

植物生态学报 ›› 2017, Vol. 41 ›› Issue (8): 914-924.DOI: 10.17521/cjpe.2016.0337
• 综述 • 上一篇
出版日期:2017-08-10
发布日期:2017-09-29
通讯作者:
张亚黎
作者简介:康璟瑶(1991-),男,江苏南京人,硕士生,主要从事旅游地理与旅游规划研究,E-mail:
基金资助:
Ji-Mei HAN1, Wang-Feng ZHANG1, Dong-Liang XIONG2, Jaume FLEXAS2, Ya-Li ZHANG1,*( )
)
Online:2017-08-10
Published:2017-09-29
Contact:
Ya-Li ZHANG
About author:KANG Jing-yao(1991-), E-mail:
摘要:
叶肉导度(gm)被用来衡量CO2从植物叶片气孔下腔到叶绿体羧化位点的传输效率, 其主要受解剖结构和生化因素的调控。近年来, gm的研究在光合作用领域受到普遍关注; 光合速率的限制因素已不再简单地划分为气孔限制和非气孔限制, 而需要从气孔限制、叶肉限制和羧化限制3个限制因素开展研究工作。该文分析了植物细胞壁、细胞膜、细胞质、叶绿体膜和叶绿体基质对gm的调控机制, 指出细胞壁厚度以及面向细胞间隙的叶绿体面积(Sc)是影响gm的重要结构因素。阐述了水孔蛋白和碳酸酐酶参与的生化过程对gm的调控机制。同时, 对外界环境因素, 如温度、光强、干旱、氮等对gm的调控机制进行了总结。在此基础上, 探讨了gm与水力导度的耦合关系。最后对gm研究中的科学问题进行了展望。
韩吉梅, 张旺锋, 熊栋梁, 张亚黎. 植物光合作用叶肉导度及主要限制因素研究进展. 植物生态学报, 2017, 41(8): 914-924. DOI: 10.17521/cjpe.2016.0337
Ji-Mei HAN, Wang-Feng ZHANG, Dong-Liang XIONG, Jaume FLEXAS, Ya-Li ZHANG. Mesophyll conductance and its limiting factors in plant leaves. Chinese Journal of Plant Ecology, 2017, 41(8): 914-924. DOI: 10.17521/cjpe.2016.0337
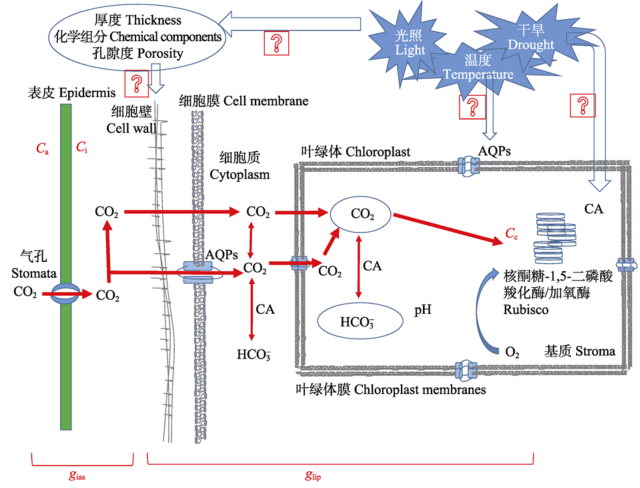
图1 CO2运输模式图。AQPs, 水孔蛋白; Ca, 大气CO2浓度; Ci, 胞间CO2浓度; CA, 碳酸酐酶; gias, 气相导度; glip, 液相导度。
Fig. 1 CO2 transport model. AQPs, aquaporins; Ca, the atmospheric CO2 concentration; Ci, intercellular CO2 concentration; CA, carbonic anhydrase; gias, the gas phase conductance; glip, the liquid phase conductance.
