

Chin J Plant Ecol ›› 2014, Vol. 38 ›› Issue (4): 311-324.DOI: 10.3724/SP.J.1258.2014.00028
SUN Shan-Wen1,2, ZHANG Yong-Jiang3, CAO Kun-Fang4,*( )
)
Received:2013-12-13
Accepted:2014-02-08
Online:2014-12-13
Published:2014-04-08
Contact:
CAO Kun-Fang
SUN Shan-Wen, ZHANG Yong-Jiang, CAO Kun-Fang. Correlations among leaf structure, drought tolerance and photosynthetic capacity in saplings of Euphorbiaceae from different micro-habitats in a seasonal tropical rainforest[J]. Chin J Plant Ecol, 2014, 38(4): 311-324.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.3724/SP.J.1258.2014.00028
| 种 Species | 分布 Distribution | 丰度 Abundance (Ind.·hm-2) |
|---|---|---|
| 长梗三宝木 Trigonostemon thyrsoideus | 沟谷 Valley | 40.45 |
| 粉绿野桐 Mallotus garrettii | 沟谷 Valley | 34.15 |
| 勐腊核果木 Drypetes hoaensis | 沟谷 Valley | 28.35 |
| 棒柄花 Cleidion brevipetiolatum | 沟谷 Valley | 48.55 |
| 秋枫 Bischofia javanica | 沟谷 Valley | 1.65 |
| 缅桐 Sumbaviopsis albicans | 山坡 Slope | 23.00 |
| 轮叶戟 Lasiococca comberi | 山坡 Slope | 9.20 |
| 风轮桐 Epiprinus siletianus | 山坡 Slope | 6.20 |
| 网脉核果木 Drypetes perreticulata | 山坡 Slope | 1.50 |
| 土蜜树 Bridelia tomentosa | 山坡 Slope | 1.05 |
| 木奶果 Baccaurea ramilflora | 遍及整个样地 Throughout the entire sample plot | 160.60 |
| 山地五月茶 Antidesma montanum | 遍及整个样地 Throughout the entire sample plot | 22.75 |
| 日本五月茶 Antidesma japonicum | 遍及整个样地 Throughout the entire sample plot | 13.75 |
| 尾叶血桐 Macaranga kurzii | 山脊 Ridge | 0.40 |
| 越南巴豆 Croton kongensis | 山脊 Ridge | 7.35 |
| 银背巴豆 Croton argyratus | 山脊 Ridge | 3.00 |
| 椴叶山麻杆 Alchornea tiliifolia | 山脊 Ridge | 18.75 |
| 云南银柴 Aporusa yunnanensis | 山脊 Ridge | 26.40 |
Table 1 A list of names, distributions, and abundance of the 18 plant species of the Euphobiaceae within study plot
| 种 Species | 分布 Distribution | 丰度 Abundance (Ind.·hm-2) |
|---|---|---|
| 长梗三宝木 Trigonostemon thyrsoideus | 沟谷 Valley | 40.45 |
| 粉绿野桐 Mallotus garrettii | 沟谷 Valley | 34.15 |
| 勐腊核果木 Drypetes hoaensis | 沟谷 Valley | 28.35 |
| 棒柄花 Cleidion brevipetiolatum | 沟谷 Valley | 48.55 |
| 秋枫 Bischofia javanica | 沟谷 Valley | 1.65 |
| 缅桐 Sumbaviopsis albicans | 山坡 Slope | 23.00 |
| 轮叶戟 Lasiococca comberi | 山坡 Slope | 9.20 |
| 风轮桐 Epiprinus siletianus | 山坡 Slope | 6.20 |
| 网脉核果木 Drypetes perreticulata | 山坡 Slope | 1.50 |
| 土蜜树 Bridelia tomentosa | 山坡 Slope | 1.05 |
| 木奶果 Baccaurea ramilflora | 遍及整个样地 Throughout the entire sample plot | 160.60 |
| 山地五月茶 Antidesma montanum | 遍及整个样地 Throughout the entire sample plot | 22.75 |
| 日本五月茶 Antidesma japonicum | 遍及整个样地 Throughout the entire sample plot | 13.75 |
| 尾叶血桐 Macaranga kurzii | 山脊 Ridge | 0.40 |
| 越南巴豆 Croton kongensis | 山脊 Ridge | 7.35 |
| 银背巴豆 Croton argyratus | 山脊 Ridge | 3.00 |
| 椴叶山麻杆 Alchornea tiliifolia | 山脊 Ridge | 18.75 |
| 云南银柴 Aporusa yunnanensis | 山脊 Ridge | 26.40 |
| 性状 Trait | 山脊 Ridge | 山坡 Slope | 沟谷 Valley | 广布种 Cosmopolitan species |
|---|---|---|---|---|
| SWC (%) | 2.660 ± 0.840ab | 1.860 ± 0.330a | 3.280 ± 1.110ab | 4.260 ± 0.770b |
| πo (MPa) | -1.670 ± 0.180a | -1.740 ± 0.250a | -1.190 ± 0.220b | -1.190 ± 0.040b |
