation-related rhythmicity genes correlation plot (B) and 159 significant photosynthesis-related rhythmicity genes correlation plot (C) of Brachythecium thraustum. Red color indicates positive correlation, blue color indicates negative correlation. The heatmap used normalized expression levels, ranging from -1 to 1. In this scheme, blue regions represent high expression levels, while red regions represent low expression levels. ACA10, Ca2+-ATPase 10; AHA5, plasma membrane H+-ATPase 5; AHA10, H+-ATPase 10; MTC, membrane-intrinsic light-harvesting complexes associated with PSII reaction centers; OST1, open stomata 1; PEX, photosystem II (PSII) extrinsic proteins; PIP2;1, aquaporin PIP2-1; Pn, photosynthetic rate; Tr, transpiration rate.
| [1] |
Brodribb TJ, McAdam SAM (2017). Evolution of the stomatal regulation of plant water content. Plant Physiology, 174, 639-649.
DOI PMID |
| [2] |
Bruce VG (1972). Mutants of the biological clock in Chlamydomonas reinhardi. Genetics, 70, 537-548.
DOI PMID |
| [3] |
Buchfink B, Xie C, Huson DH (2015). Fast and sensitive protein alignment using DIAMOND. Nature Methods, 12, 59-60.
DOI PMID |
| [4] |
Byrne TE, Wells MR, Johnson CH (1992). Circadian rhythms of chemotaxis to ammonium and of methylammonium uptake in chlamydomonas. Plant Physiology, 98, 879-886.
DOI PMID |
| [5] |
Chaves MM, Flexas J, Pinheiro C (2008). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551-560.
DOI URL |
| [6] |
Chen ZH, Chen G, Dai F, Wang YZ, Hills A, Ruan YL, Zhang GP, Franks PJ, Nevo E, Blatt MR (2017). Molecular evolution of grass stomata. Trends in Plant Science, 22, 124-139.
DOI URL |
| [7] |
Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004). A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. The Plant Journal, 37, 853-863.
DOI URL |
| [8] |
Dodd AN, Kusakina J, Hall A, Gould PD, Hanaoka M (2014). The circadian regulation of photosynthesis. Photosynthesis Research, 119, 181-190.
DOI PMID |
| [9] |
Emms DM, Kelly S (2019). OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biology, 20, 238. DOI: 10.1186/s13059-019-1832-y.
DOI PMID |
| [10] |
Endo M (2016). Tissue-specific circadian clocks in plants. Current Opinion in Plant Biology, 29, 44-49.
DOI PMID |
| [11] |
Ferrari C, Proost S, Janowski M, Becker J, Nikoloski Z, Bhattacharya D, Price D, Tohge T, Bar-Even A, Fernie A, Stitt M, Mutwil M (2019). Kingdom-wide comparison reveals the evolution of diurnal gene expression in Archaeplastida. Nature Communications, 10, 737. DOI: 10.1038/s41467-019-08703-2.
DOI PMID |
| [12] |
Fogelmark K, Troein C (2014). Rethinking transcriptional activation in the Arabidopsis circadian clock. PLoS Computational Biology, 10, e1003705. DOI: 10.1371/journal.pcbi.1003705.
DOI URL |
| [13] | Glime JM (2007). Chapter 2—Life cycles and morphology// Glime JM. Bryophyte Ecology Volume 1: Physiological Ecology. [2022-04-18]. https://digitalcommons.mtu.edu/bryophyte-ecology1/1. |
| [14] |
Goto K, Johnson CH (1995). Is the cell division cycle gated by a circadian clock? The case of Chlamydomonas reinhardtii. Journal of Cell Biology, 129, 1061-1069.
DOI PMID |
| [15] |
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng QD, Chen ZH, Mauceli E, Hacohen N, Gnirke A, Rhind N, et al. (2011). Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology, 29, 644-652.
DOI PMID |
| [16] |
Greenham K, McClung CR (2015). Integrating circadian dynamics with physiological processes in plants. Nature Reviews Genetics, 16, 598-610.
DOI PMID |
| [17] |
Hauser F, Waadt R, Schroeder JI (2011). Evolution of abscisic acid synthesis and signaling mechanisms. Current Biology, 21, R346-R355.
DOI URL |
| [18] |
Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AAR (2013). Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature, 502, 689-692.
DOI |
| [19] | Jones MA (2017). Interplay of circadian rhythms and light in the regulation of photosynthesis-derived metabolism. Progress in Botany, 79, 147-171. |
| [20] |
Kim WY, Fujiwara S, Suh SS, Kim J, Kim Y, Han LQ, David K, Putterill J, Nam HG, Somers DE (2007). ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature, 449, 356-360.
DOI |
| [21] |
Li B, Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12, 323. DOI: 10.1186/1471-2105-12-323.
DOI PMID |
| [22] |
Linde AM, Eklund DM, Kubota A, Pederson ERA, Holm K, Gyllenstrand N, Nishihama R, Cronberg N, Muranaka T, Oyama T, Kohchi T, Lagercrantz U (2017). Early evolution of the land plant circadian clock. New Phytologist, 216, 576-590.
DOI URL |
| [23] |
Más P, Kim WY, Somers DE, Kay SA (2003). Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature, 426, 567-570.