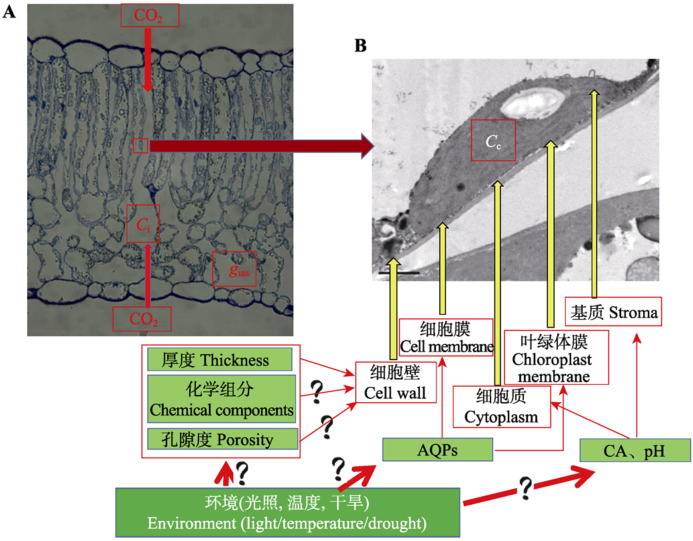
图2 gm反映的CO2扩散路径。A, 光学显微镜拍摄的棉花叶片解剖结构图,代表的是CO2从外界大气进入叶片细胞间隙,完成气相传输。B, 电子显微镜拍摄的棉花叶片超微结构图。代表的是CO2从细胞间隙进入叶绿体羧化位点所经过的部位,完成液相传输。图中简单介绍了影响传输路径的因素及需要进一步深入研究的问题。AQPs, 水孔蛋白; Ci, 胞间CO2浓度; Cc, 叶绿体羧化位点CO2浓度; CA, 碳酸酐酶, gias, 液相导度。
Fig. 2 The diffusion path of CO2 reflected by gm. A, The leaf anatomical structure in cotton by optical microscope, which represents the CO2 gas phase diffusion from the atmosphere into the leaf intercellular air layer; B, The leaf ultra-micro structure in cotton by electron microscope, which represents the CO2 liquid phase diffusion from intercellular into the chloroplast carboxylation site. AQPs, aquaporins; Ci, intercellular CO2 concentration; Cc, CO2 concentration at chloroplast carboxylation site; CA, carbonic anhydrase; gias, the gas phase conductance.
| CO2扩散方式 CO2 diffusion way | CO2运输形态 CO2 transportation form | 阻力来源 Resistance source | 动力来源 Power source | 对外界环境的响应时间 Response time to the external environment | |
|---|---|---|---|---|---|
| 细胞壁 Cell wall | 物理和生化方式 Physics and biochemical mode | CO2 | 厚度、孔隙度、果胶等组分 Thickness, porosity, pectin etc. | CO2浓度差 Difference of CO2 concentration | 最长 Longest |
| 细胞膜 Cell membrane | 物理和生化方式 Physics and biochemical mode | CO2 | 水孔蛋白、膜两侧pH差值 AQPs, the difference of pH on both sides of the membrane | CO2浓度差、跨膜蛋白主动运输 Difference of CO2 concentration, active transport of transmembrane protein | 较短 Shorter |
| 细胞液 Cytoplasm | 生化和物理方 Biochemical and physical mode | CO2, HCO3- | CA、pH、细胞液组分 CA, pH, cytosol component | pH、CA的催化 pH, catalysis of CA | 较短 Shorter |
| 叶绿体膜 Chloroplast membranes | 生化和物理方 Biochemical and physical mode | CO2 | 水孔蛋白、膜两侧CO2浓度差 AQPs, the difference of CO2 concentration on both sides of the membrane | 跨膜蛋白主动运输 Active transport of transmembrane protein | 较短 Shorter |
| 叶绿体基质 Stroma | 生化和物理方式 Biochemical and physical mode) | CO2, HCO3- | CA, pH | pH、CA的催化 pH, catalysis of CA | 最短 Shortest |
表1 CO2通过叶肉细胞中各超微组分的扩散方式、运输形态、阻力来源、动力来源和对外界环境的响应时间等的差异
Table 1 Diffusion way, transportation form, resistance source, power source when CO2 passes through the ultrastructure components of mesophyll cells and the different response time to the external environment
| CO2扩散方式 CO2 diffusion way | CO2运输形态 CO2 transportation form | 阻力来源 Resistance source | 动力来源 Power source | 对外界环境的响应时间 Response time to the external environment | |
|---|---|---|---|---|---|
| 细胞壁 Cell wall | 物理和生化方式 Physics and biochemical mode | CO2 | 厚度、孔隙度、果胶等组分 Thickness, porosity, pectin etc. | CO2浓度差 Difference of CO2 concentration | 最长 Longest |
| 细胞膜 Cell membrane | 物理和生化方式 Physics and biochemical mode | CO2 | 水孔蛋白、膜两侧pH差值 AQPs, the difference of pH on both sides of the membrane | CO2浓度差、跨膜蛋白主动运输 Difference of CO2 concentration, active transport of transmembrane protein | 较短 Shorter |
| 细胞液 Cytoplasm | 生化和物理方 Biochemical and physical mode | CO2, HCO3- | CA、pH、细胞液组分 CA, pH, cytosol component | pH、CA的催化 pH, catalysis of CA | 较短 Shorter |
| 叶绿体膜 Chloroplast membranes | 生化和物理方 Biochemical and physical mode | CO2 | 水孔蛋白、膜两侧CO2浓度差 AQPs, the difference of CO2 concentration on both sides of the membrane | 跨膜蛋白主动运输 Active transport of transmembrane protein | 较短 Shorter |
| 叶绿体基质 Stroma | 生化和物理方式 Biochemical and physical mode) | CO2, HCO3- | CA, pH | pH、CA的催化 pH, catalysis of CA | 最短 Shortest |
| [1] |
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002). Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesisin vivo. Plant Physiology, 130, 1992-1998.
