

植物生态学报 ›› 2012, Vol. 36 ›› Issue (7): 671-680.DOI: 10.3724/SP.J.1258.2012.00671
周帅1, 林富平1, 王玉魁3, 沈应柏2, 张汝民1, 高荣孚2, 高岩1,*( )
)
发布日期:2012-07-10
通讯作者:
高岩
作者简介:* E-mail: gaoyan1960@sohu.com
ZHOU Shuai1, LIN Fu-Ping1, WANG Yu-Kui3, SHEN Ying-Bai2, ZHANG Ru-Min1, GAO Rong-Fu2, GAO Yan1,*( )
)
Published:2012-07-10
Contact:
GAO Yan
摘要:
为探讨植物在机械损伤后C6-C10醛类化合物的释放机理, 及C6-C10醛类化合物对叶片光系统II (PSII)的影响, 以樟树(Cinnamomum camphora)为材料, 采用动态顶空气体循环法和热脱附/气相色谱/质谱联用技术(TDS-GC-MS), 对樟树幼苗叶片损伤后释放的C6-C10醛类化合物进行采集与分析, 并同步测定了脂氧合酶活性和损伤叶片的叶绿素荧光动力学参数。结果表明: 樟树幼苗叶片损伤后, 其挥发性有机化合物中己醛、庚醛、辛醛、壬醛和癸醛的释放量比损伤前分别增加了2.47、0.99、1.34、0.91和28.38倍(p < 0.01); 同时新增4种醛类化合物, 分别是: 2-己烯醛、2,4-己二烯醛、(E)-2-辛烯醛和(E)-2-壬烯醛。脂氧合酶活性比损伤前增加1.2倍(p < 0.01)。PSII单位反应中心复合体吸收的能量和被核心捕获的能量分别比损伤前下降12.8%和9.8% (p < 0.01)。单位面积反应中心的数量、电子传递量子产额、捕获激子能导致电子传递效率和叶片性能指数分别比损伤前增加23.3%、24.4%、22.6%和82.7% (p < 0.01)。损伤24 h后, 醛类化合物的种类、释放量、脂氧合酶活性及叶片叶绿素荧光动力学参数基本恢复到损伤前水平。说明机械损伤使PSII供体侧受损、脂氧合酶活性升高, 致使C6-C10醛类化合物大量释放, 樟树幼苗通过增加单位面积反应中心的数量来提高光合效率应对胁迫。
周帅, 林富平, 王玉魁, 沈应柏, 张汝民, 高荣孚, 高岩. 樟树幼苗机械损伤叶片对挥发性有机化合物及叶绿素荧光参数的影响. 植物生态学报, 2012, 36(7): 671-680. DOI: 10.3724/SP.J.1258.2012.00671
ZHOU Shuai, LIN Fu-Ping, WANG Yu-Kui, SHEN Ying-Bai, ZHANG Ru-Min, GAO Rong-Fu, GAO Yan. Effects of mechanical damage of leaves on volatile organic compounds and chlorophyll fluorescence parameters in seedlings of Cinnamomum camphora. Chinese Journal of Plant Ecology, 2012, 36(7): 671-680. DOI: 10.3724/SP.J.1258.2012.00671
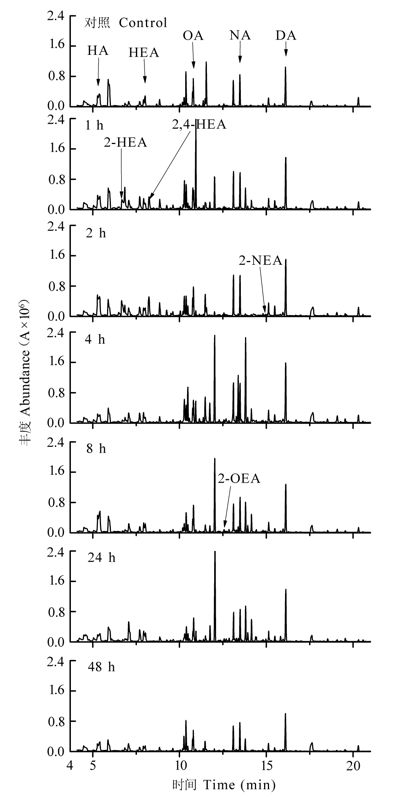
图1 机械损伤后樟树幼苗叶片挥发性有机化合物总离子流。A, 峰面积; DA, 癸醛; FA, 糠醛; HA, 己醛; HEA, 庚醛; 2-HEA, (E)-2-己烯醛; 2,4-HEA, (E,E)-2,4-己二烯醛; 2-NEA, (E)-2-壬烯醛; NA, 壬醛; OA, 辛醛; 2-OEA, (E)-2-辛烯醛。
