

植物生态学报 ›› 2023, Vol. 47 ›› Issue (11): 1600-1610.DOI: 10.17521/cjpe.2022.0490
所属专题: 光合作用
• 研究论文 • 上一篇
冯旭飞1, 雷长英1, 张玉洁1, 向导2, 杨明凤2, 张旺锋1, 张亚黎1,*( )
)
收稿日期:2022-12-06
接受日期:2023-04-06
出版日期:2023-11-20
发布日期:2023-12-22
通讯作者:
张亚黎(基金资助:
FENG Xu-Fei1, LEI Zhang-Ying1, ZHANG Yu-Jie1, XIANG Dao2, YANG Ming-Feng2, ZHANG Wang-Feng1, ZHANG Ya-Li1,*( )
)
Received:2022-12-06
Accepted:2023-04-06
Online:2023-11-20
Published:2023-12-22
Contact:
ZHANG Ya-Li(Supported by:摘要:
为探讨棉花(Gossypium spp.)叶片净光合速率(Pn)与氮分配的关系, 分析影响光合氮利用效率(PNUE)提高的限制因子, 揭示氮利用效率的光合生理调控机制, 该研究在花铃期测定包括陆地棉(G. hirsutum)、海岛棉(G. barbadense)、树棉(G. arboreum)和草棉(G. herbaceum) 16个基因型棉花的气体交换参数、不同组分氮含量与比例和单位面积细胞壁含量(CWarea)等, 分析Pn、CWarea、PNUE与各组分氮含量之间的关系。结果表明: 当单位面积氮含量(Narea)小于3.75 g·m-2时, Pn与Narea呈极显著正相关关系, 超过此值, 它们之间无相关关系; 而Pn与光合机构中的核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮含量(Nr)和生物力能学氮含量(Nb)呈显著正相关关系, 与捕光系统氮含量(NL)呈边缘性正相关关系, 表明光合机构氮含量(Np)比Narea对光合能力更为重要; 此外, Pn也与气孔导度(Gs)呈显著正相关关系。基因型‘常紫1号’低的Pn主要与低Np有关, 而基因型‘МОС-620’低的Pn主要与低Gs有关。光合机构各组分氮分配比例也与PNUE呈显著或极显著正相关关系; 但光合机构各组分氮分配比例受自身Narea和CWarea影响。相关分析表明, 生物力能学氮分配比例(Pb)和捕光系统氮分配比例(PL)与Narea极显著负相关; Rubisco氮分配比例(Pr)、PL和Pb分别与CWarea显著、极显著和边缘性负相关。表明随着Narea和CWarea增加, 叶片倾向于非光合机构氮分配, 包括储存氮组分和细胞壁组分。此外, 细胞壁可能通过影响叶肉导度(gm)降低Pn和PNUE。增加光合机构氮分配比例, 降低非光合机构的氮分配比例以及增加gm可提升基因型‘常紫1号’和‘МОС-620’的PNUE。
冯旭飞, 雷长英, 张玉洁, 向导, 杨明凤, 张旺锋, 张亚黎. 棉花花铃期叶片氮分配对光合氮利用效率的影响. 植物生态学报, 2023, 47(11): 1600-1610. DOI: 10.17521/cjpe.2022.0490
FENG Xu-Fei, LEI Zhang-Ying, ZHANG Yu-Jie, XIANG Dao, YANG Ming-Feng, ZHANG Wang-Feng, ZHANG Ya-Li. Effect of leaf nitrogen allocation on photosynthetic nitrogen use efficiency at flowering and boll stage of Gossypium spp.. Chinese Journal of Plant Ecology, 2023, 47(11): 1600-1610. DOI: 10.17521/cjpe.2022.0490
| 序号 Serial number | 种 Species | 基因型 Genotype |
|---|---|---|
| 1 | 陆地棉 G. hirsutum | GP84 |
| 2 | 陆地棉 G. hirsutum | 陆92-3 LU 92-3 |
| 3 | 陆地棉 G. hirsutum | 切尔盘111 chirpan111 |
| 4 | 陆地棉 G. hirsutum | 绉缩叶(vin5) ZHOU SUO YE (vin5) |
| 5 | 海岛棉 G. barbadense | 苏联B9103 SU LIAN B9103 |
| 6 | 海岛棉 G. barbadense | MOC-620 |
| 7 | 海岛棉 G. barbadense | 新海5号 XIN HAI 5 HAO |
| 8 | 海岛棉 G. barbadense | 新海34号 XIN HAI 34 HAO |
| 9 | 海岛棉 G. barbadense | 新海36号 XIN HAI 36 HAO |
| 10 | 树棉 G. arboreum | 常紫1号 CHANG ZI 1 HAO |
| 11 | 树棉 G. arboreum | 江宁紫花毛籽 JIANG NING ZI HUA MAO ZI |
| 12 | 树棉 G. arboreum | 119S |
| 13 | 树棉 G. arboreum | 白密拉黄花黑籽 BAI MI LA HUANG HUA HEI ZI |
| 14 | 树棉 G. arboreum | 小毛籽 XIAO MAO ZI |
| 15 | 草棉 G. herbaceum | 中草1号 ZHONG CAO 1 HAO |
| 16 | 草棉 G. herbaceum | 金塔草棉 JIN TA CAO MIAN |
表1 本研究16种基因型棉花
Table 1 16 genotypes of Gossypium spp. in this study
| 序号 Serial number | 种 Species | 基因型 Genotype |
|---|---|---|
| 1 | 陆地棉 G. hirsutum | GP84 |
| 2 | 陆地棉 G. hirsutum | 陆92-3 LU 92-3 |
| 3 | 陆地棉 G. hirsutum | 切尔盘111 chirpan111 |
| 4 | 陆地棉 G. hirsutum | 绉缩叶(vin5) ZHOU SUO YE (vin5) |
| 5 | 海岛棉 G. barbadense | 苏联B9103 SU LIAN B9103 |
| 6 | 海岛棉 G. barbadense | MOC-620 |
| 7 | 海岛棉 G. barbadense | 新海5号 XIN HAI 5 HAO |