DOI |
| [24] |
Moulager M, Monnier A, Jesson B, Bouvet R, Mosser J, Schwartz C, Garnier L, Corellou F, Bouget FY (2007). Light-dependent regulation of cell division in Ostreococcus: evidence for a major transcriptional input. Plant Physiology, 144, 1360-1369.
DOI PMID |
| [25] |
Noordally ZB, Ishii K, Atkins KA, Wetherill SJ, Kusakina J, Walton EJ, Kato M, Azuma M, Tanaka K, Hanaoka M, Dodd AN (2013). Circadian control of chloroplast transcription by a nuclear-encoded timing signal. Science, 339, 1316-1319.
DOI PMID |
| [26] |
Okada R, Kondo S, Satbhai SB, Yamaguchi N, Tsukuda M, Aoki S (2009). Functional characterization of CCA1/LHY homolog genes, PpCCA1a and PpCCA1b, in the moss Physcomitrella patens. The Plant Journal, 60, 551-563.
DOI PMID |
| [27] |
Pilgrim ML, McClung CR (1993). Differential involvement of the circadian clock in the expression of genes required for ribulose-1,5-bisphosphate carboxylase/oxygenase synthesis, assembly, and activation in Arabidopsis thaliana. Plant Physiology, 103, 553-564.
PMID |
| [28] |
Pokhilko A, Fernández AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ (2012). The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Molecular Systems Biology, 8, 574. DOI: 10.1038/msb.2012.6.
DOI URL |
| [29] |
Resco de Dios V, Arthur G, Pedro FJ, Alday JG, Michael B, Jorge DC, Sébastien D, Sonia GM, Zachary K, Damien L, Paula MG, Alexandru M, Clément P, Karin PW, Olivier R, et al. (2016). Circadian rhythms have significant effects on leaf-to-canopy scale gas exchange under field conditions. GigaScience, 5, 43. DOI: 10.1186/s13742-016-0149-y.
DOI PMID |
| [30] |
Resco de Dios V, Gessler A (2018). Circadian regulation of photosynthesis and transpiration from genes to ecosystems. Environmental and Experimental Botany, 152, 37-48.
DOI URL |
| [31] |
Resco de Dios V, Turnbull MH, Barbour MM, Ontedhu J, Ghannoum O, Tissue DT (2013). Soil phosphorous and endogenous rhythms exert a larger impact than CO2 or temperature on nocturnal stomatal conductance in Eucalyptus tereticornis. Tree Physiology, 33, 1206-1215.
DOI URL |
| [32] |
Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011). Land plants acquired active stomatal control early in their evolutionary history. Current Biology, 21, 1030-1035.
DOI PMID |
| [33] |
Schwacke R, Ponce-Soto GY, Krause K, Bolger AM, Arsova B, Hallab A, Gruden K, Stitt M, Bolger ME, Usadel B (2019). MapMan4: a refined protein classification and annotation framework applicable to multi-omics data analysis. Molecular Plant, 12, 879-892.
DOI PMID |
| [1] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [2] | 王嘉仪, 王襄平, 徐程扬, 夏新莉, 谢宗强, 冯飞, 樊大勇. 北京市行道树绒毛梣的水力结构对城市不透水表面比例的响应[J]. 植物生态学报, 2023, 47(7): 998-1009. |
| [3] | 白雪, 李玉靖, 景秀清, 赵晓东, 畅莎莎, 荆韬羽, 刘晋汝, 赵鹏宇. 谷子及其根际土壤微生物群落对铬胁迫的响应机制[J]. 植物生态学报, 2023, 47(3): 418-433. |
| [4] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [5] | 马艳泽, 杨熙来, 徐彦森, 冯兆忠. 四种常见树木叶片光合模型关键参数对臭氧浓度升高的响应[J]. 植物生态学报, 2022, 46(3): 321-329. |
| [6] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [7] | 罗丹丹, 王传宽, 金鹰. 木本植物水力系统对干旱胁迫的响应机制[J]. 植物生态学报, 2021, 45(9): 925-941. |
| [8] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [9] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [10] | 叶子飘, 于冯, 安婷, 王复标, 康华靖. 植物气孔导度对CO2响应模型的构建[J]. 植物生态学报, 2021, 45(4): 420-428. |
| [11] | 杨克彤, 常海龙, 陈国鹏, 俞筱押, 鲜骏仁. 兰州市主要绿化植物气孔性状特征[J]. 植物生态学报, 2021, 45(2): 187-196. |
| [12] | 李唐吉, 王懋林, 曹颖, 徐刚, 杨琪祺, 任思源, 胡尚连. 竹笋期竹箨和笋体的日间蒸腾特性及其对水分运输的影响[J]. 植物生态学报, 2021, 45(12): 1365-1379. |
| [13] | 陈胜楠, 陈左司南, 张志强. 北京山区油松和元宝槭冠层气孔导度特征及其环境响应[J]. 植物生态学报, 2021, 45(12): 1329-1340. |
| [14] | 李景, 王欣, 王振华, 王斌, 王成章, 邓美凤, 刘玲莉. 臭氧和气溶胶复合污染对杨树叶片光合作用的影响[J]. 植物生态学报, 2020, 44(8): 854-863. |
| [15] | 李旭, 吴婷, 程严, 谭钠丹, 蒋芬, 刘世忠, 褚国伟, 孟泽, 刘菊秀. 南亚热带常绿阔叶林4个树种对增温的生理生态适应能力比较[J]. 植物生态学报, 2020, 44(12): 1203-1214. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19