Fig. 1 Total ion current of volatile organic compounds in mechanically damaged leaves of Cinnamomum camphora seedlings. A, peak area; DA, decanal; FA, furfural; HA, hexanal; HEA, heptanal; 2-HEA, (E)-2-hexenal; 2,4-HEA, (E,E)- 2,4-hexadienal; 2-NEA, (E)-2-nonenal; NA, nonanal; OA, octanal; 2-OEA, (E)-2-octenal.
| C6-C10醛类化合物 C6-C10 aldehydes | 时间 Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | 48 | |
| 糠醛 Furfural | 1.58 ± 0.54 | 2.61 ± 0.59* | 1.18 ± 0.42 | 0.83 ± 0.25 | 1.30 ± 0.35 | 1.08 ± 0.36 | 1.32 ± 0.34 |
| 己醛 Hexanal | 9.53 ± 1.02 | 32.53 ± 4.92** | 33.05 ± 1.62** | 29.03 ± 3.19** | 18.39 ± 0.86** | 14.44 ± 3.01* | 7.06 ± 1.55 |
| 庚醛 Heptanal | 6.23 ± 0. 97 | 8.82 ± 1.21* | 11.22 ± 0.84** | 12.53 ± 2.80** | 9.45 ± 0.87* | 7.67 ± 0.95 | 6.56 ± 0.84 |
| 辛醛 Octanal | 10.11 ± 2.65 | 13.34 ± 3.67* | 23.66 ± 2.31** | 17.37 ± 3.33* | 16.43 ± 3.01* | 14.32 ± 2.95* | 11.03 ± 2.55 |
| 壬醛 Nonanal | 14.26 ± 3.43 | 22.02 ± 4.07** | 27.24 ± 3.34** | 22.91 ± 3.90* | 19.97 ± 2.85* | 18.97 ± 3.56 | 15.59 ± 2.77 |
| 癸醛 Decanal | 1.72 ± 0.67 | 44.67 ± 9.35** | 50.53 ± 4.94** | 48.84 ± 3.31** | 45.57 ± 4.06** | 36.43 ± 2.18** | 4.76 ± 1.23* |
| (E)-2-己烯醛 (E)-2-hexenal | - | 20.75 ± 2.72 | 22.05 ± 1.25 | 5.37 ± 1.63 | 1.85 ± 0.04 | 1.61 ± 0.41 | 1.22 ± 0.23 |
| (E,E)-2,4-己二烯醛 (E,E)-2,4-hexadienal | - | 12.54 ± 3.46 | 16.43 ± 2.89 | 3.81 ± 1.06 | 1.32 ± 0.36 | 0.43 ± 0.16 | - |
| (E)-2-壬烯醛 (E)-2-nonenal | - | - | 0.43 ± 0.05 | 0.51 ± 0.10 | 0.64 ± 0.14 | 0.69 ± 0.13 | 0.30 ± 0.10 |