| 8 | 海岛棉 G. barbadense | 新海34号 XIN HAI 34 HAO |
| 9 | 海岛棉 G. barbadense | 新海36号 XIN HAI 36 HAO |
| 10 | 树棉 G. arboreum | 常紫1号 CHANG ZI 1 HAO |
| 11 | 树棉 G. arboreum | 江宁紫花毛籽 JIANG NING ZI HUA MAO ZI |
| 12 | 树棉 G. arboreum | 119S |
| 13 | 树棉 G. arboreum | 白密拉黄花黑籽 BAI MI LA HUANG HUA HEI ZI |
| 14 | 树棉 G. arboreum | 小毛籽 XIAO MAO ZI |
| 15 | 草棉 G. herbaceum | 中草1号 ZHONG CAO 1 HAO |
| 16 | 草棉 G. herbaceum | 金塔草棉 JIN TA CAO MIAN |
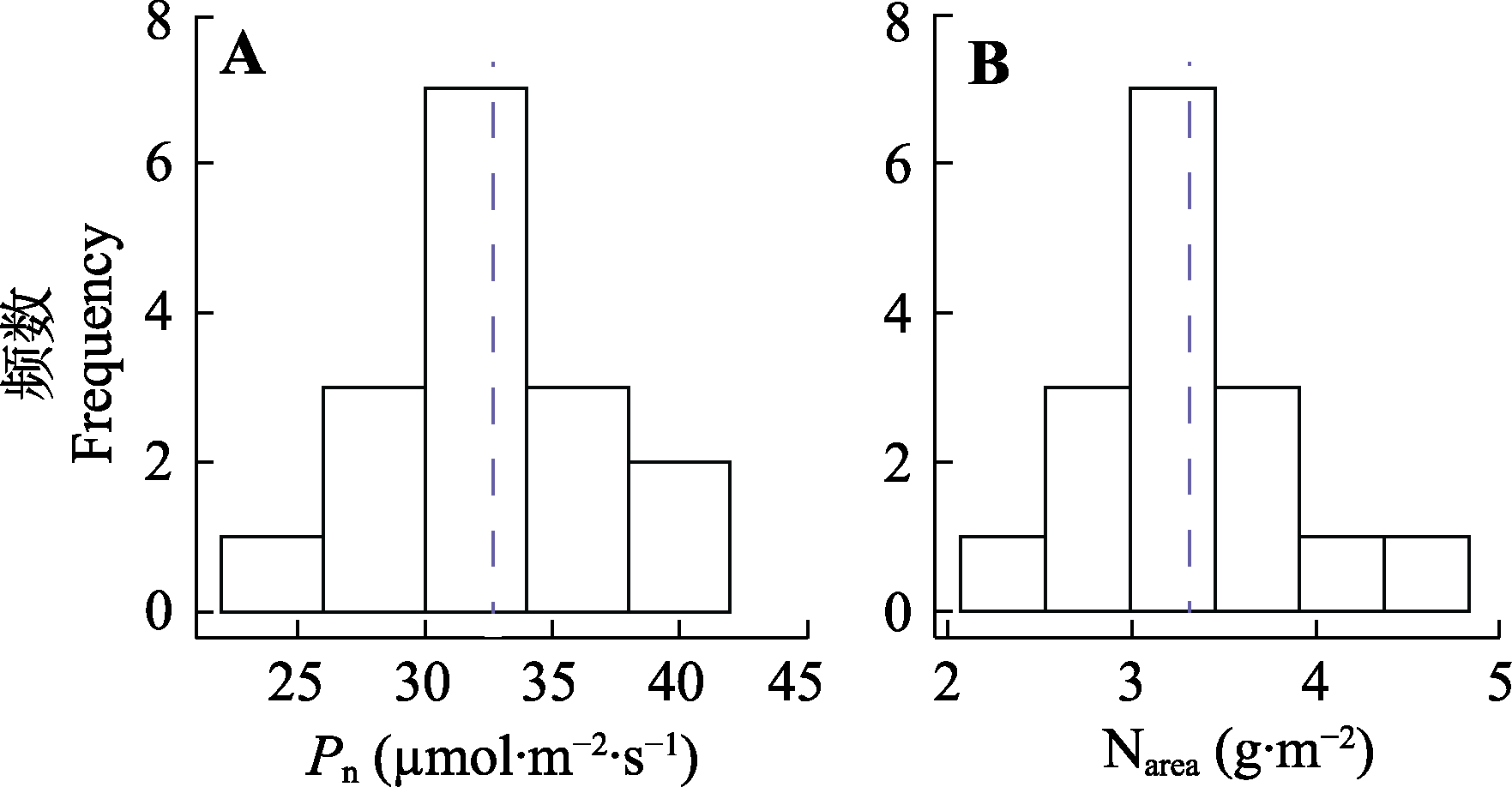
图1 16种基因型棉花净光合速率(Pn)和单位面积氮含量(Narea)的频数分布直方图。
Fig. 1 Histogram of frequency distribution in the net photosynthetic rate (Pn) and nitrogen content per unit leaf area (Narea) of 16 genotypes of Gossypium spp.
| 基因型 Genotype | Pn (μmol·m-2·s-1) | Narea (g·m-2) | Nr (g·m-2) | Nb (g·m-2) | NL (g·m-2) | Np (g·m-2) |
|---|---|---|---|---|---|---|
| GP84 | 35.01 ± 1.280 | 3.679 ± 0.055 | 2.218 ± 0.093 | 0.232 ± 0.016 | 0.224 ± 0.006 | 2.675 ± 0.109 |
| 陆92-3 LU 92-3 | 34.79 ± 1.069 | 3.554 ± 0.111 | 1.529 ± 0.001 | 0.230 ± 0.017 | 0.214 ± 0.008 | 1.973 ± 0.007 |
| 切尔盘111 chirpan111 | 31.42 ± 0.857 | 2.988 ± 0.061 | 1.138 ± 0.033 | 0.157 ± 0.001 | 0.206 ± 0.002 | 1.500 ± 0.048 |
| 绉缩叶(vin5) ZHOU SUO YE (vin5) | 39.50 ± 0.863 | 3.720 ± 0.042 | 1.535 ± 0.045 | 0.218 ± 0.005 | 0.243 ± 0.003 | 1.996 ± 0.090 |
| 苏联B9103 SU LIAN B9103 | 34.67 ± 0.960 | 3.320 ± 0.004 | 1.584 ± 0.065 | 0.202 ± 0.008 | 0.216 ± 0.001 | 2.002 ± 0.061 |
| MOC-620 | 30.65 ± 0.782 | 4.718 ± 0.107 | 1.816 ± 0.058 | 0.191 ± 0.005 | 0.240 ± 0.004 | 2.247 ± 0.065 |
| 新海5号 XIN HAI 5 HAO | 33.55 ± 0.670 | 3.336 ± 0.031 | 1.439 ± 0.028 | 0.188 ± 0.004 | 0.219 ± 0.005 | 1.846 ± 0.036 |
| 新海34号 XIN HAI 34 HAO | 33.28 ± 0.792 | 3.249 ± 0.073 | 1.514 ± 0.042 | 0.200 ± 0.004 | 0.246 ± 0.009 | 1.961 ± 0.041 |
| 新海36号 XIN HAI 36 HAO | 38.34 ± 1.853 | 3.014 ± 0.042 | 1.423 ± 0.103 | 0.196 ± 0.014 | 0.221 ± 0.011 | 1.840 ± 0.126 |
| 常紫1号 CHANG ZI 1 HAO | 28.96 ± 1.899 | 3.968 ± 0.046 | 1.192 ± 0.064 | 0.163 ± 0.003 | 0.191 ± 0.006 | 1.546 ± 0.070 |
| 江宁紫花毛籽 JIANG NING ZI HUA MAO ZI | 29.30 ± 1.131 | 3.260 ± 0.044 | 1.317 ± 0.105 | 0.197 ± 0.016 | 0.191 ± 0.005 | 1.705 ± 0.127 |
| 119S | 33.03 ± 0.400 | 2.834 ± 0.030 | 1.420 ± 0.062 | 0.218 ± 0.012 | 0.182 ± 0.008 | 1.820 ± 0.066 |
| 白密拉黄花黑籽 BAI MI LA HUANG HUA HEI ZI | 25.04 ± 0.171 | 2.623 ± 0.039 | 0.980 ± 0.076 | 0.171 ± 0.003 | 0.201 ± 0.004 | 1.352 ± 0.077 |
| 小毛籽 XIAO MAO ZI | 28.91 ± 1.065 | 2.483 ± 0.027 | 1.131 ± 0.091 | 0.185 ± 0.011 | 0.162 ± 0.005 | 1.477 ± 0.102 |
| 中草1号 ZHONG CAO 1 HAO | 33.85 ± 0.918 | 3.117 ± 0.033 | 1.586 ± 0.070 | 0.207 ± 0.002 | 0.175 ± 0.003 | 1.968 ± 0.067 |
| 金塔草棉 JIN TA CAO MIAN | 32.78 ± 0.716 | 3.171 ± 0.056 | 1.349 ± 0.099 | 0.216 ± 0.009 | 0.199 ± 0.005 | 1.764 ± 0.107 |
表2 16种基因型棉花的净光合速率(Pn)、单位面积氮含量(Narea)、核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮含量(Nr)、生物力能学氮含量(Nb)、捕光系统氮含量(NL)和光合机构氮含量(Np) (平均值±标准差)