DOI URL PMID |
| [2] |
Boex-Fontvieille E, Jossier M, Davanture M, Zivy M, Hodges M, Tcherkez G (2014). Differential protein phosphorylation regulates chloroplast movement in response to strong light and darkness inArabidopsis thaliana. Plant Molecular Biology Reporter, 32, 987-1001.
DOI URL |
| [3] |
Bongi G, Loreto F (1989). Gas-exchange properties of salt stressed olive (Olea europea L.) leaves.Plant Physiology, 90, 1408-1416.
DOI URL PMID |
| [4] |
Boron W, Endeward V, Gros G, Musa-Aziz R, Pohl P (2011). Intrinsic CO2 permeability of cell membranes and potential biological relevance of CO2 channels.Chemphyschem, 12, 1017-1019.
DOI URL PMID |
| [5] |
Burnell JN, Suzuki I, Sugiyama T (1990). Light induction and the effect of nitrogen status upon the activity of carbonic anhydrase in maize leaves.Plant Physiology, 94, 384-387.
DOI URL |
| [6] |
Clarkson DT, Carvajal M, Henzler T, Waterhouse RN, Smyth AJ, Cooke DT, Steudle E (2000). Root hydraulic conductance: Diurnal aquaporin expression and the effects of nutrient stress.Journal of Experimental Botany, 51, 61-70.
DOI URL PMID |
| [7] |
Cochard H, Nardini A, Coll L (2004). Hydraulic architecture of leafblades: Where is the main resistance?Plant, Cell & Environment, 27, 1257-1267.
DOI URL |
| [8] |
Diaz-Espejo A, Nicolás E, Fernández JE (2007). Seasonal evolution of diffusional limitations and photosynthetic capacity in olive under drought.Plant, Cell & Environment, 30, 922-933.
DOI URL PMID |
| [9] | Ethier GJ, Livingston NJ (2004). On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model.Plant, Cell & Environment, 27, 137-153. |
| [10] |
Evans JR, Kaldenhoff R, Genty B, Terashima I (2009). Resistances along the CO2 diffusion pathway inside leaves. Journal of Experimental Botany, 60, 2235-2248.
DOI URL PMID |
| [11] |
Evans JR, Shatrkey TD, Berry JA, Farquhar GD (1986). Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants.Australian Journal of Plant Physiology, 13, 281-292.
DOI URL |
| [12] |
Evans JR, von Caemmere S (1996). Carbon dioxide diffusion inside leaves.Plant Physiology, 110, 339-346.
DOI URL PMID |
| [13] |
Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994). The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco.Australian Journal of Plant Physiology, 21, 475-495.
DOI URL |
| [14] |
Farquhar GD, von Caemmerer S, Berry JA (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.Planta, 149, 78-90.
DOI URL |
| [15] |
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets ü, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012). Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Science, 193-194, 70-84.
DOI URL PMID |
| [16] |
Flexas J, Bota J, Cifre J, Escalona JM, Galmés J, Gulías J, Lefi EK, Martinez-Canellas SF, Moreno MT, Ribas-Carbo M (2004). Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Annal of Applied Biology, 144, 273-283.
