

植物生态学报 ›› 2012, Vol. 36 ›› Issue (7): 662-670.DOI: 10.3724/SP.J.1258.2012.00662
发布日期:2012-07-10
通讯作者:
张旺锋
作者简介:*E-mail: zhwf_agr@shzu.edu.cn; zwf_shzu@163.com
LI Wei, ZHANG Ya-Li, HU Yuan-Yuan, YANG Mei-Sen, WU Jie, ZHANG Wang-Feng*( )
)
Published:2012-07-10
Contact:
ZHANG Wang-Feng
摘要:
通过比较棉花(Gossypium hirsutum)幼叶和完全展开叶气体交换参数及叶绿素荧光特性的差异, 探讨高光强下幼叶的光抑制程度及明确光保护机制间的协调机理。在田间自然条件下, 以棉花刚展平的幼嫩叶片(幼叶)和面积已达到最大的完全展开叶片为研究对象, 通过测定不同发育阶段叶片气体交换参数及叶绿素a荧光参数的变化, 并运用Dual-PAM100对不同发育阶段的叶片进行快速光响应曲线的拟合。结果表明: 幼叶和完全展开叶片在光合、荧光特性方面表现出明显的差异。与完全展开叶相比, 较低的叶绿素(Chl)含量和气孔导度(Gs)是幼叶较低净光合速率(Pn)的限制因素, 从而直接导致其光系统II (PSII)实际光化学效率(ΦPSII)和光化学猝灭系数(qP)的降低。在1800 μmol·m-2·s-1光强以下, 完全展开叶具有较强的围绕PSI循环的电子流(CEF), 有利于合成ATP, 是其具有较高光合能力的原因之一。相同光强下, 幼叶较低的光饱和点(LSP)更易受光抑制, 但其PSII原初光化学效率(Fv/Fm)的日变化幅度显著小于完全展开叶, 说明强光下幼叶通过类胡萝卜素(Car)猝灭单线态氧、光呼吸(Pr)、热耗散(NPQ)以及PSI-CEF等光保护机制能有效地耗散过剩的光能, 从而避免其光合机构发生光抑制。
李维, 张亚黎, 胡渊渊, 杨美森, 吴洁, 张旺锋. 田间条件下棉花幼叶光合特性及光保护机制. 植物生态学报, 2012, 36(7): 662-670. DOI: 10.3724/SP.J.1258.2012.00662
LI Wei, ZHANG Ya-Li, HU Yuan-Yuan, YANG Mei-Sen, WU Jie, ZHANG Wang-Feng. Research on the photoprotection and photosynthesis characteristics of young cotton leaves under field conditions. Chinese Journal of Plant Ecology, 2012, 36(7): 662-670. DOI: 10.3724/SP.J.1258.2012.00662
| 叶型 Leaf type | 叶绿素 Chl a + b (mg·dm-2) | 类胡萝卜素 Car (mg·dm-2) | Car/Chl a + b | 气孔导度 Gs (mol H2O·m-2·s-1) | 胞间CO2浓度 Ci (μmol·mol-1) | 净光合速率 Pn (μmol CO2·m-2·s-1) |
|---|---|---|---|---|---|---|