| πtlp (MPa) | -1.950 ± 0.180a | -1.990 ± 0.220a | -1.400 ± 0.270b | -1.400 ± 0.020b |
| ε (MPa) | 15.21 ± 6.540a | 22.32 ± 7.480a | 18.70 ± 6.300a | 11.88 ± 2.190a |
| RWCtlp (%) | 84.62 ± 5.280a | 90.70 ± 4.600ab | 92.61 ± 3.560b | 86.99 ± 0.810ab |
| LT (mm) | 0.670 ± 0.380a | 0.540 ± 0.140a | 0.900 ± 0.300a | 1.100 ± 0.460a |
| UET (mm) | 0.069 ± 0.036a | 0.061 ± 0.010a | 0.083 ± 0.017a | 0.164 ± 0.053b |
| PT (mm) | 0.240 ± 0.130a | 0.150 ± 0.060a | 0.190 ± 0.090a | 0.280 ± 0.060a |
| ST (mm) | 0.300 ± 0.230a | 0.260 ± 0.100a | 0.540 ± 0.220a | 0.550 ± 0.340a |
| LET (mm) | 0.063 ± 0.022a | 0.063 ± 0.015a | 0.080 ± 0.018a | 0.101 ± 0.023a |
| P/S (%) | 1.214 ± 0.611a | 0.670 ± 0.345a | 0.432 ± 0.270a | 0.618 ± 0.208a |
| LD (g·cm-3) | 940.4 ± 391.2ab | 1 175.0 ± 385.5a | 514.2 ± 94.2b | 529.5 ± 171.1ab |
| LMA (g·cm-2) | 51.36 ± 12.810a | 59.56 ± 14.080a | 44.91 ± 14.750a | 53.49 ± 6.640a |
| Aa (μmol·m-2·s-1) | 10.380 ± 2.310a | 8.400 ± 3.410a | 7.690 ± 1.520a | 7.710 ± 0.750a |
| Am (nmol·g-1·s-1) | 0.210 ± 0.150a | 0.160 ± 0.110a | 0.190 ± 0.110a | 0.150 ± 0.030a |
| R (μmol·m-2·s-1) | 0.600 ± 0.074a | 0.470 ± 0.074b | 0.500 ± 0.064ab | 0.480 ± 0.046ab |
| Rm (nmol·g-1·s-1) | 0.012 ± 0.003a | 0.008 ± 0.003a | 0.012 ± 0.006a | 0.009 ± 0.001a |
Table 2 Traits values of the Euphobiaceae plants in different habitats and summary of ANOVA analysis (mean ± SD)
| 性状 Trait | 山脊 Ridge | 山坡 Slope | 沟谷 Valley | 广布种 Cosmopolitan species |
|---|---|---|---|---|
| SWC (%) | 2.660 ± 0.840ab | 1.860 ± 0.330a | 3.280 ± 1.110ab | 4.260 ± 0.770b |
| πo (MPa) | -1.670 ± 0.180a | -1.740 ± 0.250a | -1.190 ± 0.220b | -1.190 ± 0.040b |
| πtlp (MPa) | -1.950 ± 0.180a | -1.990 ± 0.220a | -1.400 ± 0.270b | -1.400 ± 0.020b |
| ε (MPa) | 15.21 ± 6.540a | 22.32 ± 7.480a | 18.70 ± 6.300a | 11.88 ± 2.190a |
| RWCtlp (%) | 84.62 ± 5.280a | 90.70 ± 4.600ab | 92.61 ± 3.560b | 86.99 ± 0.810ab |
| LT (mm) | 0.670 ± 0.380a | 0.540 ± 0.140a | 0.900 ± 0.300a | 1.100 ± 0.460a |
| UET (mm) | 0.069 ± 0.036a | 0.061 ± 0.010a | 0.083 ± 0.017a | 0.164 ± 0.053b |
| PT (mm) | 0.240 ± 0.130a | 0.150 ± 0.060a | 0.190 ± 0.090a | 0.280 ± 0.060a |
| ST (mm) | 0.300 ± 0.230a | 0.260 ± 0.100a | 0.540 ± 0.220a | 0.550 ± 0.340a |
| LET (mm) | 0.063 ± 0.022a | 0.063 ± 0.015a | 0.080 ± 0.018a | 0.101 ± 0.023a |
| P/S (%) | 1.214 ± 0.611a | 0.670 ± 0.345a | 0.432 ± 0.270a | 0.618 ± 0.208a |
| LD (g·cm-3) | 940.4 ± 391.2ab | 1 175.0 ± 385.5a | 514.2 ± 94.2b | 529.5 ± 171.1ab |
| LMA (g·cm-2) | 51.36 ± 12.810a | 59.56 ± 14.080a | 44.91 ± 14.750a | 53.49 ± 6.640a |
| Aa (μmol·m-2·s-1) | 10.380 ± 2.310a | 8.400 ± 3.410a | 7.690 ± 1.520a | 7.710 ± 0.750a |
| Am (nmol·g-1·s-1) | 0.210 ± 0.150a | 0.160 ± 0.110a | 0.190 ± 0.110a | 0.150 ± 0.030a |
| R (μmol·m-2·s-1) | 0.600 ± 0.074a | 0.470 ± 0.074b | 0.500 ± 0.064ab | 0.480 ± 0.046ab |
| Rm (nmol·g-1·s-1) | 0.012 ± 0.003a | 0.008 ± 0.003a | 0.012 ± 0.006a | 0.009 ± 0.001a |