Table 2 Net photosynthetic rate (Pn), nitrogen content per unit area (Narea), Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) nitrogen content (Nr), bioenergetics nitrogen content (Nb), light harvesting system nitrogen content (NL) and photosynthetic apparatus nitrogen content (Np) of 16 genotypes of Gossypium spp. (mean ± SD)
| 基因型 Genotype | Pn (μmol·m-2·s-1) | Narea (g·m-2) | Nr (g·m-2) | Nb (g·m-2) | NL (g·m-2) | Np (g·m-2) |
|---|---|---|---|---|---|---|
| GP84 | 35.01 ± 1.280 | 3.679 ± 0.055 | 2.218 ± 0.093 | 0.232 ± 0.016 | 0.224 ± 0.006 | 2.675 ± 0.109 |
| 陆92-3 LU 92-3 | 34.79 ± 1.069 | 3.554 ± 0.111 | 1.529 ± 0.001 | 0.230 ± 0.017 | 0.214 ± 0.008 | 1.973 ± 0.007 |
| 切尔盘111 chirpan111 | 31.42 ± 0.857 | 2.988 ± 0.061 | 1.138 ± 0.033 | 0.157 ± 0.001 | 0.206 ± 0.002 | 1.500 ± 0.048 |
| 绉缩叶(vin5) ZHOU SUO YE (vin5) | 39.50 ± 0.863 | 3.720 ± 0.042 | 1.535 ± 0.045 | 0.218 ± 0.005 | 0.243 ± 0.003 | 1.996 ± 0.090 |
| 苏联B9103 SU LIAN B9103 | 34.67 ± 0.960 | 3.320 ± 0.004 | 1.584 ± 0.065 | 0.202 ± 0.008 | 0.216 ± 0.001 | 2.002 ± 0.061 |
| MOC-620 | 30.65 ± 0.782 | 4.718 ± 0.107 | 1.816 ± 0.058 | 0.191 ± 0.005 | 0.240 ± 0.004 | 2.247 ± 0.065 |
| 新海5号 XIN HAI 5 HAO | 33.55 ± 0.670 | 3.336 ± 0.031 | 1.439 ± 0.028 | 0.188 ± 0.004 | 0.219 ± 0.005 | 1.846 ± 0.036 |
| 新海34号 XIN HAI 34 HAO | 33.28 ± 0.792 | 3.249 ± 0.073 | 1.514 ± 0.042 | 0.200 ± 0.004 | 0.246 ± 0.009 | 1.961 ± 0.041 |
| 新海36号 XIN HAI 36 HAO | 38.34 ± 1.853 | 3.014 ± 0.042 | 1.423 ± 0.103 | 0.196 ± 0.014 | 0.221 ± 0.011 | 1.840 ± 0.126 |
| 常紫1号 CHANG ZI 1 HAO | 28.96 ± 1.899 | 3.968 ± 0.046 | 1.192 ± 0.064 | 0.163 ± 0.003 | 0.191 ± 0.006 | 1.546 ± 0.070 |
| 江宁紫花毛籽 JIANG NING ZI HUA MAO ZI | 29.30 ± 1.131 | 3.260 ± 0.044 | 1.317 ± 0.105 | 0.197 ± 0.016 | 0.191 ± 0.005 | 1.705 ± 0.127 |
| 119S | 33.03 ± 0.400 | 2.834 ± 0.030 | 1.420 ± 0.062 | 0.218 ± 0.012 | 0.182 ± 0.008 | 1.820 ± 0.066 |
| 白密拉黄花黑籽 BAI MI LA HUANG HUA HEI ZI | 25.04 ± 0.171 | 2.623 ± 0.039 | 0.980 ± 0.076 | 0.171 ± 0.003 | 0.201 ± 0.004 | 1.352 ± 0.077 |
| 小毛籽 XIAO MAO ZI | 28.91 ± 1.065 | 2.483 ± 0.027 | 1.131 ± 0.091 | 0.185 ± 0.011 | 0.162 ± 0.005 | 1.477 ± 0.102 |
| 中草1号 ZHONG CAO 1 HAO | 33.85 ± 0.918 | 3.117 ± 0.033 | 1.586 ± 0.070 | 0.207 ± 0.002 | 0.175 ± 0.003 | 1.968 ± 0.067 |
| 金塔草棉 JIN TA CAO MIAN | 32.78 ± 0.716 | 3.171 ± 0.056 | 1.349 ± 0.099 | 0.216 ± 0.009 | 0.199 ± 0.005 | 1.764 ± 0.107 |
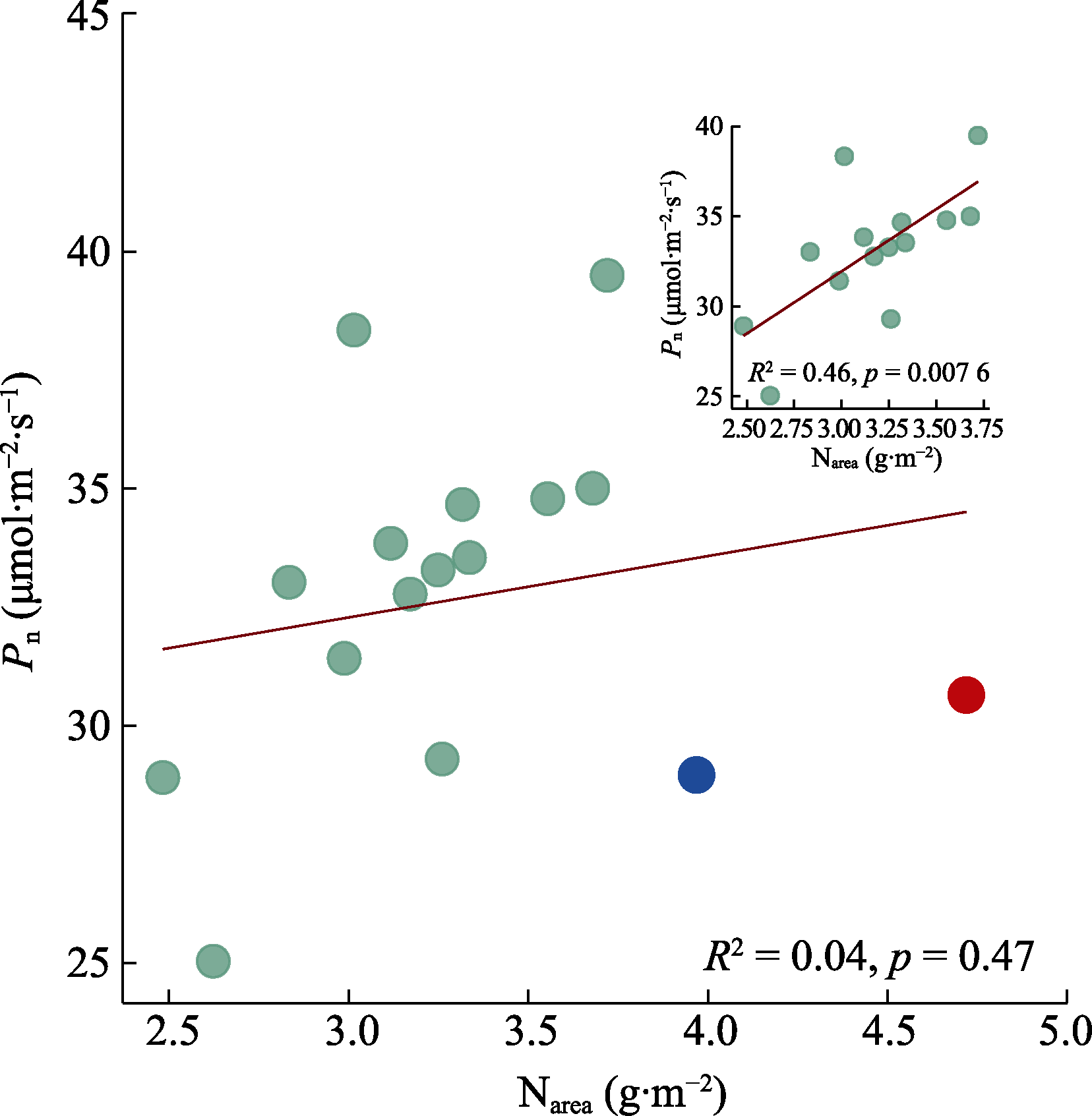
图2 16种基因型棉花净光合速率(Pn)和单位面积氮含量(Narea)之间的相关关系。右上角小图表示Narea小于3.75 g·m-2时, Pn与Narea之间的相关关系。红点与蓝点表示Narea大于3.75 g·m-2的基因型; 红点为基因型‘МОС-620’, 蓝点为基因型‘常紫1号’。
Fig. 2 Correlation between net photosynthetic rate (Pn) and nitrogen content per unit leaf area (Narea) for 16 genotypes of Gossypium spp. The small figure in the upper right corner shows the correlation between Pn and Narea for genotypes with Narea less than 3.75 g·m-2. Red dot and blue dot indicate genotypes with Narea greater than 3.75 g·m-2. The red dot represents the genotype ‘МОС-620’, and the blue dot represents the genotype ‘CHANG ZI 1 HAO’.