| YL | 2.53 ± 0.07b | 0.41 ± 0.02b | 0.20 ± 0.01a | 0.45 ± 0.01b | 283.13 ± 0.23a | 21.09 ± 0.058 3b |
| ML | 4.3 ± 0.13a | 0.51 ± 0.05a | 0.15 + 0.01b | 0.64 ± 0.08a | 235.82 ± 1.17b | 37.61 ± 0.027 1a |
表1 棉花幼叶和完全展开叶光合色素含量、气体交换参数的变化(平均值±标准偏差)
Table 1 Changes of chlorophyll content and gas exchange parameter in young and mature leaves of cotton (mean ± SD)
| 叶型 Leaf type | 叶绿素 Chl a + b (mg·dm-2) | 类胡萝卜素 Car (mg·dm-2) | Car/Chl a + b | 气孔导度 Gs (mol H2O·m-2·s-1) | 胞间CO2浓度 Ci (μmol·mol-1) | 净光合速率 Pn (μmol CO2·m-2·s-1) |
|---|---|---|---|---|---|---|
| YL | 2.53 ± 0.07b | 0.41 ± 0.02b | 0.20 ± 0.01a | 0.45 ± 0.01b | 283.13 ± 0.23a | 21.09 ± 0.058 3b |
| ML | 4.3 ± 0.13a | 0.51 ± 0.05a | 0.15 + 0.01b | 0.64 ± 0.08a | 235.82 ± 1.17b | 37.61 ± 0.027 1a |
| 叶型 Leaf type | 最大光化学效率 Fv/Fm | 有效光化学效率 ΦPSII | 光化学猝灭系数 qP | 非光化学猝灭系数 NPQ |
|---|---|---|---|---|
| YL | 0.812 ± 0.01b | 0.30 ± 0.02b | 0.52 ± 0.04b | 2.84 ± 0.03a |
| ML | 0.840 ± 0.05a | 0.35 ± 0.003a | 0.57 ± 0.01a | 2.15 ± 0.01b |
表2 棉花幼叶和完全展开叶中叶绿素荧光参数的比较(平均值±标准偏差) (PAR = 1957 μmol·m-2·s-1)
Table 2 Comparison of chlorophyll fluorescence parameters in young and mature leaves of cotton (mean ± SD) (PAR = 1957 μmol·m-2·s-1)
| 叶型 Leaf type | 最大光化学效率 Fv/Fm | 有效光化学效率 ΦPSII | 光化学猝灭系数 qP | 非光化学猝灭系数 NPQ |
|---|---|---|---|---|
| YL | 0.812 ± 0.01b | 0.30 ± 0.02b | 0.52 ± 0.04b | 2.84 ± 0.03a |
| ML | 0.840 ± 0.05a | 0.35 ± 0.003a | 0.57 ± 0.01a | 2.15 ± 0.01b |
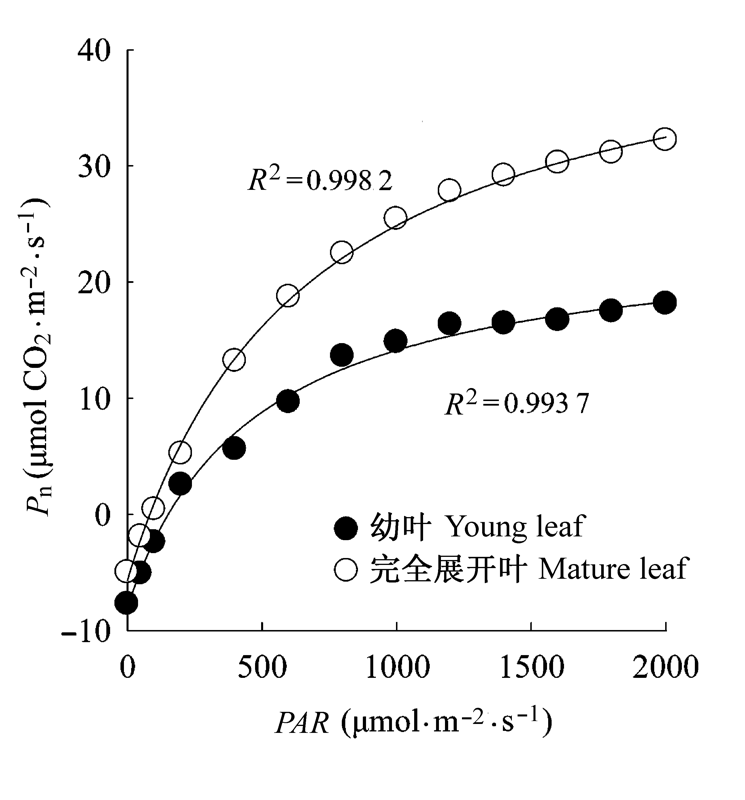
图1 棉花幼叶与完全展开叶的光合-光响应曲线(平均值±标准偏差)。PAR, 光合有效辐射; Pn, 净光合速率.