| (E)-2-辛烯醛 (E)-2-octenal | - | - | - | - | 0.68 ± 0.08 | 0.55 ± 0.06 | 0.30 ± 0. 10 |
表1 机械损伤后樟树幼苗叶片C6-C10醛类化合物释放量的变化(平均值±标准误差)
Table 1 Variation of the release amount of C6-C10 aldehydes in mechanically damaged leaves of Cinnamomum camphora seedlings (mean ± SE)
| C6-C10醛类化合物 C6-C10 aldehydes | 时间 Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | 48 | |
| 糠醛 Furfural | 1.58 ± 0.54 | 2.61 ± 0.59* | 1.18 ± 0.42 | 0.83 ± 0.25 | 1.30 ± 0.35 | 1.08 ± 0.36 | 1.32 ± 0.34 |
| 己醛 Hexanal | 9.53 ± 1.02 | 32.53 ± 4.92** | 33.05 ± 1.62** | 29.03 ± 3.19** | 18.39 ± 0.86** | 14.44 ± 3.01* | 7.06 ± 1.55 |
| 庚醛 Heptanal | 6.23 ± 0. 97 | 8.82 ± 1.21* | 11.22 ± 0.84** | 12.53 ± 2.80** | 9.45 ± 0.87* | 7.67 ± 0.95 | 6.56 ± 0.84 |
| 辛醛 Octanal | 10.11 ± 2.65 | 13.34 ± 3.67* | 23.66 ± 2.31** | 17.37 ± 3.33* | 16.43 ± 3.01* | 14.32 ± 2.95* | 11.03 ± 2.55 |
| 壬醛 Nonanal | 14.26 ± 3.43 | 22.02 ± 4.07** | 27.24 ± 3.34** | 22.91 ± 3.90* | 19.97 ± 2.85* | 18.97 ± 3.56 | 15.59 ± 2.77 |
| 癸醛 Decanal | 1.72 ± 0.67 | 44.67 ± 9.35** | 50.53 ± 4.94** | 48.84 ± 3.31** | 45.57 ± 4.06** | 36.43 ± 2.18** | 4.76 ± 1.23* |
| (E)-2-己烯醛 (E)-2-hexenal | - | 20.75 ± 2.72 | 22.05 ± 1.25 | 5.37 ± 1.63 | 1.85 ± 0.04 | 1.61 ± 0.41 | 1.22 ± 0.23 |
| (E,E)-2,4-己二烯醛 (E,E)-2,4-hexadienal | - | 12.54 ± 3.46 | 16.43 ± 2.89 | 3.81 ± 1.06 | 1.32 ± 0.36 | 0.43 ± 0.16 | - |
| (E)-2-壬烯醛 (E)-2-nonenal | - | - | 0.43 ± 0.05 | 0.51 ± 0.10 | 0.64 ± 0.14 | 0.69 ± 0.13 | 0.30 ± 0.10 |
| (E)-2-辛烯醛 (E)-2-octenal | - | - | - | - | 0.68 ± 0.08 | 0.55 ± 0.06 | 0.30 ± 0. 10 |