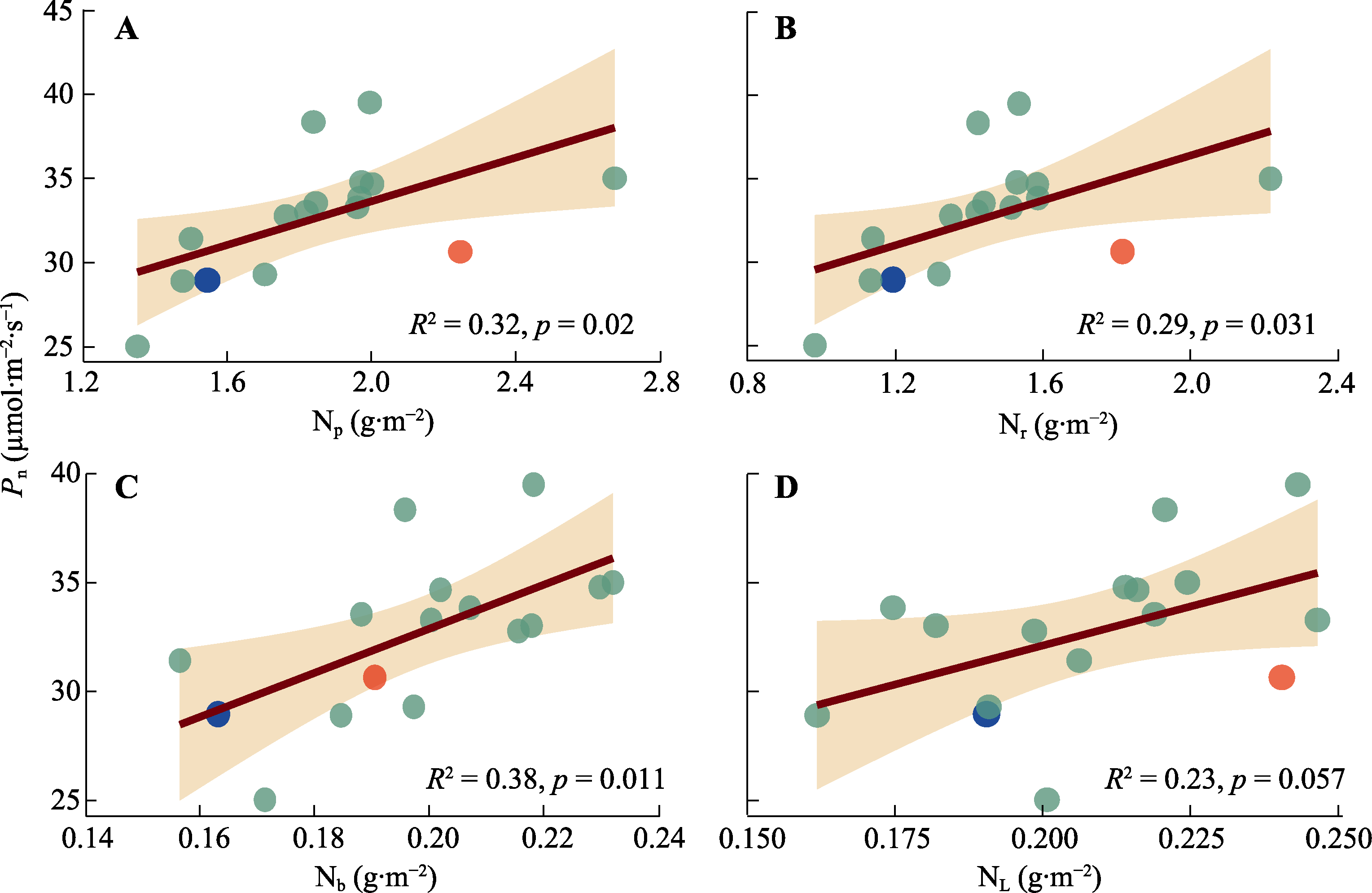
图3 棉花光合机构不同组分氮含量与净光合速率(Pn)的相关性。A, 光合机构氮含量(Np)。B, 核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮含量(Nr)。C, 生物力能学氮含量(Nb)。D, 捕光系统氮含量(NL)。红点与蓝点表示Narea大于3.75 g·m-2的基因型; 红点为基因型‘МОС-620’, 蓝点为基因型‘常紫1号’。
Fig. 3 Correlation between nitrogen content of different components in photosynthetic apparatus and net photosynthetic rate (Pn) of Gossypium spp.. A, Photosynthetic apparatus nitrogen content (Np). B, Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) nitrogen content (Nr). C, Bioenergetics nitrogen content (Nb). D, Light harvesting system nitrogen content (NL). Red dot and blue dot indicate genotypes with Narea greater than 3.75 g·m-2. The red dot represents the genotype ‘МОС-620’, and the blue dot represents the genotype ‘CHANG ZI 1 HAO’.