Fig. 1 Light response curves in young and mature leaves of cotton (mean ± SD). PAR, photosynthetic active radiation; Pn, net photosynthetic rate.
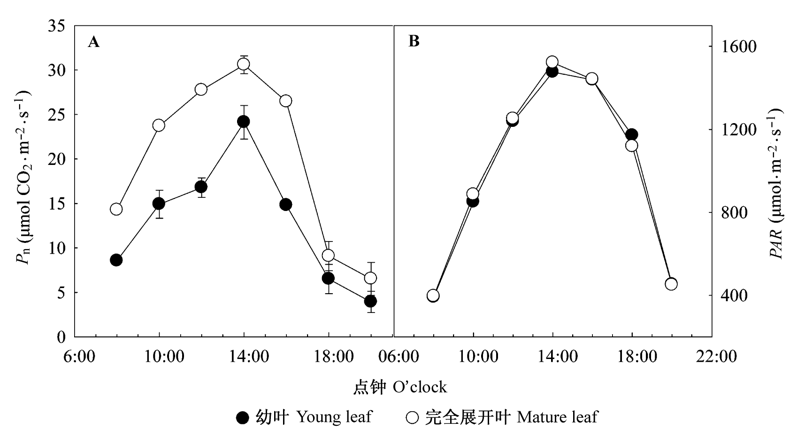
图2 棉花幼叶和完全展开叶Pn和PAR的日变化(平均值±标准偏差)。PAR, 光合有效辐射; Pn, 净光合速率。
Fig. 2 Diurnal variations of Pn and PAR in young and mature leaves of cotton (mean ± SD). PAR, photosynthetic active radiation; Pn, net photosynthetic rate.
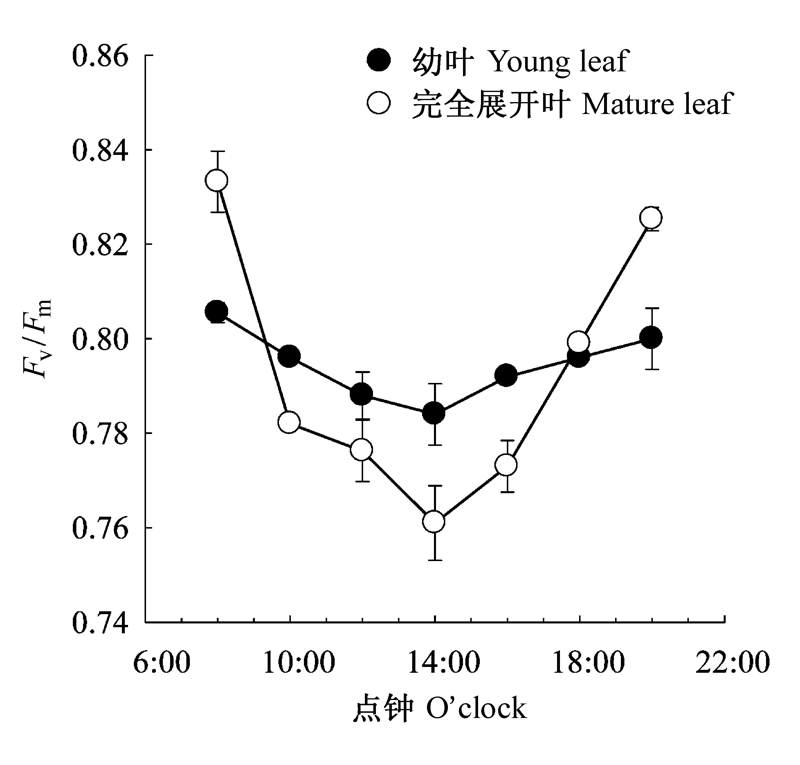
图3 棉花幼叶和完全展开叶PSII最大光化学效率(Fv/Fm)的日变化(平均值±标准偏差)。
Fig. 3 Diurnal changes of maximum photochemical efficiency of PSII (Fv/Fm) in young and mature leaves of cotton (mean ± SD).