| SWC | πo | πtlp | ε | RWCtlp | LT | UET | PT | ST | LET | P/S | LD | LMA | Aa | Am | R | Rm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWC | 1 | 0.81*** | 0.82*** | -0.25 | 0.07 | 0.62** | 0.61** | -0.05 | 0.70** | 0.60* | -0.81*** | -0.71*** | -0.44 | 0.25 | 0.51* | 0.19 | 0.57* |
| πo | 0.70** | 1 | 0.98*** | -0.25 | 0.20 | 0.59* | 0.52* | -0.23 | 0.74*** | 0.37 | -0.76*** | -0.71*** | -0.86*** | 0.27 | 0.63** | -0.11 | 0.66** |
| πtlp | 0.77*** | 0.98*** | 1 | -0.20 | 0.28 | 0.55* | 0.53* | -0.24 | 0.69** | 0.41 | -0.82*** | -0.70** | -0.79*** | 0.18 | 0.57** | -0.17 | 0.61** |
| ε | -0.47 | -0.68** | -0.57* | 1 | 0.81*** | -0.65** | -0.45 | 0.01 | -0.74*** | -0.44 | 0.43 | 0.24 | 0.66** | -0.65** | -0.74*** | -0.37 | -0.69** |
| RWCtlp | -0.05 | -0.26 | -0.10 | 0.77*** | 1 | -0.31 | -0.18 | -0.05 | -0.34 | -0.06 | -0.12 | -0.13 | 0.47 | -0.56* | -0.54* | -0.44 | -0.52* |
| LT | 0.64** | 0.60** | 0.60** | -0.13 | 0.25 | 1 | 0.84*** | 0.74*** | 0.95*** | 0.91*** | -0.56* | -0.78*** | 0.33 | 0.01 | -0.25 | -0.03 | -0.33 |
| UET | 0.66** | 0.55* | 0.56* | -0.31 | 0 | 0.71** | 1 | 0.71*** | 0.69** | 0.85*** | -0.45 | -0.62** | 0.23 | -0.06 | -0.23 | -0.10 | -0.28 |
| PT | 0.65** | 0.42 | 0.43 | -0.23 | -0.06 | 0.51* | 0.27 | 1 | 0.49* | 0.69** | 0.20 | -0.61** | 0.23 | 0.40 | 0.05 | 0.36 | -0.08 |
| ST | 0.50* | 0.56* | 0.55* | -0.03 | 0.37 | 0.95*** | 0.66** | 0.23 | 1 | 0.82*** | -0.70** | -0.73*** | 0.32 | -0.13 | -0.33 | -0.17 | -0.37 |
| LET | 0.54* | 0.52* | 0.54* | -0.20 | 0.20 | 0.83*** | 0.63** | 0.60* | 0.71** | 1 | -0.50* | -0.76*** | 0.34 | -0.03 | -0.25 | -0.16 | -0.38 |
| P/S | -0.21 | -0.37 | -0.37 | -0.37 | -0.68** | -0.54* | -0.30 | -0.00 | -0.70** | -0.57* | 1 | 0.69** | 0.44 | -0.21 | -0.45 | 0.06 | -0.41 |
| LD | -0.70** | -0.89*** | -0.87*** | 0.75*** | 0.36 | -0.75** | -0.56* | -0.10 | -0.81*** | -0.60* | 0.48* | 1 | 0.76*** | -0.53* | -0.81*** | -0.23 | -0.82*** |
| LMA | -0.16 | -0.29 | -0.27 | 0.31 | 0.33 | -0.29 | -0.24 | 0.40 | -0.47 | 0 | -0.37 | 0.14 | 1 | -0.22 | -0.77*** | 0.16 | -0.75** |
| Aa | 0.16 | 0 | -0.04 | -0.39 | -0.42 | 0.69** | 0.22 | 0.58* | 0.61** | 0.54* | 0.35 | -0.20 | -0.28 | 1 | 0.77*** | 0.76*** | 0.44 |
| Am | 0.14 | 0.10 | 0.10 | -0.37 | -0.37 | 0.60* | 0.27 | 0.18 | 0.62** | 0.40 | 0.44 | -0.18 | -0.68** | 0.80*** | 1 | 0.49* | 0.87*** |
| R | 0.20 | -0.08 | -0.14 | -0.41 | -0.56* | 0.53* | 0.10 | 0.70** | 0.38 | 0.52* | 0.48* | -0.18 | -0.24 | 0.59** | 0.52* | 1 | -0.62** |
| Rm | 0.16 | 0.12 | 0.12 | -0.36 | -0.40 | 0.50* | 0.25 | 0.04 | 0.56* | 0.30 | 0.49* | -0.15 | -0.86*** | 0.58* | 0.92*** | -0.45 | 1 |
Table 3 Correlations among leaf traits in plant species of the Euphobiaceae. The lower left corner shows conventional Pearson correlation, and the upper right corner shows correlations given by the phylogenetic independent contrasts analysis
| SWC | πo | πtlp | ε | RWCtlp | LT | UET | PT | ST | LET | P/S | LD | LMA | Aa | Am | R | Rm | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWC | 1 | 0.81*** | 0.82*** | -0.25 | 0.07 | 0.62** | 0.61** | -0.05 | 0.70** | 0.60* | -0.81*** | -0.71*** | -0.44 | 0.25 | 0.51* | 0.19 | 0.57* |