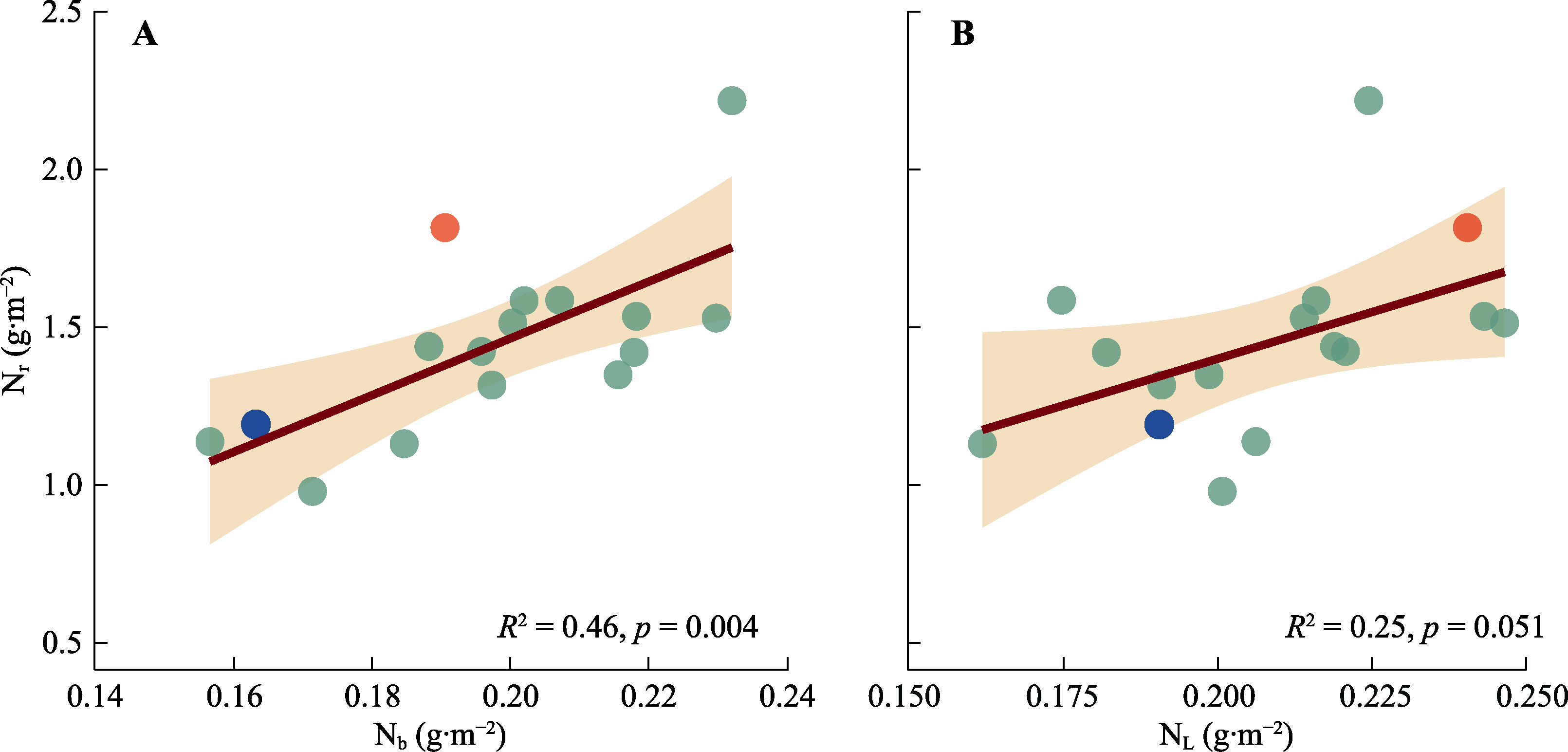
图4 棉花生物力能学氮含量(Nb)、捕光系统氮含量(NL)与核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮含量(Nr)的相关性。红点与蓝点表示Narea大于3.75 g·m-2的基因型; 红点为基因型‘МОС-620’, 蓝点为基因型‘常紫1号’。
Fig. 4 Correlation between the bioenergetics nitrogen content (Nb), light harvesting system nitrogen content (NL) and Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) nitrogen content (Nr) of Gossypium spp. Red dot and blue dot indicate genotypes with Narea greater than 3.75 g·m-2. The red dot represents the genotype ‘МОС-620’, and the blue dot represents the genotype ‘CHANG ZI 1 HAO’.
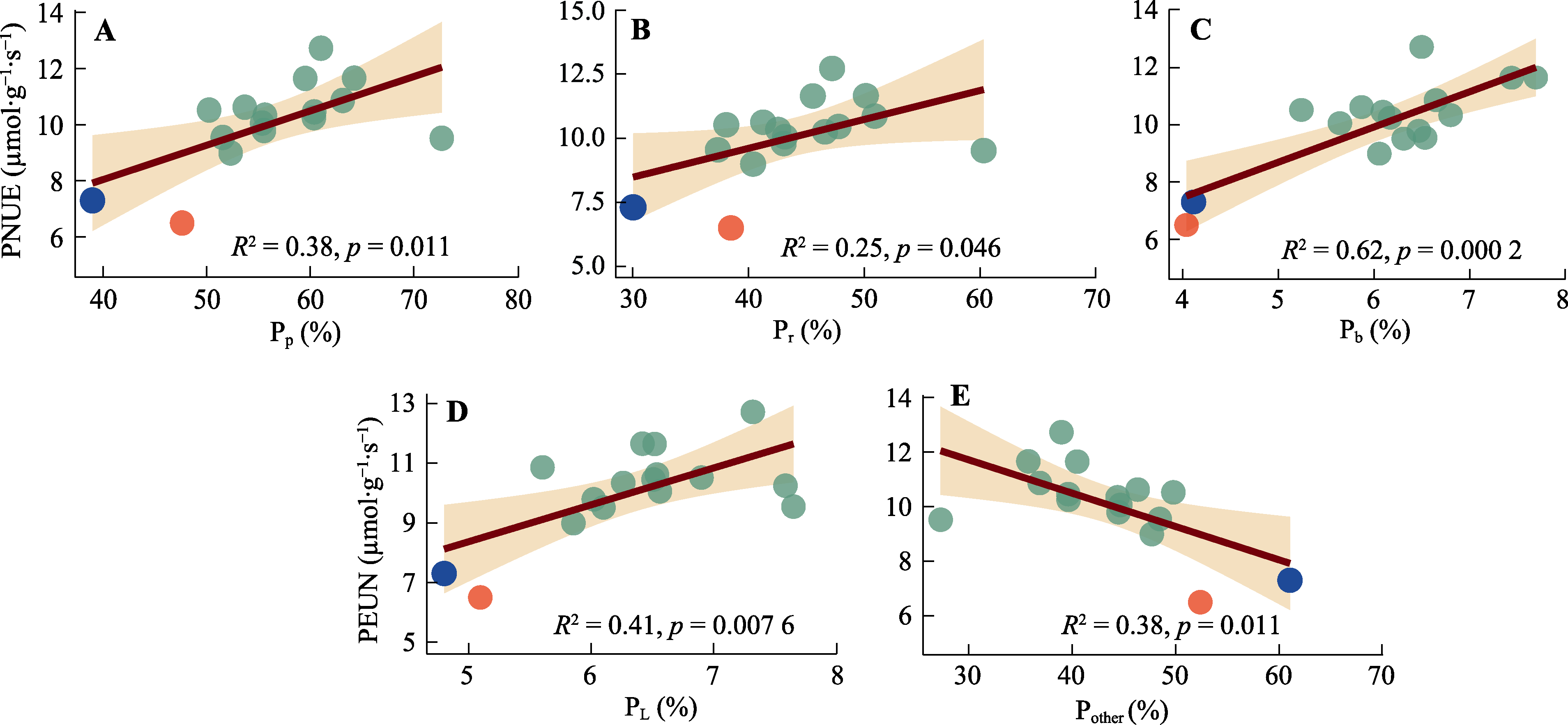
图5 棉花不同组分氮分配比例与光合氮利用效率(PNUE)的相关性。A, 光合机构氮分配比例(Pp)。B, 核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮分配比例(Pr)。C, 生物力能学氮分配比例(Pb)。D, 捕光系统氮分配比例(PL)。E, 非光合系统氮分配比例(Pother)。红点与蓝点表示Narea大于3.75 g·m-2的基因型; 红点为基因型‘МОС-620’, 蓝点为基因型‘常紫1号’。
Fig. 5 Correlation between the fraction of nitrogen allocation to different components and photosynthetic nitrogen use efficiency (PNUE) of Gossypium spp. A, Fraction of nitrogen allocation to photosynthetic apparatus (Pp). B, Fraction of nitrogen allocation to Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Pr). C, Fraction of nitrogen allocation to bioenergetics (Pb). D, Fraction of nitrogen allocation to light-harvesting components (PL). E, Fraction of nitrogen allocation to non-photosynthetic apparatus (Pother). Red dot and blue dot indicate genotypes with Narea greater than 3.75 g·m-2. The red dot represents the genotype ‘МОС-620’, and the blue dot represents the genotype ‘CHANG ZI 1 HAO’.