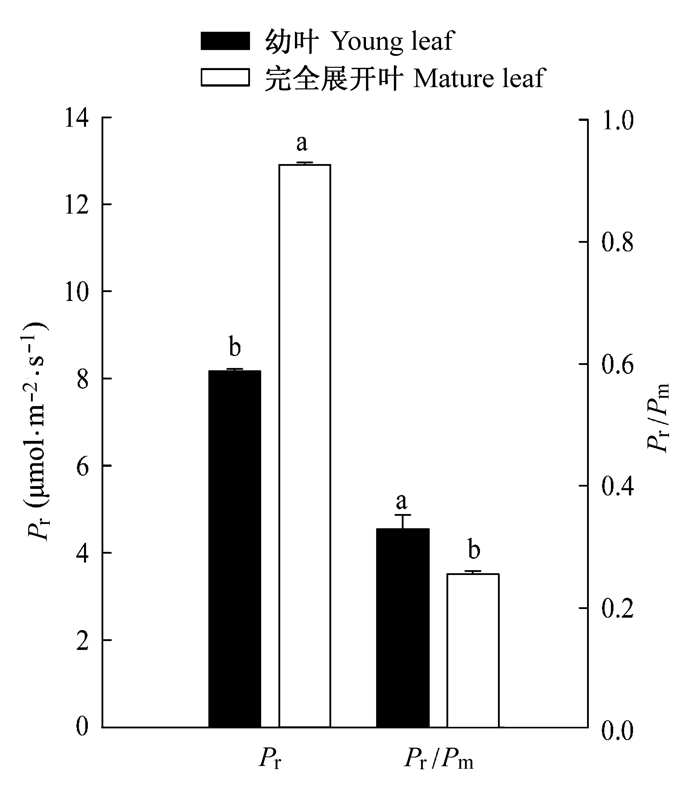
图4 1800 μmol·m-2·s-1光照下棉花幼叶和完全展开叶光呼吸(Pr)、光呼吸与总光合速率比例(Pr/Pm)的变化(平均值±标准偏差)。
Fig. 4 Variation of photorespiration (Pr) and ratio of photo- respiration/mass photosynthesis (Pr/Pm) in young and mature leaves of cotton under 1800 μmol·m-2·s-1 irradiance (mean ± SD).
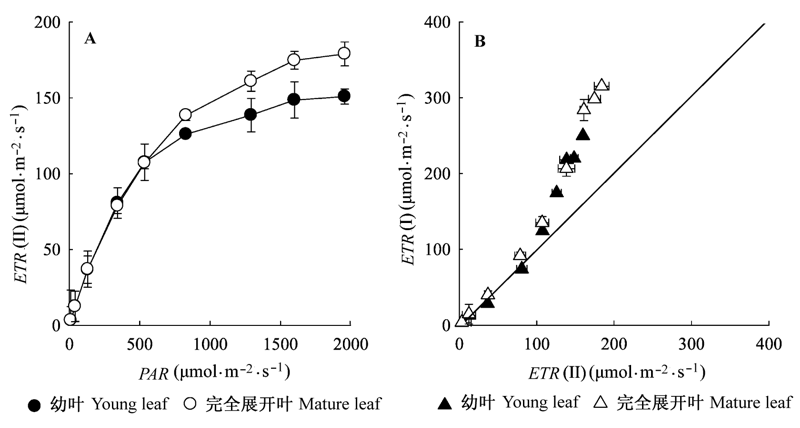
图5 棉花幼叶和完全展开叶PSI和PSII中ETRs快速光响应曲线(平均值±标准偏差)。ETR(I)、ETR(II), 光系统I、光系统II电子传递速率; PAR, 光合有效辐射。
Fig. 5 Rapid light response curves of the ETRs around PSI and PSII in young and mature leaves of cotton (mean ± SD). ETR(I), ETR(II), apparent electron transport rate at PSI and PSII. PAR, photosynthetic active radiation.