| πo | 0.70** | 1 | 0.98*** | -0.25 | 0.20 | 0.59* | 0.52* | -0.23 | 0.74*** | 0.37 | -0.76*** | -0.71*** | -0.86*** | 0.27 | 0.63** | -0.11 | 0.66** |
| πtlp | 0.77*** | 0.98*** | 1 | -0.20 | 0.28 | 0.55* | 0.53* | -0.24 | 0.69** | 0.41 | -0.82*** | -0.70** | -0.79*** | 0.18 | 0.57** | -0.17 | 0.61** |
| ε | -0.47 | -0.68** | -0.57* | 1 | 0.81*** | -0.65** | -0.45 | 0.01 | -0.74*** | -0.44 | 0.43 | 0.24 | 0.66** | -0.65** | -0.74*** | -0.37 | -0.69** |
| RWCtlp | -0.05 | -0.26 | -0.10 | 0.77*** | 1 | -0.31 | -0.18 | -0.05 | -0.34 | -0.06 | -0.12 | -0.13 | 0.47 | -0.56* | -0.54* | -0.44 | -0.52* |
| LT | 0.64** | 0.60** | 0.60** | -0.13 | 0.25 | 1 | 0.84*** | 0.74*** | 0.95*** | 0.91*** | -0.56* | -0.78*** | 0.33 | 0.01 | -0.25 | -0.03 | -0.33 |
| UET | 0.66** | 0.55* | 0.56* | -0.31 | 0 | 0.71** | 1 | 0.71*** | 0.69** | 0.85*** | -0.45 | -0.62** | 0.23 | -0.06 | -0.23 | -0.10 | -0.28 |
| PT | 0.65** | 0.42 | 0.43 | -0.23 | -0.06 | 0.51* | 0.27 | 1 | 0.49* | 0.69** | 0.20 | -0.61** | 0.23 | 0.40 | 0.05 | 0.36 | -0.08 |
| ST | 0.50* | 0.56* | 0.55* | -0.03 | 0.37 | 0.95*** | 0.66** | 0.23 | 1 | 0.82*** | -0.70** | -0.73*** | 0.32 | -0.13 | -0.33 | -0.17 | -0.37 |
| LET | 0.54* | 0.52* | 0.54* | -0.20 | 0.20 | 0.83*** | 0.63** | 0.60* | 0.71** | 1 | -0.50* | -0.76*** | 0.34 | -0.03 | -0.25 | -0.16 | -0.38 |
| P/S | -0.21 | -0.37 | -0.37 | -0.37 | -0.68** | -0.54* | -0.30 | -0.00 | -0.70** | -0.57* | 1 | 0.69** | 0.44 | -0.21 | -0.45 | 0.06 | -0.41 |
| LD | -0.70** | -0.89*** | -0.87*** | 0.75*** | 0.36 | -0.75** | -0.56* | -0.10 | -0.81*** | -0.60* | 0.48* | 1 | 0.76*** | -0.53* | -0.81*** | -0.23 | -0.82*** |
| LMA | -0.16 | -0.29 | -0.27 | 0.31 | 0.33 | -0.29 | -0.24 | 0.40 | -0.47 | 0 | -0.37 | 0.14 | 1 | -0.22 | -0.77*** | 0.16 | -0.75** |
| Aa | 0.16 | 0 | -0.04 | -0.39 | -0.42 | 0.69** | 0.22 | 0.58* | 0.61** | 0.54* | 0.35 | -0.20 | -0.28 | 1 | 0.77*** | 0.76*** | 0.44 |
| Am | 0.14 | 0.10 | 0.10 | -0.37 | -0.37 | 0.60* | 0.27 | 0.18 | 0.62** | 0.40 | 0.44 | -0.18 | -0.68** | 0.80*** | 1 | 0.49* | 0.87*** |
| R | 0.20 | -0.08 | -0.14 | -0.41 | -0.56* | 0.53* | 0.10 | 0.70** | 0.38 | 0.52* | 0.48* | -0.18 | -0.24 | 0.59** | 0.52* | 1 | -0.62** |
| Rm | 0.16 | 0.12 | 0.12 | -0.36 | -0.40 | 0.50* | 0.25 | 0.04 | 0.56* | 0.30 | 0.49* | -0.15 | -0.86*** | 0.58* | 0.92*** | -0.45 | 1 |
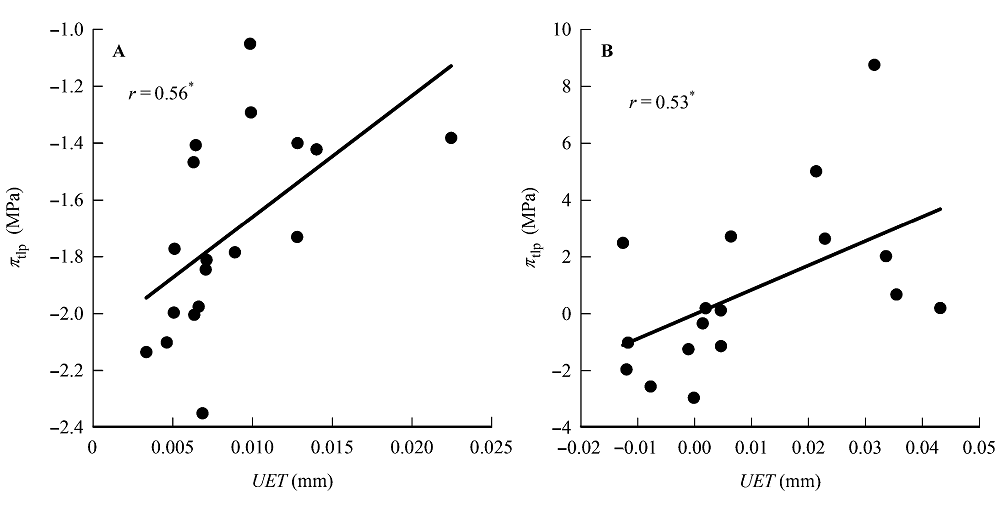
Fig. 1 Correlations between upper epidermis thickness (UET) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. *, 0.01 < p < 0.05.
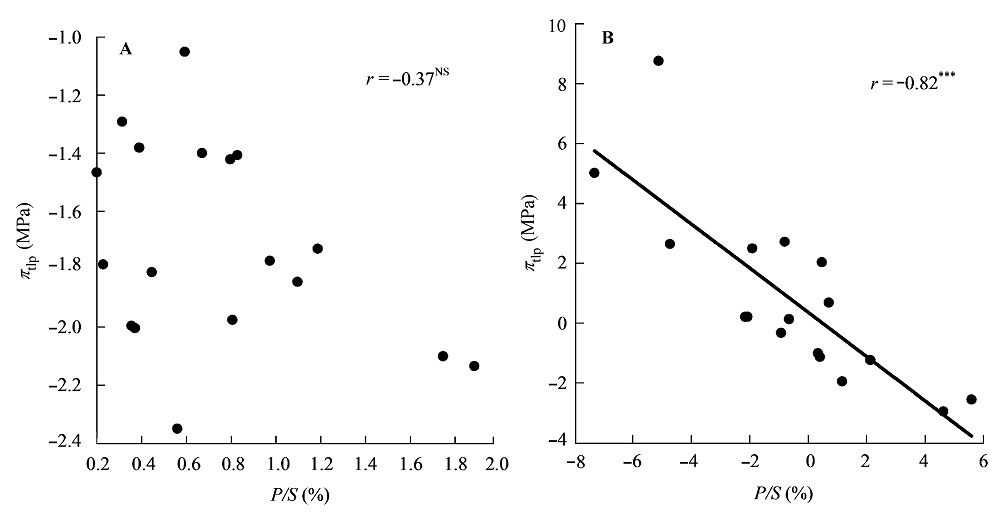
Fig. 2 Correlations between the palisade thickness/spongy thickness (P/S) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. ***, p < 0.001; NS, p > 0.05.
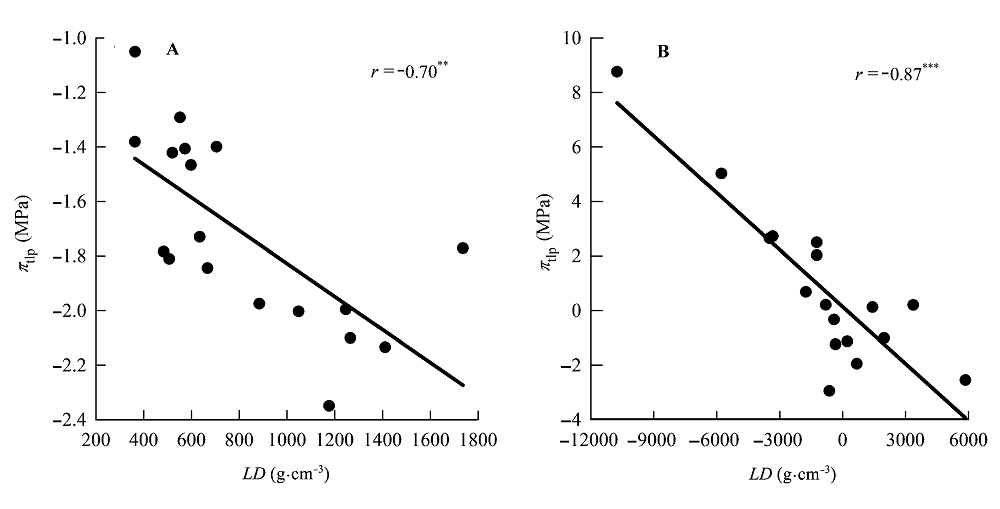
Fig. 3 Correlations between leaf density (LD) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. **, 0.001 < p < 0.01; ***, p < 0.001.