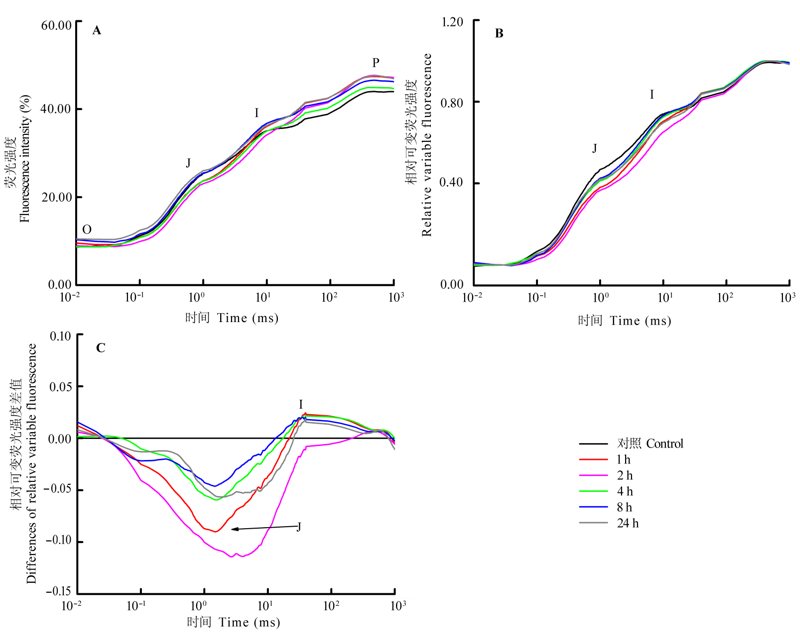
图2 机械损伤后樟树幼苗叶片叶绿素荧光动力学曲线的变化。A, 机械损伤后樟树幼苗叶片O-J-I-P荧光诱导曲线(3次重复的平均值)。B, Fo与Fm间相对可变荧光强度(Vt)随时间的变化, Vt = (Ft -Fo)/(Fm-Fo), Ft表示t时的荧光强度, Fo表示暗适应后的最小荧光强度, Fm表示暗适应后的最大荧光强度。C, Vt与对照的差值△Vt, △Vt = Vt(处理) -Vt (对照)。O、J、I、P各点含义详见李鹏民等(2005)。
Fig. 2 Variation of chlorophyll fluorescence transients in mechanically damaged leaves of Cinnamomum camphora seedlings. A, O-J-I-P transient recorded in seedlings leaves of C. camphora after mechanical damage (average of three samples). B, relative variable fluorescence (Vt) between Fo and Fm, Vt = (Ft -Fo)/(Fm-Fo), Ft description fluorescence at time t, Fo description minimum fluorescence intensity after dark adaptation, Fm description maximum fluorescence intensity after dark adaptation. C, differences (?Vt) of Vt to the CK, ?Vt = Vt (treatment) - Vt (CK). Meaning of O, J, I and P referred to Li et al. (2005).
| 参数 Parameter | 时间 Time (h) | |||||
|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | |
| ABS/RC | 2.35 ± 0.17 | 2.21 ± 0.15 | 2.05 ± 0.04** | 2.30 ± 0.06 | 2.37 ± 0.07 | 2.40 ± 0.17 |
| TRo/RC | 1.84 ± 0.08 | 1.71 ± 0.05 | 1.66 ± 0.03** | 1.91 ± 0.07 | 1.86 ± 0.06 | 1.96 ± 0.14 |
| ETo/RC | 0.96 ± 0.14 | 1.04 ± 0.09 | 1.01 ± 0.11 | 1.03 ± 0.01 | 1.01 ± 0.01 | 1.15 ± 0.06** |
| ψo | 0.52 ± 0.01 | 0.61 ± 0.01* | 0.64 ± 0.08** | 0.54 ± 0.03 | 0.50 ± 0.06 | 0.51 ± 0.00 |
| ΦEo | 0.45 ± 0.19 | 0.43 ± 0.05 | 0.56 ± 0.01** | 0.41 ± 0.01 | 0.40 ± 0.00 | 0.48 ± 0.06 |
| RC/CS | 3.82 ± 0.37 | 4.26 ± 0.19* | 4.71 ± 0.59** | 3.77 ± 0.37 | 4.21 ± 0.08 | 3.91 ± 0.11 |