DOI URL |
| [17] |
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H (2002). Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Functional Plant Biology, 29, 461-471.
DOI URL |
| [18] |
Flexas J, Diaz-Espejo A (2015). Interspecific differences in temperature response of mesophyll conductance: Food for thought on its origin and regulation.Plant, Cell & Environment, 38, 625-628.
DOI URL |
| [19] |
Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano HO, Ribas-Carbó M (2007). Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves.Plant, Cell & Environment, 30, 1284-1298.
DOI URL PMID |
| [20] |
Flexas J, Ribas-Carbó M, Bota J, Galmés J, Henkle M, Martínez-Ca?ellas S, Medrano H (2006a). Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration.New Phytologist, 172, 73-82.
DOI URL PMID |
| [21] | Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008). Mesophyll conductance to CO2: Current knowledge and future prospects.Plant, Cell & Environment, 31, 602-621. |
| [22] | Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R (2006b). Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo. The Plant Journal, 48, 427-439. |
| [23] |
Flexas J, Scoffoni C, Gago J, Sack L (2013). Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination.Journal of Experimental Botany, 64, 3965-3981.
DOI URL PMID |
| [24] |
Galmés J, Medrano H, Flexas J (2006). Acclimation of Rubisco specificity factor to drought in tobacco: Discrepancies between in vitro and in vivo estimations.Journal of Experimental Botany, 57, 3659-3667.
DOI URL PMID |
| [25] |
Galmés J, Medrano H, Flexas J (2007). Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytologist, 175, 81-93.
DOI URL |
| [26] |
Gillon JS, Yakir D (2000). Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves.Plant Physiology, 123, 201-213.
DOI URL |
| [27] |
Gu L, Sun Y (2013). Artefactual responses of mesophyll conductance to CO2 and irradiance estimated with the variable J and online isotope discrimination methods.Plant, Cell & Environment, 37, 1231-1249.
DOI URL PMID |
| [28] |
Han JM, Meng HF, Wang SY, Jiang CD, Liu F, Zhang WF, Zhang YL (2016). Variability of mesophyll conductance and its relationship with water use efficiency in cotton leaves under drought pretreatment.Journal of Plant Physiology, 194, 61-71.
DOI URL PMID |
| [29] | Hanba YT, Kogami H, Terashima I (2002). The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand.Plant, Cell & Environment, 25, 1021-1030. |
| [30] |
Hanba YT, Miyazawa SI, Terashima I (1999). The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm temperate forests.Functional Ecology, 13, 632-639.
DOI URL |
| [31] |
Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004). Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants.Plant Cell Physiology, 45, 521-529.
URL PMID |
| [32] |
Harley PC, Loreto F, Marco GD, Sharkey TD (1992). Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2.Plant Physiology, 98, 1429-1436.
DOI URL PMID |
| [33] |
Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR (2009). Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls.Journal of Experimental Botany, 60, 2303-2314.
DOI URL PMID |
| [34] |
Heckwolf M, Pater D, Hanson DT, Kaldenhoff R (2011). The Arabidopsis thaliana aquaporin AtPIP1;2 is a physiologically relevant CO2 transport facilitator.Plant Journal, 67, 795-804.
DOI URL PMID |
| [35] |
Hub JS, de Groot BL (2006). Does CO2 permeate through aquaporin-1?Biophysical Journal, 91, 842-848.
DOI URL PMID |
| [36] |
Hub JS, de Groot BL (2008). Mechanism of selectivity in aquaporins and aquaglyceroporins.Proceedings of the National Academy of Sciences of the United States of America, 105, 1198-1203.
DOI URL PMID |
| [37] |
Jia WS, Davies WJ (2007). Modification of leaf apoplastic pH in relation to stomatal sensitivity to root-sourced abscisic acid signals.Plant Physiology, 143, 68-77.
DOI URL PMID |
| [38] |
Kelly G, Sade N, Attia Z, Secchi F, Zwieniecki M, Holbrook NM, Levi A, Alchanatis V, Moshelion M, Granot D (2014). Relationship between hexokinase and the aquaporin PIP1 in the regulation of photosynthesis and plant growth.PLOS ONE, 9, e87888. doi:10.1371/journal.pone. 0087888.