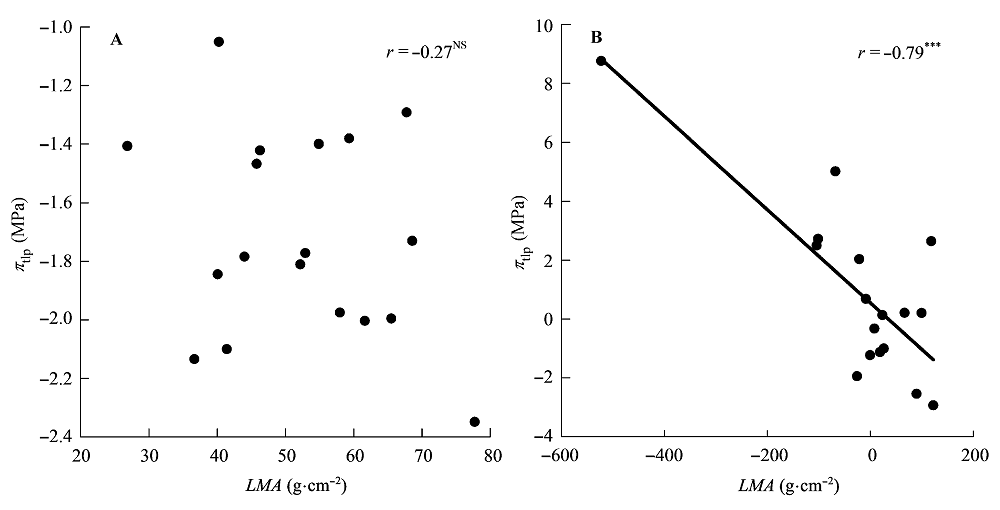
Fig. 4 Correlations between leaf mass per area (LMA) and water potential at turgor loss point (πtlp). A, Traditional Pearson correlation. B, Correlation given by the phylogenetic independent contrasts analysis. ***, p < 0.001; NS, p > 0.05.
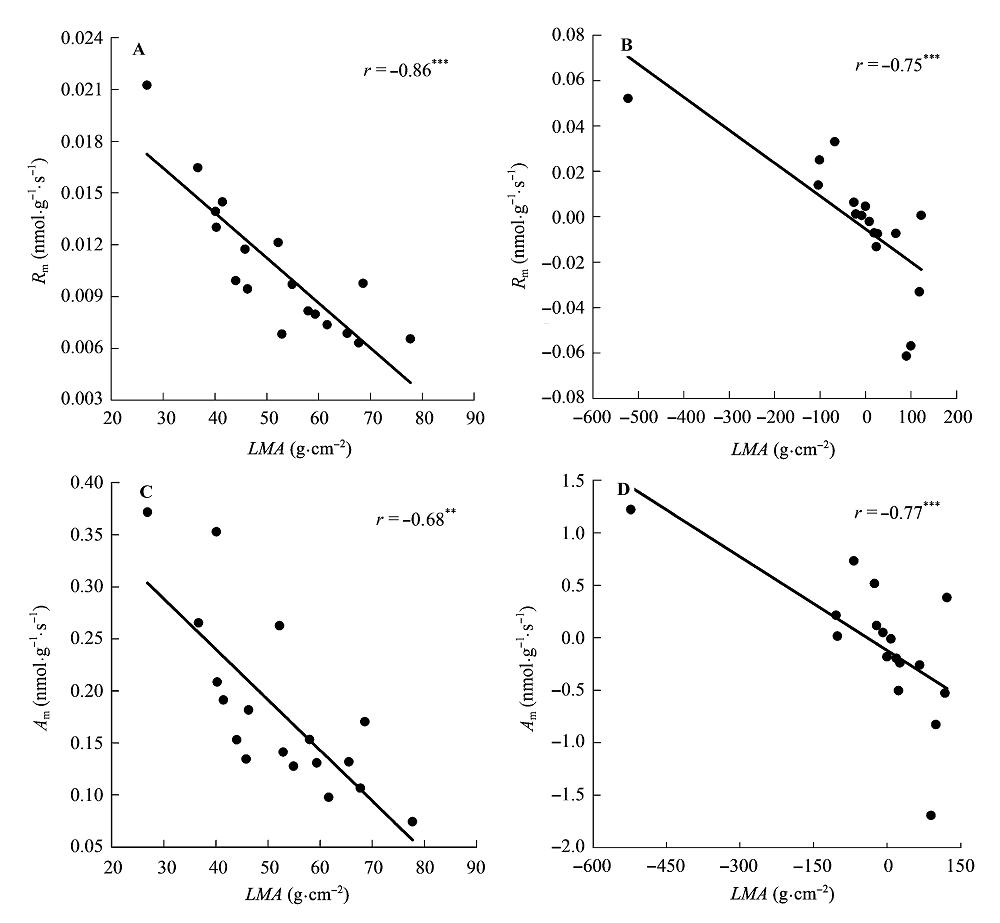
Fig. 5 Correlations of leaf mass per area (LMA) with maximum photosynthesis per leaf dry mass (Am) and dark respiration per leaf dry mass (Rm). A, B, Traditional Pearson correlation. C, D, Correlation given by the phylogenetic independent contrasts analysis. **, 0.001 < p < 0.01; ***, p < 0.001.