| FV/Fm | 0.87 ± 0.01 | 0.89 ± 0.07 | 0.89 ± 0.06 | 0.85 ± 0.01 | 0.75 ± 0.07 | 0.74 ± 0.03 |
| PIABS | 1.86 ± 0.46 | 2.77 ± 1.06** | 3.41 ± 0.83** | 2.27 ± 0.16* | 1.99 ± 0.36 | 1.89 ± 0.51 |
表2 机械损伤后樟树幼苗叶片主要叶绿素荧光参数的变化(平均值±标准误差)
Table 2 Variations of main chlorophyll ?uorescence parameters in mechanically damaged leaves of Cinnamomum camphora seedlings (mean ± SE)
| 参数 Parameter | 时间 Time (h) | |||||
|---|---|---|---|---|---|---|
| 对照 Control | 1 | 2 | 4 | 8 | 24 | |
| ABS/RC | 2.35 ± 0.17 | 2.21 ± 0.15 | 2.05 ± 0.04** | 2.30 ± 0.06 | 2.37 ± 0.07 | 2.40 ± 0.17 |
| TRo/RC | 1.84 ± 0.08 | 1.71 ± 0.05 | 1.66 ± 0.03** | 1.91 ± 0.07 | 1.86 ± 0.06 | 1.96 ± 0.14 |
| ETo/RC | 0.96 ± 0.14 | 1.04 ± 0.09 | 1.01 ± 0.11 | 1.03 ± 0.01 | 1.01 ± 0.01 | 1.15 ± 0.06** |
| ψo | 0.52 ± 0.01 | 0.61 ± 0.01* | 0.64 ± 0.08** | 0.54 ± 0.03 | 0.50 ± 0.06 | 0.51 ± 0.00 |
| ΦEo | 0.45 ± 0.19 | 0.43 ± 0.05 | 0.56 ± 0.01** | 0.41 ± 0.01 | 0.40 ± 0.00 | 0.48 ± 0.06 |
| RC/CS | 3.82 ± 0.37 | 4.26 ± 0.19* | 4.71 ± 0.59** | 3.77 ± 0.37 | 4.21 ± 0.08 | 3.91 ± 0.11 |
| FV/Fm | 0.87 ± 0.01 | 0.89 ± 0.07 | 0.89 ± 0.06 | 0.85 ± 0.01 | 0.75 ± 0.07 | 0.74 ± 0.03 |
| PIABS | 1.86 ± 0.46 | 2.77 ± 1.06** | 3.41 ± 0.83** | 2.27 ± 0.16* | 1.99 ± 0.36 | 1.89 ± 0.51 |
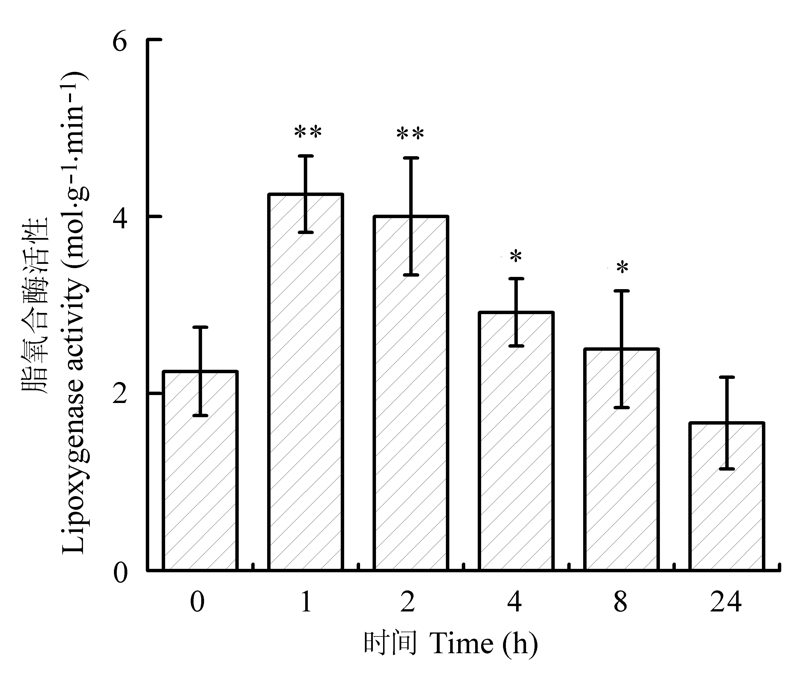
图3 机械损伤后不同时间樟树幼苗叶片脂氧合酶的活性(平均值±标准误差)。
Fig. 3 Activity of lipoxygenase in leaves of Cinnamomum camphora seedlings at different time after mechanical damage (mean ± SE). *, p < 0.05; **, p < 0.01.
| [1] |
Apel K, Hirt H (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373-399.