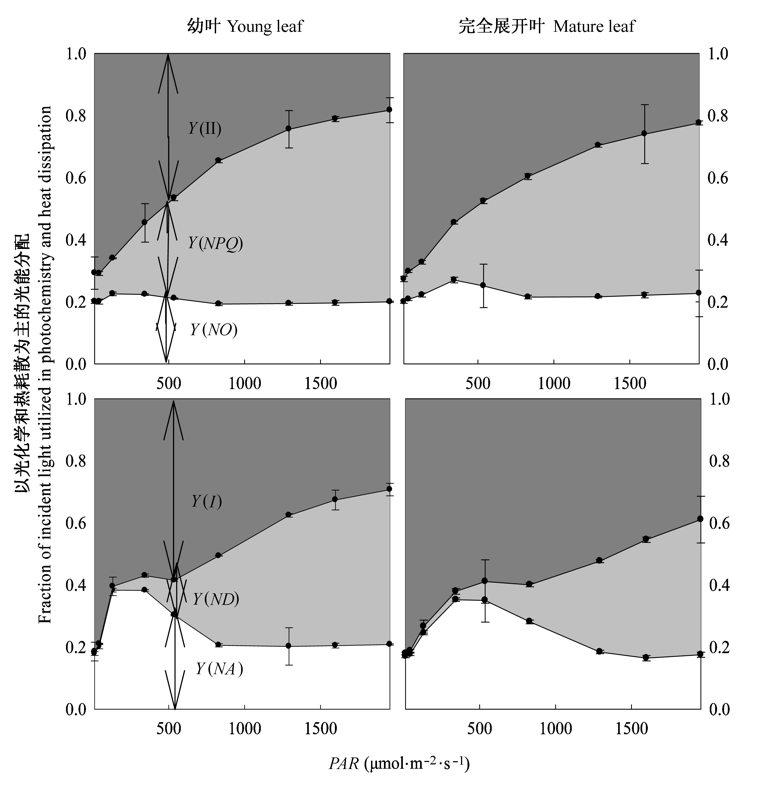
图6 棉花幼叶和完全展开叶中PSII和PSI量子产量随光合有效辐射(PAR)升高的转化(平均值±标准偏差)。Y(I), 光系统I光化学量子产量; Y(II), 光系统II光化学量子产量; Y(ND), PSI中依赖于非光化学能力耗散的供体侧限制; Y(NA), PSI中依赖于非光化学能力耗散的受体侧限制; Y(NO), PSII中荧光和不依赖光的基础热耗散量子产量; Y(NPQ), PSII中ΔpH和叶黄素调节的热耗散量子产量。
Fig. 6 Conversion of quantum yields in PSII and PSI in young and mature leaves of cotton with increasing photosynthetic active radiation (PAR) (mean ± SD). Y(I), photochemical quantum yields in PSI; Y(II), photochemical quantum yields in PSII; Y(ND), quantum yield of non-photochemical energy dissipation in PSI due to donor side limitation; Y(NA), quantum yield of non-photochemical energy dissipation in PSI due to acceptor side limitation; Y(NO), quantum yield of fluorescence and light-independent constitution thermal dissipation; Y(NPQ), quantum yield of ΔpH-and xanthophyll-regulated thermal dissipation.
| [1] |
Allakhverdiev SI, Nishiyama Y, Takahashi S, Miyairi S, Suzuki I, Murata N (2005). Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in synechocystis. Plant Physiology, 137, 263-273.
DOI URL PMID |
| [2] |
Aro EM, Virgin I, Andersson B (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochimica et Biophysica Acta, 1143, 113-134.
URL PMID |
| [3] |
Bartley GE, Scolnik PA (1995). Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell, 7, 1027-1038.
DOI URL PMID |
| [4] | Bendall DS, Manasse RS (1995). Cyclic photophosphorylation and electron transport. Biochimica et Biophysica Acta, 1229, 23-38. |
| [5] |
Bertamini M, Nedunchezhian N (2003). Photoinhibition of photosynthesis in mature and young leaves of grapevine ( Vitis vinifera L.). Plant Science, 164, 635-644.
DOI URL |
| [6] |
Bilger W, Björkman O (1990). Role of the xanthophyll cycle in photoprotection elucidated by measurements of light- induced absorbance changes, fluorescence and photosyn- thesis in leaves of Hedera canariensis. Photosynthesis Research, 25, 173-185.