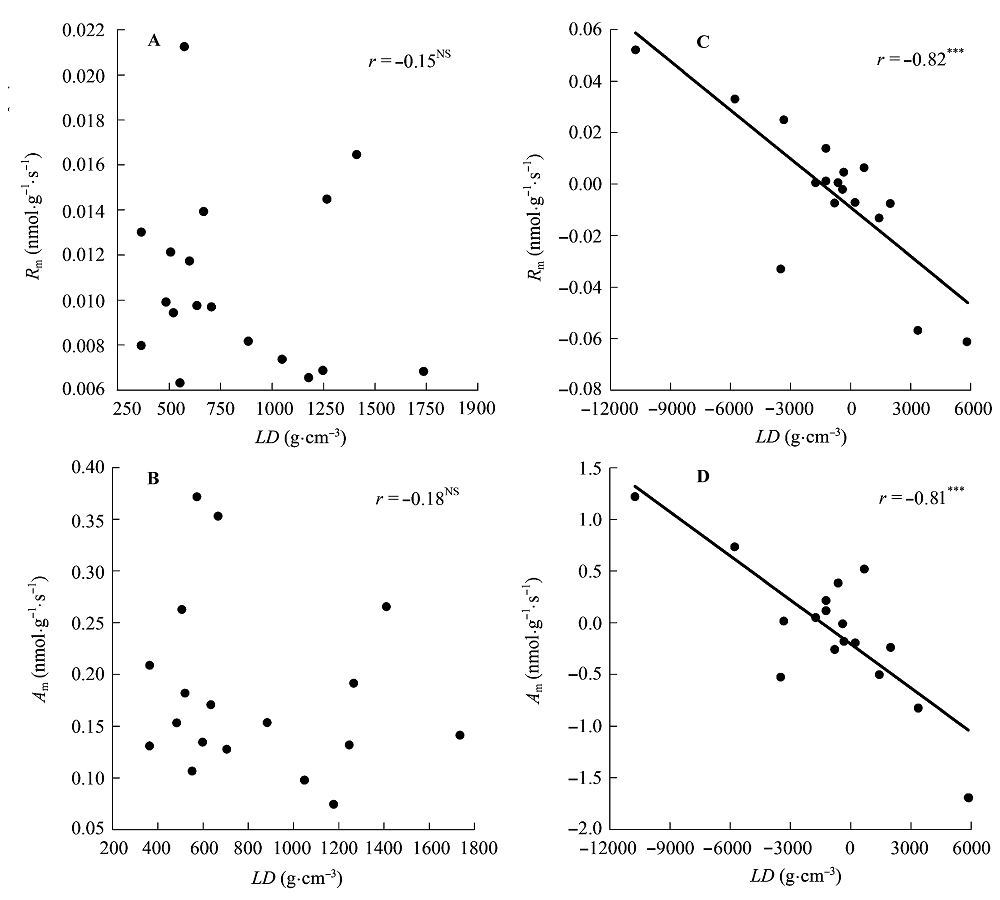
Fig. 6 Correlations of leaf density (LD) with maximum photosynthesis per leaf dry mass (Am) and dark respiration per leaf dry mass (Rm). A, B, Traditional Pearson correlation. C, D, Correlation given by the phylogenetic independent contrasts analysis. ***, p < 0.001; NS, p > 0.05.
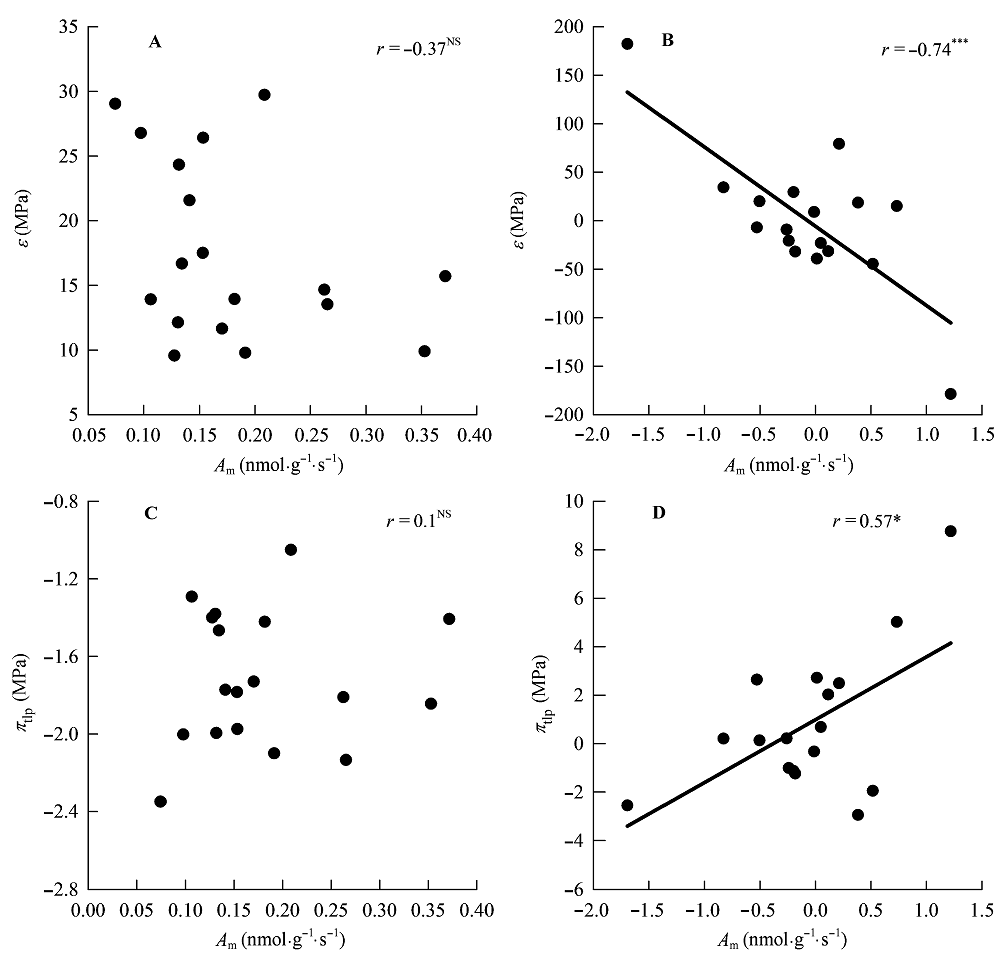
Fig. 7 Correlations of maximum photosynthesis per leaf dry mass (Am) with water potential at turgor loss point (πtlp) and modulus of elasticity at full turgor (ε). A, B, Traditional Pearson correlation. C, D, Correlation given by the phylogenetic independent contrasts analysis. *, 0.01 < p < 0.05; ***, p < 0.001; NS, p > 0.05.
| [1] | Aranda I, Castro L, Pardos M, Gil L, Pardos JA (2005). Effects of the interaction between drought and shade on water relations, gas exchange and morphological traits in cork oak (Quercus suber L.) seedlings. Forest Ecology and Management, 210, 117-129. |
| [2] |
Bacelar EA, Correia CM, Moutinho-Pereira JM, Goncalves BC, Lopes JI, Torres-Pereira JM (2004). Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiology, 24, 233-239.
URL PMID |
| [3] | Bartlett MK, Scoffoni C, Ardy R, Zhang Y, Sun SW, Cao KF, Sack L (2012a). Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods in Ecology and Evolution, 3, 880-888. |
| [4] |
Bartlett MK, Scoffoni C, Sack L (2012b). The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecology Letters, 15, 393-405.