DOI URL PMID |
| [39] |
Laisk A, Eichelmann H, Oja V, Rasulov B, Padu E, Bichele I, Pettai H, Kull O (2005). Adjustment of leaf photosynthesis to shade in a natural canopy: Rate parameters.Plant, Cell & Environment, 28, 375-388.
DOI URL |
| [40] |
Li Y, Gao YX, Xu XM, Shen QR, Guo SW (2009). Light- saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration.Journal of Experimental Botany, 60, 2351-2360.
DOI URL PMID |
| [41] |
Loreto F, Harley PC, Di Marco G, Sharkey TD (1992). Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiology, 98, 1437-1443.
DOI URL PMID |
| [42] |
Loreto F, Tsonev T, Centritto M (2009). The impact of blue light on leaf mesophyll conductance.Journal of Experimental Botany, 60, 2283-2290.
DOI URL PMID |
| [43] |
Makino A, Sakashita H, Hidema J, Mae T, Ojima K, Osmond B (1992). Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance.Plant Physiology, 100, 1737-1743.
DOI URL PMID |
| [44] |
Missner A, Kugler P, Antonenko YN, Pohl P (2008). Passive transport across bilayer lipid membranes: Overton continues to rule.Proceedings of the National Academy of Sciences of the United States of America, 1778, 2154-2156.
DOI URL |
| [45] | Miyazawa SI, Yoshimura S, Shinzaki Y, Maeshima M, Miyake C (2008). Relationship between mesophyll CO2 gas diffusion conductance and leaf plasma-membrane-type aquaporin contents in tobacco plants grown under drought conditions.Photosynthesis, 91, 805-808. |
| [46] |
Moualeu-Ngangue DP, Chen T-W, Stutzel H (2016). A new method to estimate photosynthetic parameters through net assimilation reteintercellular space CO2 concentration (A-Ci) curve and chlorophyll fluorescence measurements.New Phytologist, 213, 1543-1554.
DOI URL PMID |
| [47] |
Niinemets ü, Reichstein M (2003a). Controls on the emission of plant volatiles through stomata: A sensitivity analysis.Journal of Geophysical Research, 108, 4211. doi: 4210.1029/2002JD002626.
DOI URL |
| [48] |
Niinemets ü, Reichstein M (2003b). Controls on the emission of plant volatiles through stomata: Sensitivity or insensitivity of the emission rates to stomatal closure explained.Journal of Geophysical Research, 108, 4208. doi: 4210.1029/2002JD002620.
DOI URL |
| [49] |
Niinemets ü, Diaz-Espejo A, Flexas J, Galmés J, Warren CR (2009). Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field.Journal of Experimental Botany, 60, 2271-2282.
DOI URL |
| [50] |
Otto B, Uehlein N, Sdorra S, Fischer M, Ayaz M, Belastegui- Macadam X, Heckwolf M, Lachnit M, Pede N, Priem N (2010). Aquaporin tetramer composition modifies the function of tobacco aquaporins.Journal of Biological Chemistry, 285, 31253-31260.
DOI URL |
| [51] |
Pakatas A, Stavrakas D, Fisarakis I (2003). Relationship between CO2 assimilation and leafanatomical characteristics of two grapevine cultivars.Agronomie, 23, 293-296.
DOI URL |
| [52] |
Perez-Martin A, Michelazzo C, Torres-Ruiz JM, Flexas J, Fernández JE, Sebastiani L, Diaz-Espejo A (2014). Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins.Journal of Experimental Botany, 65, 3143-3156.
DOI URL |
| [53] |
Piel C, Frak E, Le Roux X, Genty B (2002). Effect of local irradiance on CO2 transfer conductance of mesophyll in walnut.Journal of Experimental Botany, 53, 2423-2430.
DOI URL PMID |
| [54] |
Price DG, von Caemmerer S, Evans JR, Yu JW, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger M (1994). Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation.Planta, 193, 331-340.
DOI URL |
| [55] |
Sack L, Holbrook NM (2006). Leaf hydraulics.Annual Review of Plant Biology, 57, 361-381.
DOI URL |
| [56] |
Sack L, Streeter CM, Holbrook NM (2004). Hydraulic analysis of water flow through leaves of sugar maple and red oak.Plant Physiology, 134, 1824-1833.