DOI URL PMID |
| [7] |
Bulley NR, Nelson CD, Tregunna EB (1969). Photosynthesis: action spectra for leaves in normal and low oxygen. Plant Physiology, 44, 678-684.
URL PMID |
| [8] | Cai ZQ (蔡志全), Cao KF (曹坤芳), Qi X (齐欣) (2003). Photo- inhibition of photosynthesis in leaves of two developing stages of a tropical rain forest canopy tree, Pometia tomentoscs. Acta Phytoecologica Sinica (植物生态学报), 27, 210217. (in Chinese with English abstract) |
| [9] |
Cleland RE, Melis A, Neale PJ (1986). Mechanism of photoinhibition: photochemical reaction center inactivation in system II of chloroplasts. Photosynthesis Research, 9, 79-88.
URL PMID |
| [10] |
Cornic G, Briantais JM (1991). Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf ( Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta, 183, 178-184.
DOI URL PMID |
| [11] | Cornic G, Fresneau C (2002). Photosynthetic carbon reduction and carbon oxidation cycles are the main electron sinks for photosystem II activity during a mild drought. Annals of Botany, 89, 887-894. |
| [12] | Critchley C, Russell AW (1994). Photoinhibition of photosyn- thesis in vivo: the role of protein turnover in photosystem II. Physiologia Plantarum, 92, 188-196. |
| [13] | Da Matta FM, Maestri M, Barros RS (1997). Photosynthetic performance of two coffee species under drought. Photosynthetica, 34, 257-264. |
| [14] | Demmig-Adams B, Adams WW III (1992). Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology, 43, 599-626. |
| [15] | Demming-Adams B, Adams WW III (1996). Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species. Planta, 198, 460-470. |
| [16] | Dong GF (董高峰), Chen YZ (陈贻竹), Jiang YM (蒋跃明) (1999). Plant xanthophyll cycle and radiationless energy dissipation. Plant Physiology Communications (植物生理学通讯), 35, 141-144. (in Chinese with English abstract) |
| [17] | Drumm-Herrel H, Mohr H (1985). Photosensitivity of seedlings differing in their potential to synthesize anthocyanin. Physiologia Plantarum, 64, 60-66. |
| [18] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [19] | Gilmore AM, Björkman O (1994). Adenine nucleotides and the xanthophyll cycle in leaves. Planta, 192, 526-536. |
| [20] | Gilmore AM, Yamamoto HY (1993). Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosyn- thesis Research, 35, 67-78. |
| [21] |
Heber U, Walker D (1992). Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiology, 100, 1621-1626.
DOI URL PMID |
| [22] |
Hendrickson L, Furbank RT, Chow WS (2004). A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynthesis Research, 82, 73-81.
DOI URL PMID |
| [23] |
Huang W, Zhang SB, Cao KF (2010). Stimulation of cyclic electron flow during recovery after chilling-induced photoinhibition of photosystem II. Plant and Cell Physiology, 51, 1922-1928.
DOI URL PMID |
| [24] |
Jahns P, Krause GH (1994). Xanthophyll cycle and energy- dependent fluorescence quenching in leaves from pea plants grown under intermittent light. Planta, 192, 176-182.
DOI URL |
| [25] | Jiang CD (姜闯道), Gao HY (高辉远), Zou Q (邹琦), Jiang GM (蒋高明), Li LH (李凌浩) (2005). The co-operation of leaf orientation, photorespiration and thermal dissipation alleviate photoinhibition in young leaves of soybean plants. Acta Ecologica Sinica (生态学报), 25, 319-325. (in Chinese with English abstract) |
| [26] |
Kim SJ, Lee CH, Hope AB, Chow WS (2001). Inhibition of photosystems I and II and enhanced back flow of photosystem I electrons in cucumber leaf discs chilled in the light. Plant and Cell Physiology, 42, 842-848.