URL PMID |
| [5] | Becker P, Wong M (1994). Drought induced mortality in tropical heath forest. Journal of Tropical Sciences, 5, 416-417. |
| [6] | Brodersen CR, Vogelmann TC (2007). Do epidermal lens cells facilitate the absorptance of diffuse light? American Journal of Botang, 94, 1061-1066. |
| [7] |
Brown C, Burslem DFRP, Illian JB, Bao L, Brockelman W, Cao M, Chang LW, Dattaraja HS, Davies S, Gunatilleke CVS, Gunatilleke IAUN, Huang J, Kassim AR, LaFrankie JV, Lian J, Lin L, Ma K, Mi X, Nathalang A, Noor S, Ong P, Sukumar R, Su SH, Sun IF, Suresh HS, Tan S, Thompson J, Uriarte M, Valencia R, Yap SL, Ye W, Law R (2013). Multispecies coexistence of trees in tropical forests: spatial signals of topographic niche differentiation increase with environmental heterogeneity. Proceedings of the Royal Society B: Biological Sciences, 280, 20130502.
DOI URL PMID |
| [8] |
Bucci S, Goldstein G, Meinzer F, Scholz F, Franco A, Bust- amante M (2004). Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiology, 24, 891-899.
URL PMID |
| [9] |
Cao KF (2000). Leaf anatomy and chlorophyll content of 12 woody species in contrasting light conditions in a bornean heath forest. Canadian Journal of Botany, 78, 1245-1253.
DOI URL |
| [10] |
Cao M, Zou XM, Warren M, Zhu H (2006). Tropical forests of Xishuangbanna, China. Biotropica, 38, 306-309.
DOI URL |
| [11] |
Chartzoulakis K, Patakas A, Kofidis G, Bosabalidis A, Nastou A (2002). Water stress affects leaf anatomy, gas exchange, water relations and growth of two avocado cultivars. Scientia Horticulturae, 95, 39-50.
DOI URL |
| [12] | Choat B, Medek DE, Stuart SA, Pasquet-Kok J, Egerton JJG, Salari H, Sack L, Ball MC (2011). Xylem traits mediate a trade-off between resistance to freeze-thaw-induced embolism and photosynthetic capacity in overwintering evergreens. New Phytologist, 191, 996-1005. |
| [13] | Cutler J, Rains D, Loomis R (1977). The importance of cell size in the water relations of plants. Physiologia Plantarum, 40, 255-260. |
| [14] | DeLucia EH, Nelson K, Vogelmann TC, Smith WK (1996). Contribution of intercellular reflectance to photosynthesis in shade leaves. Plant, Cell & Environment, 19, 159-170. |
| [15] | Evans J, Vogelmann T, Williams W, Gorton H (2004). Chloroplast to leaf. In: Smith W, Vogelmann T, Critchley C eds. Photosynthetic Adaptation. Springer, New York. 178. 15-41. |
| [16] | Felsenstein J (1985). Phylogenies and the comparative method. The American Naturalist, 125, 1-15. |
| [17] |
Feng YL, Lei YB, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit , Li YP, Zheng YL (2009). Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106, 1853-1856.
URL PMID |
| [18] |
Fu PL, Jiang YJ, Wang AY, Brodribb TJ, Zhang JL, Zhu SD, Cao KF (2012). Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an asian tropical dry karst forest. Annals of Botany, 110, 189-199.
DOI URL PMID |
| [19] | Garland T, Harvey PH, Ives AR (1992). Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology, 41, 18-32. |
| [20] |
Green DS, Kruger EL (2001). Light-mediated constraints on leaf function correlate with leaf structure among deciduous and evergreen tree species. Tree Physiology, 21, 1341-1346.
DOI URL PMID |
| [21] | Hanba YT, Miyazawa SI, Terashima I (1999). The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Functional Ecology, 13, 632-639. |
| [22] |
Hassiotou F, Renton M, Ludwig M, Evans JR, Veneklaas EJ (2010). Photosynthesis at an extreme end of the leaf trait spectrum: How does it relate to high leaf dry mass per area and associated structural parameters? Journal of Experimental Botany, 61, 3015-3028.
DOI URL PMID |
| [23] | Hessini K, Martínez JP, Gandour M, Albouchi A, Soltani A, Abdelly C (2009). Effect of water stress on growth, osmo- tic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environmental and Experimental Botany, 67, 312-319. |
| [24] |
Hu YH, Sheng DY, Xiang YZ, Yang ZJ, Xu DP, Zhang NN, Shi LL (2013). The environment, not space, dominantly structures the landscape patterns of the richness and composition of the tropical understory vegetation. PLoS ONE, 8, e81308.
URL PMID |
| [25] | Karabourniotis G (1998). Light-guiding function of foliar sclereids in the evergreen sclerophyll phillyrea latifolia: a quantitative approach. Journal of Experimental Botany, 49, 739-746. |
| [26] | Karabourniotis G, Bornman JF (1999). Penetration of UV-A, UV-B and blue light through the leaf trichome layers of two xeromorphic plants, olive and oak, measured by optical fibre microprobes. Physiologia Plantarum, 105, 655-661. |
| [27] | Karabourniotis G, Papastergiou N, Kabanopoulou E, Fasseas C (1994). Foliar sclereids of Olea europaea may function as optical fibres. Canadian Journal of Botany, 72, 330-336. |
| [28] | Kubiske ME, Abrams MD (1990). Pressure-volume relation- ships in non-rehydrated tissue at various water deficits. Plant, Cell & Environment, 13, 995-1000. |
| [29] | Kursar TA, Engelbrecht BMJ, Burke A, Tyree MT, Omari BE, Giraldo JP (2009). Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Functional Ecology, 23, 93-102. |
| [30] |
Lan GY, Getzin S, Wiegand T, Hu YH, Xie GS, Zhu H, Cao M (2012). Spatial distribution and interspecific associations of tree species in a tropical seasonal rain forest of China. PLoS ONE, 7, e46074.
DOI URL PMID |
| [31] | Lan YG, Hu YH, Cao M, Zhu H, Wang H, Zhou SS, Deng SS, Deng XB, Cui JY, Huang JG, Liu LY, Xu HL, Song JP, He YC (2008). Establishment of Xishuangbanna tropical forest dynamics plot: species compositions and spatial distribution patterns. Journal of Plant Ecology (Chinese Version), 32, 287-298. (in Chinese with English abstract) |
| [ 兰国玉, 胡跃华, 曹敏, 朱华, 王洪, 周仕顺, 邓晓保, 崔景云, 黄建国, 刘林云, 许海龙, 宋军平, 何有才 (2008). 西双版纳热带森林动态监测样地——树种组成与空间分布格局. 植物生态学报, 32, 287-298.] | |
| [32] | Lin LX, Comita LS, Zheng Z, Cao M (2012). Seasonal differentiation in density-dependent seedling survival in a tropical rain forest. Journal of Ecology, 100, 905-914. |
| [33] | Markesteijn L, Poorter L, Paz H, Sack L, Bongers F (2011). Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant, Cell & Environment, 34, 137-148. |
| [34] |
Mediavilla S, Escudero A, Heilmeier H (2001). Internal leaf anatomy and photosynthetic resource-use efficiency: inter- specific and intraspecific comparisons. Tree Physiology, 21, 251-259.