DOI URL PMID |
| [57] |
Sade N, Gallé A, Flexas J, Lerner S, Peleg G, Yaaran A, Moshelion M (2014). Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions.Planta, 239, 357-366.
DOI URL |
| [58] |
Sade N, Gebretsadik M, Seligmann R, Schwartz A, Wallach R, Moshelion M (2010). The Role of tobacco aquaporin 1 in improving water use efficiency, hydraulic conductivity, and yield production under salt stress.Plant Physiology, 152, 245-254.
DOI URL PMID |
| [59] |
Sage TL, Sage RF (2009). The functional anatomy of rice leaves: Implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice.Plant Cell Physiology, 50, 756-772.
DOI URL PMID |
| [60] |
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves.Plant, Cell & Environment, 30, 1035-1040.
DOI URL PMID |
| [61] | Syvertsen JP, Lloyd J, Meconchie C, Kriedbmann PE, Farquhar GD (1995). On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves.Plant Cell Physiology, 18, 149-157. |
| [62] |
Terashima I, Araya T, Miyazawa S-I, Sone K, Yano S (2005). Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: An eco-developmental treatise.Annals of Botany, 95, 507-519.
DOI URL PMID |
| [63] |
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006). Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. Journal of Experimental Botany, 57, 343-354.
DOI URL PMID |
| [64] |
Terashima I, Hanba YT, Tholen D, Niinemets U (2011). Leaf functional anatomy in relation to photosynthesis.Plant Physiology, 155, 108-116.
DOI URL PMID |
| [65] | Terashima I, Hikosaka K (1995). Comparative ecophysiology/ anatomy of leaf and canopy photosynthesis.Plant, Cell & Environment, 18, 1111-1128. |
| [66] |
Terashima I, Ono K (2002). Effects of HgCl2 on CO2 dependence of leaf photosynthesis: Evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane.Plant Cell Physiology, 43, 70-78.
URL PMID |
| [67] |
Théroux-Rancourt G, Gilbert ME (2017). The light response of mesophyll conductance is controlled by structure across leaf profiles. Plant, Cell & Environment, 40, 726-740.
DOI URL PMID |
| [68] |
Tholen D, Boom C, Noguchi K, Ueda S, Katase T, Terashima I (2008). The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell & Environment, 31, 1688-1700.
DOI URL PMID |
| [69] |
Tholen D, Ethier G, Genty B, Pepin S, Zhu XG (2012). Variable mesophyll conductance revisited: Theoretical background and experimental implications.Plant, Cell & Environment, 35, 2087-2103.
DOI URL PMID |
| [70] |
Tholen D, Zhu XG (2011). The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiology, 156, 90-105.
DOI URL |
| [71] |
Tomás M, Flexas J, Copolovici L, Galmes J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets ü (2013). Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models.Journal of Experimental Botany, 64, 2269-2281.
DOI URL |
| [72] |
Tomás M, Medrano H, Brugnoli E, Escalona JM, Martorell S, Pou A, Ribas-Carbó M, Flexas J (2014). Variability of mesophyll conductance in grapevine cultivars under water stress conditions in relation to leaf anatomy and water use efficiency.Australian Journal of Grape and Wine Research, 20, 272-280.
DOI URL |
| [73] |
Tosens T, Niinemets ü, Vislap V, Eichelmann H, Castro Diez P (2012a). Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: How structure constrains function.Plant, Cell & Environment, 35, 839-856.
DOI URL PMID |
| [74] |
Tosens T, Niinemets ü, Westoby M, Wright IJ (2012b). Anatomicalbasis of variation in mesophyll resistance in eastern Australian sclerophylls: News of a long and winding path.Journal of Experimental Botany, 63, 5105-5119.
DOI URL PMID |
| [75] |
Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R (2008). Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell, 20, 648-657.
DOI URL |
| [76] |
von Caemmerer S, Evans JR (1991). Determination of the average partial pressure of CO2 in chloroplast from leaves of several C3 plants.Australian Journal of Plant Physiology, 18, 287-305.
DOI URL |
| [77] |
Warren CR (2004). The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply.Journal of Experimental Botany, 55, 2313-2321.
DOI URL PMID |
| [78] |
Warren CR (2008). Stand aside stomata, another actor deserves centre stage: The forgotten role of the internal conductance to CO2 transfer.Journal of Experimental Botany, 59, 1475-1487.