DOI URL PMID |
| [27] | Klughammer C, Schreiber U (1994). An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700 +-absorbance changes at 830 nm . Planta, 192, 261-268. |
| [28] | Kositsup B, Kasemsap P, Thanisawanyangkura S, Chairungsee N, Satakhun D, Teerawatanasuk K, Ameglio T, Thaler P (2010). Effect of leaf age and position on light-saturated CO2 assimilation rate, photosynthetic capacity, and stomatal conductance in rubber trees. Photosynthetica, 48, 67-78. |
| [29] |
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004). New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynthesis Research, 79, 209-218.
DOI URL PMID |
| [30] | Krause GH, Virgo A, Winter K (1995). High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta, 197, 583-591. |
| [31] | Krause GH, Weis E (1991). Chlorophyll fluorescence and photosynthesis: the basics. Annual Review of Plant Physiology and Plant Molecular Biology, 42, 313-349. |
| [32] | Lepeduš H, Jurković V, Štolfa I, Ćurković-Perica M, Fulgosi H, Cesar V (2010). Changes in photosystem II photo- chemistry in senescing maple leaves. Croatica Chemica Acta, 83, 379-386. |
| [33] | Liu ZM, Radin JW, Turcotte EL, Percy R, Zeiger E (1994). High yields in advanced lines of pima cotton are associated with higher stomatal conductance, reduced leaf area and lower leaf temperature. Physiologia Plantarum, 92, 266-272. |
| [34] | Marchi S, Tognetti R, Minnocci A, Borghi M, Sebastiani L (2008). Variation in mesophyll anatomy and photosyn- thetic capacity during leaf development in a deciduous mesophyte fruit tree (Prunus persica) and an evergreen sclerophyllous Mediterranean shrub (Olea europaea). Trees, 22, 559-571. |
| [35] | Meng QW (孟庆伟), Zhao SJ (赵世杰), Xu CC (许长成) (1996). Photoinhibition of photosynthesis and protective effect of photorespiration in winter wheat leaves under field conditions. Acta Agronomica Sinica (作物学报), 22, 470-475. (in Chinese with English abstract) |
| [36] |
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005). CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves-relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching ( NPQ) of Chl fluorescence. Plant Cell Physiology, 46, 629-637.
DOI URL PMID |
| [37] |
Miyake C, Shinzaki Y, Miyata M, Tomizawa K (2004). Enhancement of cyclic electron flow around PSI at high light and its contribution to the induction of non-photochemical quenching of Chl fluorescence in intact leaves of tobacco plants. Plant and Cell Physiology, 45, 1426-1433.
URL PMID |
| [38] | Niyogi KK, Grossman AR, Björkman O (1998). Arabidopsis mutants define a central role for the xanthopylls cycle in the regulation of photosynthetic energy conversion. Plant Cell, 10, 1121-1134. |
| [39] |
Nobel PS, Zaragoza LJ, Smith WK (1975). Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiology, 55, 1067-1070.
DOI URL PMID |
| [40] | Oxborough K, Baker NR (1997). Resolving chlorophyll a fluo- rescence images of photosynthetic efficiency into photo- chemical and non-photochemical components―calcula- tion of qP and Fv/Fm′; without measuring Fo′. Photosynthesis Research, 54, 135-142. |
| [41] | Schreiber U ( 2004). Pulse-amplitude modulation (PAM) fluorom ETRy and saturation pulse method. In: Papageorgiou G, Govindjee eds. Chlorophyll Fluorescence: a Signature of Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands. 279-319. |
| [42] | Schreiber U, Bilger W, Neubauer C ( 1994). Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM eds. Ecophysiology of Photosynthesis. Springer-Verlag, Berlin. |
| [43] |
Vos J, Oyarzún PJ (1987). Photosynthesis and stomatal conductance of potato leaves―effects of leaf age, irradiance, and leaf water potential. Photosynthesis Research, 11, 253-264.
DOI URL PMID |
| [44] | Wang KB (王可玢), Xu CH (许春辉), Zhao FH (赵福洪), Tang CQ (唐崇钦), Dai YL (戴云玲) (1997). The effects of water stress on some in vivo chlorophyll a fluorescence parameters of wheat flag leaves. Acta Biophysica Sinica (生物物理学报), 13, 273-278. (in Chinese with English abstract) |
| [45] |
Warren CR (2006). Estimation the internal conductance to CO2 movement. Functional Plant Biology, 33, 431-442.