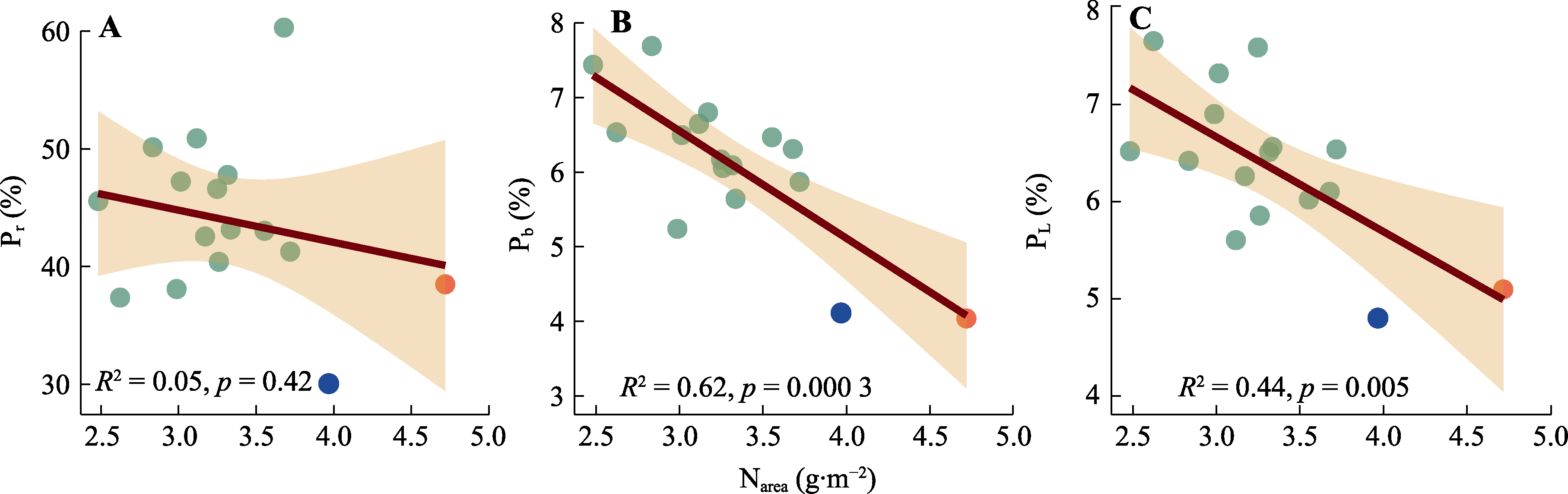
图6 棉花光合机构不同组分氮分配比例与单位面积氮含量(Narea)的相关性。A, 核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮分配比例(Pr)。B, 生物力能学氮分配比例(Pb)。C, 捕光系统氮分配比例(PL)。红点与蓝点表示Narea大于3.75 g·m-2的基因型; 红点为基因型‘МОС-620’, 蓝点为基因型‘常紫1号’。
Fig. 6 Correlation between the fraction of nitrogen allocation to different components and nitrogen content per unit area (Narea) of Gossypium spp. A, Fraction of nitrogen allocation to Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Pr). B, Fraction of nitrogen allocation to bioenergetics (Pb). C, Fraction of nitrogen allocation to light-harvesting components (PL). Red dot and blue dot indicate genotypes with Narea greater than 3.75 g·m-2. The red dot represents the genotype ‘МОС-620’, and the blue dot represents the genotype ‘CHANG ZI 1 HAO’.
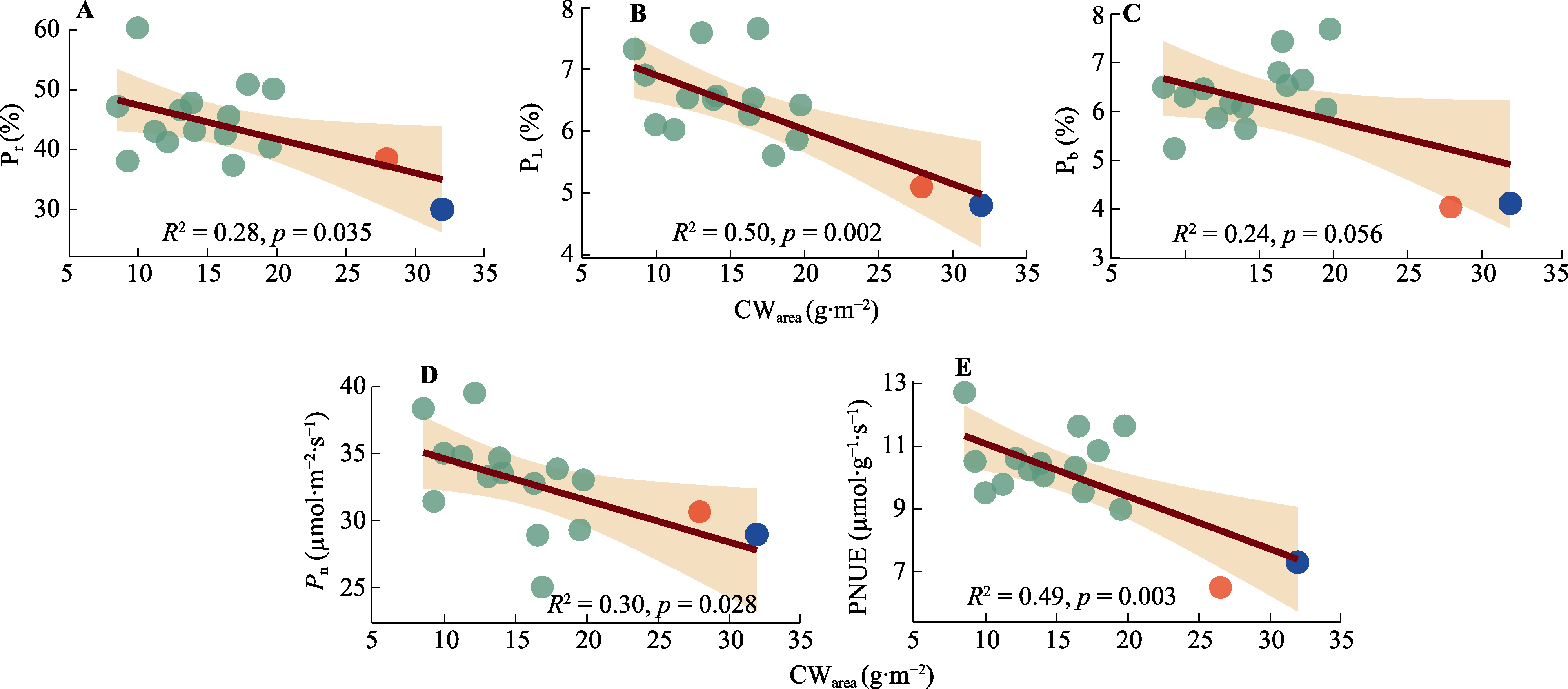
图7 棉花单位面积细胞壁含量(CWarea)与光合系统氮组分分配比例及光合速率(Pn)的相关性。A, 核酮糖-1,5-双磷酸羧化酶/加氧酶(Rubisco)氮分配比例(Pr)。B, 捕光系统氮分配比例(PL)。C, 生物力能学氮分配比例(Pb)。D, 净光合速率(Pn)。E, 光合氮利用效率(PNUE)。红点与蓝点表示Narea大于3.75 g·m-2的基因型; 红点为基因型‘МОС-620’, 蓝点为基因型‘常紫1号’。
Fig. 7 Correlation between the cell wall content per unit area (CWarea) and the fraction of nitrogen allocation to different photosynthetic apparatus component and the net photosynthetic rate (Pn) of Gossypium spp. A, Fraction of nitrogen allocation to Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) (Pr). B, Fraction of nitrogen allocation to light-harvesting component (PL). C, Fraction of nitrogen allocation to bioenergetics (Pb). D, Net photosynthetic rate (Pn). E, Photosynthetic nitrogen use efficiency (PNUE). Red dot and blue dot indicate genotypes with Narea greater than 3.75 g·m-2. The red dot represents the genotype ‘МОС-620’, and the blue dot represents the genotype ‘CHANG ZI 1 HAO’.