DOI URL PMID |
| [79] |
Warren CR, Low M, Matyssek R, Tausz M (2007). Internal conductance to CO2 transfer of adult Fagus sylvatica: Variation between sun and shade leaves and due to free-air ozone fumigation.Environmental and Experimental Botany, 59, 130-138.
DOI URL |
| [80] |
Williams TG, Flanagan LB, Coleman JR (1996). Photosynthetic gas exchange and discrimination against 13CO2, and C18O16O in tobacco plants modified by an antisense construct to have low chloroplastic carbonic anhydrase.Plant Physiology, 112, 319-326.
DOI URL PMID |
| [81] |
Xiong D, Flexas J, Yu T, Peng S, Huang J (2016). Leaf anatomy mediates coordination of leaf hydraulic conductance and mesophyll conductance to CO2 inOryza. New Phytologist, 213, 572-583.
DOI URL PMID |
| [82] | Xiong D, Liu X, Liu L, Douthe C, Li Y, Peng S, Huang J (2015b). Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice.Plant, Cell & Environment, 38, 2541-2550. |
| [83] |
Xiong D, Yu T, Zhang T, Li Y, Peng S, Huang J (2015a). Leaf hydraulic conductance is coordinated with leaf morpho- anatomical traits and nitrogen status in the genusOryza. Journal of Experimental Botany, 66, 741-748.
DOI URL PMID |
| [84] |
Yamori W, Nagai T, Makino A (2011). The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species.Plant, Cell & Environment, 34, 764-777.
DOI URL PMID |
| [85] |
Yamori W, Noguchi K, Hanba YT, Terashima I (2006). Effects of internal conductance on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures.Plant Cell Physiology, 47, 1069-1080.
DOI URL PMID |
| [1] | 萨其拉, 张霞, 朱琳, 康萨如拉. 长期不同放牧强度下荒漠草原优势种无芒隐子草叶片解剖结构变化[J]. 植物生态学报, 2024, 48(3): 331-340. |
| [2] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [3] | 蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用[J]. 植物生态学报, 2023, 47(7): 988-997. |
| [4] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [5] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [6] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [7] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [8] | 叶子飘, 于冯, 安婷, 王复标, 康华靖. 植物气孔导度对CO2响应模型的构建[J]. 植物生态学报, 2021, 45(4): 420-428. |
| [9] | 吴建波, 王小丹. 高寒草原优势种紫花针茅叶片解剖结构对青藏高原高寒干旱环境适应性分析[J]. 植物生态学报, 2021, 45(3): 265-273. |
| [10] | 李景, 王欣, 王振华, 王斌, 王成章, 邓美凤, 刘玲莉. 臭氧和气溶胶复合污染对杨树叶片光合作用的影响[J]. 植物生态学报, 2020, 44(8): 854-863. |
| [11] | 纪若璇, 于笑, 常远, 沈超, 白雪卡, 夏新莉, 尹伟伦, 刘超. 蒙古莸叶片解剖结构的地理种源变异及其对环境变化响应的意义[J]. 植物生态学报, 2020, 44(3): 277-286. |
| [12] | 李旭, 吴婷, 程严, 谭钠丹, 蒋芬, 刘世忠, 褚国伟, 孟泽, 刘菊秀. 南亚热带常绿阔叶林4个树种对增温的生理生态适应能力比较[J]. 植物生态学报, 2020, 44(12): 1203-1214. |
| [13] | 刘校铭, 杨晓芳, 王璇, 张守仁. 暖温带落叶阔叶林辽东栎和五角枫生长和光合生理生态特征对模拟氮沉降的响应[J]. 植物生态学报, 2019, 43(3): 197-207. |
| [14] | 李鑫豪, 闫慧娟, 卫腾宙, 周文君, 贾昕, 查天山. 油蒿资源利用效率在生长季的相对变化及对环境因子的响应[J]. 植物生态学报, 2019, 43(10): 889-898. |
| [15] | 张娜, 朱阳春, 李志强, 卢信, 范如芹, 刘丽珠, 童非, 陈静, 穆春生, 张振华. 淹水和干旱生境下铅对芦苇生长、生物量分配和光合作用的影响[J]. 植物生态学报, 2018, 42(2): 229-239. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19