DOI URL PMID |
| [2] |
Appenroth KJ, Stöckel J, Srivastava A, Strasser RJ (2001). Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environmental Pollution, 115, 49-64.
URL PMID |
| [3] |
Bi JL, Felton GW (1995). Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. Journal of Chemical Ecology, 21, 1511-1529.
URL PMID |
| [4] |
Bown AW, Hall DE, MacGregor KB (2002). Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiology, 129, 1430-1434.
DOI URL PMID |
| [5] |
Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH (2004). Airborne signals prime plants against insect herbivore attack. Proceedings of the National Academy of Sciences of the United States of America, 101, 1781-1785.
DOI URL PMID |
| [6] |
Feussner I, Wasternack C (2002). The lipoxygenase pathway. Annual Review of Plant Biology, 53, 275-297.
DOI URL PMID |
| [7] | Gao Y, Jin YJ, Li HD, Chen HJ (2005). Volatile organic compounds and their roles in bacteriostasis in five conifer species. Journal of Integrated Plant Biology, 47, 499-507. |
| [8] |
Gouinguené SP, Turlings TCJ (2002). The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiology, 129, 1296-1307.
DOI URL PMID |
| [9] |
Graus M, Schnitzler JP, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J (2004). Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiology, 135, 1967-1975.
URL PMID |
| [10] | Grote R, Keenan T, Lavoir AV, Staudt M (2010). Process- based simulation of seasonality and drought stress in monoterpene emission models. Biogeosciences, 7, 257-274. |
| [11] |
Hartikainen K, Nerg A, Kivimäenpää M, Kontunen-soppela S, Mäenpää M, Oksanen E, Rousi M, Holopainen T (2009). Emissions of volatile organic compounds and leaf structural characteristics of European aspen ( Populus tremula) grown under elevated ozone and temperature. Tree Physiology, 29, 1163-1173.
URL PMID |
| [12] | Hatanaka A (1993). The biogeneration of green odour by green leaves. Phytochemistry, 34, 1201-1218. |
| [13] |
Holopainen JK (2011). Can forest trees compensate for stress-generated growth losses by induced production of volatile compounds? Tree Physiology, 31, 1356-1377.
DOI URL PMID |
| [14] | Hu ZH, Shen YB, Luo YQ, Shen FY, Gao HB, Gao RF (2008). Aldehyde volatiles emitted in succession from mechanically damaged leaves of poplar cuttings. Plant Biology, 51, 269-275. |
| [15] | Hu ZH, Shen YB, Su XH (2009). Saturated aldehydes C6-C10 emitted from ashleaf maple ( Acer negundo L.) leaves at different levels of light intensity, O2, and CO2. Journal of Plant Biology, 52, 289-297. |
| [16] |
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2005). Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against botrytis cinerea in Arabidopsis thaliana. Plant and Cell Physiology, 46, 1093-1102.
URL PMID |
| [17] |
León J, Rojo E, Sánchez-Serrano JJ (2001). Wound signaling in plants. Journal of Experimental Botany, 52, 1-9.
DOI URL PMID |
| [18] | Li PM (李鹏民), Gao HY (高辉远), Strasser RJ (2005). Application of the fast chlorophyll fluorescence induction dynamics analysis in photosynthesis study. Journal of Plant Physiology and Molecular Biology (植物生理与分子生物学学报), 31, 559-566. (in Chinese with English abstract) |
| [19] |
Loreto F, Barta C, Brilli F, Nogues I (2006). On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant, Cell & Environment, 29, 1820-1828.
DOI URL PMID |
| [20] |
Loreto F, Schnitzler JP (2010). Abiotic stresses and induced BVOCs. Trends in Plant Science, 15, 154-166.
DOI URL PMID |
| [21] |
Matsui K, Kurishita S, Hisamitsu A, Kajiwara T (2000). A lipid-hydrolysing activity involved in hexenal formation. Biochemical Society Transactions, 28, 857-860.