DOI URL PMID |
| [46] | Wullschleger SD, Oosterhuis DM (1990). Photosynthetic carbon production and use by developing cotton leaves and bolls. Crop Science, 30, 1259-1264. |
| [47] | Yamamoto HY, Bugos RC, Hieber AD (1999). Biochemistry and molecular biology of the xanthophyll cycle. Biomedical and Life Sciences, 8, 293-303. |
| [48] | Yang CW (阳成伟), Chen YZ (陈贻竹) (2000). The mechanism of photoprotection during light energy transformation associated with metabolism of dissipating energy in photosynthesis. Journal of Tropical and Subtropical Botany (热带亚热带植物学报), 8, 346-352. (in Chinese with English abstract) |
| [49] | Zhang QD (张其德), Lu CM (卢从明), Kuang TY (匡廷云) (1992). Effects of the rising CO2 levels on photosynthesis. Chinese Bulletin Botany (植物学通报), 9(4), 18-23. (in Chinese with English abstract) |
| [50] | Zhang YL (张亚黎), Luo HH (罗宏海), Fan DY (樊大勇), He ZJ (何在菊), Bai HD (白慧东), Zhang WF (张旺锋) (2008). Effects of water deficit on photochemical activity and excitation energy dissipation of photosynthetic apparatus in cotton leaves during flowering and boll-setting stages. Journal of Plant Ecology (Chinese Version) ( 植物生态学报), 32, 681-689. (in Chinese with English abstract) |
| [51] | Zhang YL, Zhang HZ, Feng GY, Tian JS, Zhang WF (2009). Leaf diaheliotropic movement can improve carbon gain and water use efficiency and not intensify photoinhibition in upland cotton ( Gossypium hirsutum L.). Photosynthetica, 47, 609-615. |
| [1] | 杜英东, 袁相洋, 冯兆忠. 不同形态氮对杨树光合特性及生长的影响[J]. 植物生态学报, 2023, 47(3): 348-360. |
| [2] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [3] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [4] | 冯旭飞, 雷长英, 张玉洁, 向导, 杨明凤, 张旺锋, 张亚黎. 棉花花铃期叶片氮分配对光合氮利用效率的影响[J]. 植物生态学报, 2023, 47(11): 1600-1610. |
| [5] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [6] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| [7] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [8] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [9] | 周稳, 迟永刚, 周蕾. 基于日光诱导叶绿素荧光的北半球森林物候研究[J]. 植物生态学报, 2021, 45(4): 345-354. |
| [10] | 丁键浠, 周蕾, 王永琳, 庄杰, 陈集景, 周稳, 赵宁, 宋珺, 迟永刚. 叶绿素荧光主动与被动联合观测应用前景[J]. 植物生态学报, 2021, 45(2): 105-118. |
| [11] | 郭庆华, 胡天宇, 马勤, 徐可心, 杨秋丽, 孙千惠, 李玉美, 苏艳军. 新一代遥感技术助力生态系统生态学研究[J]. 植物生态学报, 2020, 44(4): 418-435. |
| [12] | 刘校铭, 杨晓芳, 王璇, 张守仁. 暖温带落叶阔叶林辽东栎和五角枫生长和光合生理生态特征对模拟氮沉降的响应[J]. 植物生态学报, 2019, 43(3): 197-207. |
| [13] | 蔡建国, 韦孟琪, 章毅, 魏云龙. 遮阴对绣球光合特性和叶绿素荧光参数的影响[J]. 植物生态学报, 2017, 41(5): 570-576. |
| [14] | 樊大勇, 付增娟, 谢宗强, 李荣贵, 张淑敏. 调制式荧光影像新技术: 叶片内部最大光化学量子效率及其异质性的活体测定[J]. 植物生态学报, 2016, 40(9): 942-951. |
| [15] | 刘畅, 孙鹏森, 刘世荣. 植物反射光谱对水分生理变化响应的研究进展[J]. 植物生态学报, 2016, 40(1): 80-91. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19