| [1] |
Evans JR (1989). Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia, 78, 9-19.
DOI PMID |
| [2] |
Evans JR, Clarke VC (2019). The nitrogen cost of photosynthesis. Journal of Experimental Botany, 70, 7-15.
DOI PMID |
| [3] |
Evans JR, Kaldenhoff R, Genty B, Terashima I (2009). Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany, 60, 2235-2248.
DOI PMID |
| [4] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta, 149, 78-90.
DOI PMID |
| [5] | Feng YL, Lei YB, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit, Li YP, Zheng YL (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106, 1853-1856. |
| [6] |
Gao XB, Wang YH, Chen BL, Li J, Zhou ZG (2012). Effect of nitrogen concentration in the subtending leaves of cotton bolls on the strength of source and sink during boll development. Acta Ecologica Sinica, 32, 238-246.
DOI URL |
| [高相彬, 王友华, 陈兵林, 李健, 周治国 (2012). 棉铃发育期棉花源库活性对棉铃对位叶氮浓度的响应. 生态学报, 32, 238-246.] | |
| [7] |
Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S (2005). Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology, 137, 638-650.
DOI PMID |
| [8] |
Ghimire B, Riley WJ, Koven CD, Kattge J, Rogers A, Reich PB, Wright IJ (2017). A global trait-based approach to estimate leaf nitrogen functional allocation from observations. Ecological Applications, 27, 1421-1434.
DOI PMID |
| [9] |
Gu JF, Zhou ZX, Li ZK, Chen Y, Wang ZQ, Zhang H (2017). Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crops Research, 200, 58-70.
DOI URL |
| [10] |
Guan LL, Wen DZ (2011). More nitrogen partition in structural proteins and decreased photosynthetic nitrogen-use efficiency of Pinus massoniana under in situ polluted stress. Journal of Plant Research, 124, 663-673.
DOI URL |
| [11] |
Hasper TB, Dusenge ME, Breuer F, Uwizeye FK, Wallin G, Uddling J (2017). Stomatal CO2 responsiveness and photosynthetic capacity of tropical woody species in relation to taxonomy and functional traits. Oecologia, 184, 43-57.
DOI URL |
| [12] |
Hirasawa T, Ozawa S, Taylaran R, Ookawa T (2010). Varietal differences in photosynthetic rates in rice plants, with special reference to the nitrogen content of leaves. Plant Production Science, 13, 53-57.
DOI URL |
| [13] |
Huang GJ, Peng SB, Li Y (2022). Variation of photosynthesis during plant evolution and domestication: implications for improving crop photosynthesis. Journal of Experimental Botany, 73, 4886-4896.
DOI URL |
| [14] | Lai B, Tang MY, Chai ZP, Chen BL, Li QJ, Dong JH, Wang F, Tian CY (2014). Investigation and evaluation of the chemical fertilizer application situation of farmland in Xinjiang. Arid Zone Research, 31, 1024-1030. |
| [赖波, 汤明尧, 柴仲平, 陈波浪, 李青军, 董巨河, 王飞, 田长彦 (2014). 新疆农田化肥施用现状调查与评价. 干旱区研究, 31, 1024-1030.] | |
| [15] |
Lei ZY, Wang H, Wright IJ, Zhu XG, Niinemets Ü, Li ZL, Sun DS, Dong N, Zhang WF, Zhou ZL, Liu F, Zhang YL (2021). Enhanced photosynthetic nitrogen use efficiency and increased nitrogen allocation to photosynthetic machinery under cotton domestication. Photosynthesis Research, 150, 239-250.
DOI PMID |
| [16] |
Liu T, Lu JW, Ren T, Wang W, Wang Z, Wang SH (2016). Characteristics of photosynthetic nitrogen allocation in leaves of different positions in winter oilseed rape at seedling stage under suitable nitrogen level. Scientia Agricultura Sinica, 49, 3532-3541.
DOI |
|
[刘涛, 鲁剑巍, 任涛, 汪威, 王振, 王少华 (2016). 适宜氮水平下冬油菜苗期不同叶位叶片光合氮分配特征. 中国农业科学, 49, 3532-3541.]
DOI |
|
| [17] |
Liu T, Ren T, White PJ, Cong RH, Lu JW (2018). Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. Journal of Experimental Botany, 69, 2995-3007.
DOI PMID |
| [18] |
Makino A, Sakuma H, Sudo E, Mae T (2003). Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant and Cell Physiology, 44, 952-956.
PMID |
| [19] |
Milroy SP, Bange MP (2003). Nitrogen and light responses of cotton photosynthesis and implications for crop growth. Crop Science, 43, 904-913.
DOI URL |
| [20] | Mu XH, Chen QW, Chen FJ, Yuan LX, Mi GH (2016). Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage. Frontiers in Plant Science, 7, 699. DOI: 10.3389/fpls.2016.00699. |
| [21] | Niinemets U, Tenhunen JD (1997). A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant, Cell & Environment, 20, 845-866. |
| [22] |
Onoda Y, Hikosaka K, Hirose T (2004). Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Functional Ecology, 18, 419-425.
DOI URL |
| [23] |
Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets Ü, Poorter H, Tosens T, Westoby M (2017). Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytologist, 214, 1447-1463.
DOI PMID |
| [24] | Park M, Cho S, Park J, Lee H, Song W, Park IK, Kim HS (2019). Size-dependent variation in leaf functional traits and nitrogen allocation trade-offs in Robinia pseudoacacia and Cornus controversa. Tree Physiology, 39, 755-766. |
| [25] |
Perchlik M, Tegeder M (2018). Leaf amino acid supply affects photosynthetic and plant nitrogen use efficiency under nitrogen stress. Plant Physiology, 178, 174-188.
DOI PMID |
| [26] |
Poorter H, Remkes C, Lambers H (1990). Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiology, 94, 621-627.
DOI PMID |
| [27] | Reddy KR, Hodges HF, McKinion JM (1997). Crop modeling and applications: a cotton example//Sparks DL. Advances in Agronomy: Vol. 59. Elsevier, Amsterdam. 226-290. |
| [28] |
Roucou A, Violle C, Fort F, Roumet P, Ecarnot M, Vile D (2018). Shifts in plant functional strategies over the course of wheat domestication. Journal of Applied Ecology, 55, 25-37.