URL PMID |
| [22] |
Niinemets Ü, Loreto F, Reichstein M (2004). Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science, 9, 180-186.
URL PMID |
| [23] |
Pegoraro E, Rey A, Bobich EG, Barron-Gafford G, Grieve KA, Malhi Y, Murthy R (2004). Effect of elevated CO2 concentration and vapour pressure deficit on isoprene emission from leaves of Populus deltoides during drought. Functional Plant Biology, 31, 1137-1147.
DOI URL PMID |
| [24] |
Peñuelas J, Staudt M (2010). BVOCs and global change. Trends in Plant Science, 15, 133-144.
DOI URL PMID |
| [25] |
Piesik D, Pańka D, Delaney KJ, Skoczek A, Lamparski R, Weaver DK (2011). Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.) Journal of Plant Physiology, 168, 878-886.
URL PMID |
| [26] | Ping LY (平立岩), Shen YB (沈应柏), Jin YJ (金幼菊), Hao JH (郝建华) (2001). Leaf volatiles induced by mechanical damage from diverse taxonomic tree species. Acta Botanica Sinica (植物学报), 43, 261-266. (in Chinese with English abstract) |
| [27] | Sallas L, Kainulainen P, Utriainen J, Holopainen T, Holopainen JK (2001). The influence of elevated O3 and CO2 concentrations on secondary metabolites of Scots pine ( Pinus sylvestris L.) seedlings. Global Change Biology, 7, 303-311. |
| [28] |
Sallas L, Luomala EM, Ultriainen J, Kainulainen P, Holopainen JK (2003). Contrasting effects of elevated carbon dioxide concentration and temperature on rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiology, 23, 97-108.
DOI URL PMID |
| [29] |
Schaub A, Blande JD, Graus M, Oksanen E, Holopainen JK, Hansel A (2010). Real-time monitoring of herbivore induced volatile emissions in the field. Physiologia Plantarum, 138, 123-133.
DOI URL PMID |
| [30] | Sharkey TD, Wiberley AE, Donohue AR (2008). Isoprene emission from plants: why and how. Annals of the Missouri Botanical Garden, 101, 5-18. |
| [31] | Shen YB (沈应柏) (2008). Responses of Populus simonii × Populus pyramidalis ‘Opera 8277’ Cuttings to Wounding and Airborne Defensive Signals . (合作杨苗木对伤害和气体防御信号的响应) PhD dissertation, Beijing Forestry University, Beijing, 7-35. (in Chinese with English abstract) |
| [32] |
Shiojiri K, Kishimoto K, Ozawa R, Kugimiya S, Urashimo S, Arimura G, Horiuchi J, Nishioka T, Matsui K, Takabayashi J (2006). Changing green leaf volatile biosynthesis in plants: an approach for improving plant resistance against both herbivores and pathogens. Proceedings of the National Academy of Sciences of the United States of America, 103, 16672-16676.
URL PMID |
| [33] | Strasser RJ, Srivastava A, Tsimilli-Michael M (2000). The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P eds. Probing Photosynthesis: Mechanisms, Regulation and Adaptation. Taylor and Francis, London. 445-483. |
| [34] | Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal, 11, 1187-1194. |
| [35] | van Heerden PDR, Strasser RJ, Krüger GHJ (2004). Reduction of dark chilling stress in N2-fixing soybean by nitrate as indicated by chlorophyll a fluorescence kinetics. Physiolo- gia Plantarum, 121, 239-249. |
| [36] |
van Heerden PDR, Tsimilli-Michael M, Krüger GHJ, Strasser RJ (2003). Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiologia Plantarum, 117, 476-491.
DOI URL PMID |
| [37] |
Wildt J, Kobel K, Schuh-Thomas G, Heiden AC (2003). Emissions of oxygenated volatile organic compounds from plants. Part II: Emissions of saturated aldehydes. Journal of Atmospheric Chemistry, 45, 173-196.
DOI URL |
| [38] |
Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R (2009). Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Global Change Biology, 15, 1189-1200.