DOI URL |
| [29] | Sharkey TD (2016). What gas exchange data can tell us about photosynthesis. Plant, Cell & Environment, 39, 1161-1163. |
| [30] | Showalter AM (1993). Structure and function of plant cell wall proteins. The Plant Cell, 5, 9-23. |
| [31] |
Simkin AJ, McAusland L, Lawson T, Raines CA (2017). Overexpression of the Rieske FeS protein increases electron transport rates and biomass yield. Plant Physiology, 175, 134-145.
DOI URL |
| [32] |
Staswick PE (1994). Storage proteins of vegetative plant tissues. Annual Review of Plant Physiology and Plant Molecular Biology, 45, 303-322.
DOI URL |
| [33] |
Sudo E, Suzuki Y, Makino A (2014). Whole-plant growth and N utilization in transgenic rice plants with increased or decreased rubisco content under different CO2partial pressures. Plant and Cell Physiology, 55, 1905-1911.
DOI PMID |
| [34] | Takashima T, Hikosaka K, Hirose T (2004). Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant, Cell & Environment, 27, 1047-1054. |
| [35] |
Tosens T, Niinemets Ü, Westoby M, Wright IJ (2012). Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. Journal of Experimental Botany, 63, 5105-5119.
DOI PMID |
| [36] |
van Ommen Kloeke AEE, Douma JC, Ordoñez JC, Reich PB, van Bodegom PM (2012). Global quantification of contrasting leaf life span strategies for deciduous and evergreen species in response to environmental conditions. Global Ecology and Biogeography, 21, 224-235.
DOI URL |
| [37] |
Vos J,van der Putten PEL (1998). Effect of nitrogen supply on leaf growth, leaf nitrogen economy and photosynthetic capacity in potato. Field Crops Research, 59, 63-72.
DOI URL |
| [38] |
Wolf S (2022). Cell wall signaling in plant development and defense. Annual Review of Plant Biology, 73, 323-353.
DOI PMID |
| [39] |
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M (2005). Assessing the generality of global leaf trait relationships. New Phytologist, 166, 485-496.
DOI PMID |
| [40] |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, et al.(2004). The worldwide leaf economics spectrum. Nature, 428, 821-827.
DOI |
| [41] |
Yan S, Zhang L, Jing YS, He HL, Yu GR (2014). Variations in the relationship between maximum leaf carboxylation rate and leaf nitrogen concentration. Chinese Journal of Plant Ecology, 38, 640-652.
DOI |
|
[闫霜, 张黎, 景元书, 何洪林, 于贵瑞 (2014). 植物叶片最大羧化速率与叶氮含量关系的变异性. 植物生态学报, 38, 640-652.]
DOI |
|
| [42] |
Ye M, Peng SB, Li Y (2019). Intraspecific variation in photosynthetic nitrogen-use efficiency is positively related to photosynthetic rate in rice (Oryza sativa L.) plants. Photosynthetica, 57, 311-319.
DOI URL |
| [43] |
Yin LJ, Xu HC, Dong SX, Chu JP, Dai XL, He MR (2019). Optimised nitrogen allocation favours improvement in canopy photosynthetic nitrogen-use efficiency: evidence from late-sown winter wheat. Environmental and Experimental Botany, 159, 75-86.
DOI URL |
| [44] |
Yoon DK, Ishiyama K, Suganami M, Tazoe Y, Watanabe M, Imaruoka S, Ogura M, Ishida H, Suzuki Y, Obara M, Mae T, Makino A (2020). Transgenic rice overproducing Rubisco exhibits increased yields with improved nitrogen- use efficiency in an experimental paddy field. Nature Food, 1, 134-139.
DOI |
| [45] |
Zhong C, Jian SF, Huang J, Jin QY, Cao XC (2019). Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiology and Biochemistry, 135, 41-50.
DOI PMID |
| [46] |
Zhu XG, de Sturler E, Long SP (2007). Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiology, 145, 513-526.
DOI URL |
| [47] | Ziegler C, Dusenge ME, Nyirambangutse B, Zibera E, Wallin G, Uddling J (2020). Contrasting dependencies of photosynthetic capacity on leaf nitrogen in early- and late-successional tropical montane tree species. Frontiers in Plant Science, 11, 1428. DOI: 10.3389/fpls.2020.500479. |
| [1] | 朱启林, 向蕊, 汤利, 龙光强. 间作对氮调控玉米光合速率和光合氮利用效率的影响[J]. 植物生态学报, 2018, 42(6): 672-680. |
| [2] | 司晓林, 王文银, 高小刚, 徐当会. 氮硅添加对高寒草甸垂穗披碱草叶片全氮含量及净光合速率的影响[J]. 植物生态学报, 2016, 40(12): 1238-1244. |
| [3] | 唐海萍, 薛海丽, 房飞. 叶片和群落尺度净光合速率关系的探讨[J]. 植物生态学报, 2015, 39(9): 924-931. |
| [4] | 雒珺瑜, 刘传亮, 张帅, 王春义, 吕丽敏, 李春花, 李付广, 崔金杰. 转RRM2基因棉生长势和产量及对棉田节肢动物群落的影响[J]. 植物生态学报, 2014, 38(7): 785-794. |
| [5] | 张超, 占东霞, 张鹏鹏, 张亚黎, 罗宏海, 张旺锋. 棉花苞叶光呼吸和PSII热耗散对土壤水分的响应[J]. 植物生态学报, 2014, 38(4): 387-395. |
| [6] | 武辉, 戴海芳, 张巨松, 焦晓玲, 刘翠, 石俊毅, 范志超, 阿丽艳·肉孜. 棉花幼苗叶片光合特性对低温胁迫及恢复处理的响应[J]. 植物生态学报, 2014, 38(10): 1124-1134. |
| [7] | 陶先萍, 罗宏海, 杨海, 丁全盛, 张亚黎, 张旺锋. 根域限制下水氮供应对膜下滴灌棉花根系及叶片衰老特性的影响[J]. 植物生态学报, 2013, 37(3): 256-267. |
| [8] | 王荣荣, 夏江宝, 杨吉华, 赵艳云, 刘京涛, 孙景宽. 贝壳砂生境干旱胁迫下杠柳叶片光合光响应模型比较[J]. 植物生态学报, 2013, 37(2): 111-121. |
| [9] | 李维, 张亚黎, 胡渊渊, 杨美森, 吴洁, 张旺锋. 田间条件下棉花幼叶光合特性及光保护机制[J]. 植物生态学报, 2012, 36(7): 662-670. |
| [10] | 陈卫英, 陈真勇, 罗辅燕, 彭正松, 余懋群. 光响应曲线的指数改进模型与常用模型比较[J]. 植物生态学报, 2012, 36(12): 1277-1285. |
| [11] | 夏江宝, 张光灿, 孙景宽, 刘霞. 山杏叶片光合生理参数对土壤水分和光照强度的阈值效应[J]. 植物生态学报, 2011, 35(3): 322-329. |
| [12] | 魏雅芬, 方杰, 赵学勇, 李胜功. 科尔沁沙地樟子松人工林不同年龄针叶生理生态性状[J]. 植物生态学报, 2011, 35(12): 1271-1280. |
| [13] | 刘瑞显, 陈兵林, 王友华, 郭文琦, 周治国. 氮素对花铃期干旱再复水后棉花根系生长的影响[J]. 植物生态学报, 2009, 33(2): 405-413. |
| [14] | 叶子飘, 于强. 光合作用光响应模型的比较[J]. 植物生态学报, 2008, 32(6): 1356-1361. |
| [15] | 张亚黎, 罗宏海, 张旺锋, 樊大勇, 何在菊, 白慧东. 土壤水分亏缺对陆地棉花铃期叶片光化学活性和激发能耗散的影响[J]. 植物生态学报, 2008, 32(3): 681-689. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 769
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 760
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19 51La