DOI URL |
| [39] | Wojtaszek P (1997). Oxidative burst: an early plant response to pathogen infection. Biochemical Journal, 322, 681-692. |
| [40] | Xue W (薛伟), Li XY (李向义), Lin LS (林丽莎), Wang YJ (王迎菊), Li L (李磊) (2011). Effects of short time heat stress on photosystem II, rubisco activities and oxidative radicals in Alhagi sparsifolia. Chinese Journal of Plant Ecology (植物生态学报), 35, 441-451. (in Chinese with English abstract) |
| [41] |
Zeringue HJ (1991). Effect of C6 to C9 alkenals on aflatoxin production in corn, cottonseed, and peanuts. Applied and Environmental Microbiology, 57, 2433-2434.
DOI URL PMID |
| [42] | Zhang FJ (张风娟), Li JQ (李继泉), Xu XY (徐兴友), Meng XD (孟宪东), Chen FJ (陈发菊) (2007). The volatiles of two greening tree species and the antimicrobial activity. Acta Horticulturae Sinica (园艺学报), 34, 4973-4978. (in Chinese with English abstract) |
| [43] | Zuo ZJ (左照江), Zhang RM (张汝民), Wang Y (王勇), Hou P (侯平), Wen GS (温国胜), Gao Y (高岩) (2010). Analysis of main volatile organic compounds and study of aboveground structures in Artemisia frigida. Chinese Journal of Plant Ecology (植物生态学报), 34, 462-468. (in Chinese with English abstract) |
| [1] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [2] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [3] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [4] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| [5] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [6] | 周稳, 迟永刚, 周蕾. 基于日光诱导叶绿素荧光的北半球森林物候研究[J]. 植物生态学报, 2021, 45(4): 345-354. |
| [7] | 丁键浠, 周蕾, 王永琳, 庄杰, 陈集景, 周稳, 赵宁, 宋珺, 迟永刚. 叶绿素荧光主动与被动联合观测应用前景[J]. 植物生态学报, 2021, 45(2): 105-118. |
| [8] | 郭庆华, 胡天宇, 马勤, 徐可心, 杨秋丽, 孙千惠, 李玉美, 苏艳军. 新一代遥感技术助力生态系统生态学研究[J]. 植物生态学报, 2020, 44(4): 418-435. |
| [9] | 刘校铭, 杨晓芳, 王璇, 张守仁. 暖温带落叶阔叶林辽东栎和五角枫生长和光合生理生态特征对模拟氮沉降的响应[J]. 植物生态学报, 2019, 43(3): 197-207. |
| [10] | 蔡建国, 韦孟琪, 章毅, 魏云龙. 遮阴对绣球光合特性和叶绿素荧光参数的影响[J]. 植物生态学报, 2017, 41(5): 570-576. |
| [11] | 刘盟盟, 贾丽, 程路芸, 张洪芹, 臧晓琳, 宝音陶格涛, 张汝民, 高岩. 冷蒿酚酸及其抗氧化防御酶活性对机械损伤的响应[J]. 植物生态学报, 2017, 41(2): 219-230. |
| [12] | 樊大勇, 付增娟, 谢宗强, 李荣贵, 张淑敏. 调制式荧光影像新技术: 叶片内部最大光化学量子效率及其异质性的活体测定[J]. 植物生态学报, 2016, 40(9): 942-951. |
| [13] | 刘畅, 孙鹏森, 刘世荣. 植物反射光谱对水分生理变化响应的研究进展[J]. 植物生态学报, 2016, 40(1): 80-91. |
| [14] | 安东升, 曹娟, 黄小华, 周娟, 窦美安. 基于Lake模型的叶绿素荧光参数在甘蔗苗期抗旱性研究中的应用[J]. 植物生态学报, 2015, 39(4): 398-406. |
| [15] | 李志真, 刘东焕, 赵世伟, 姜闯道, 石雷. 环境强光诱导玉簪叶片光抑制的机制[J]. 植物生态学报, 2014, 38(7): 720-728. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19