DOI URL PMID |
| [35] |
Meinzer FC (2003). Functional convergence in plant responses to the environment. Oecologia, 134, 1-11.
DOI URL PMID |
| [36] |
Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A (2008). Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiologia Plantarum, 134, 237-245.
URL PMID |
| [37] | Morgan JM (1984). Osmoregulation and water stress in higher plants. Annual Review of Plant Physiology, 35, 299-319. |
| [38] | Niinemets Ü (1999). Research review. Components of leaf dry mass per area-thickness and density-alter leaf photo- synthetic capacity in reverse directions in woody plants. New Phytologist, 144, 35-47. |
| [39] | Niinemets Ü (2001). Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology, 82, 453-469. |
| [40] |
Niinemets Ü, Diaz-Espejo A, Flexas J, Galmes J, Warren CR (2009). Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. Journal of Experimental Botany, 60, 2249-2270.
DOI URL PMID |
| [41] |
Nikolopoulos D, Liakopoulos G, Drossopoulos I, Karabour- niotis G (2002). The relationship between anatomy and photosynthetic performance of heterobaric leaves. Plant Physiology, 129, 235-243.
DOI URL PMID |
| [42] | Onoda Y, Hikosaka K, Hirose T (2004). Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Functional Ecology, 18, 419-425. |
| [43] | Parkhurst DF (1994). Diffusion of CO2 and other gases inside leaves. New Phytologist, 126, 449-479. |
| [44] | Patakas A, Nikolaou N, Zioziou E, Radoglou K, Noitsakis B (2002). The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Science, 163, 361-367. |
| [45] |
Poorter H, Evans JR (1998). Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia, 116, 26-37.
URL PMID |
| [46] | Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist, 182, 565-588. |
| [47] | Poulson ME, Vogelmann TC (1990). Epidermal focussing and effects upon photosynthetic light-harvesting in leaves of oxalis. Plant, Cell & Environment, 13, 803-811. |
| [48] | Reich P, Wright I, Cavender-Bares J, Craine J, Oleksyn J, Westoby M, Walters M (2003). The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences, 164, S143-S164. |
| [49] |
Richardson AD, Berlyn GP (2002). Changes in foliar spectral reflectance and chlorophyll fluorescence of four temperate species following branch cutting. Tree Physiology, 22, 499-506.
DOI URL PMID |
| [50] |
Sack L, Frole K (2006). Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology, 87, 483-491.
DOI URL PMID |
| [51] | Sack L, Pasquet-Kok J (2011). Leaf pressure-volume curve parameters. http://prometheuswiki.publish.csiro.au/tiki- index.php?page=Pressure-volume+curves. Cited: 23 Dec. 2013. |
| [52] |
Savé R, Biel C, de Herralde F (2000). Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus creticus L. Biologia Plantarum, 43, 239-244.
DOI URL |
| [53] | Scafaro AP, von Caemmerer S, Evans JR, Atwell BJ (2011). Temperature response of mesophyll conductance in cultiv- ated and wild oryza species with contrasting mesophyll cell wall thickness. Plant, Cell & Environment, 34, 1999-2008. |
| [54] | Smith T, Huston M (1989). A theory of the spatial and temporal dynamics of plant communities. Plant Ecology, 83, 49-69. |
| [55] | Smith WK, Vogelmann TC, DeLucia EH, Bell DT, Shepherd KA (1997). Leaf form and photosynthesis. BioScience, 47, 785-793. |
| [56] |
Swenson NG, Enquist BJ (2007). Ecological and evolutionary determinants of a key plant functional trait: wood density and its community-wide variation across latitude and elevation. American Journal of Botany, 94, 451-459.
DOI URL PMID |
| [57] |
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731-2739.
URL PMID |
| [58] |
Terashima I, Hanba YT, Tholen D, Niinemets U (2011). Leaf functional anatomy in relation to photosynthesis. Plant Physiology (Rockville), 155, 108-116.
DOI URL PMID |
| [59] |
Tomas M, Flexas J, Copolovici L, Galmes J, Hallik L, Medrano H, Ribas-Carbo M, Tosens T, Vislap V, Niinemets Ü (2013). Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany, 64, 2269-2281.
DOI URL PMID |
| [60] | Vogelmann TC (1993). Plant tissue optics. Annual Review of Plant Physiology and Plant Molecular Biology, 44, 231-251. |
| [61] | Vogelmann TC, Bornman JF, Yates DJ (1996a). Focusing of light by leaf epidermal cells. Physiologia Plantarum, 98, 43-56. |
| [62] | Vogelmann TC, Martin G (1993). The functional-significance of palisade tissue—Penetration of directional versus diffuse light. Plant, Cell & Environment, 16, 65-72. |
| [63] | Vogelmann TC, Nishio JN, Smith WK (1996b). Leaves and light capture: light propagation and gradients of carbon fixation within leaves. Trends in Plant Science, 1, 65-70. |
| [64] | Walters M, Reich P (2000). Trade-offs in low-light CO2 exchange: a component of variation in shade tolerance among cold temperate tree seedlings. Functional Ecology, 14, 155-165. |
| [65] | Winter H, Robinson DG, Heldt HW (1993). Subcellular volumes and metabolite concentrations in barley leaves. Planta, 191, 180-190. |
| [66] |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M (2004). The worldwide leaf economics spectrum. Nature, 428, 821-827.
DOI URL PMID |
| [1] |
Jia WEN Xin-Na ZHANG 娟 王 Xiu-Hai ZHAO Chun-Yu ZHANG.
Responses of seedling survival rate to neighbor competition and environmental variables regulated by traits [J]. Chin J Plant Ecol, 2024, 48(预发表): 0-0. |
| [2] | MA Chang-Qin, HUANG Hai-Long, PENG Zheng-Lin, WU Chun-Ze, WEI Qing-Yu, JIA Hong-Tao, WEI Xing. Response of compound leaf types and photosynthetic function of male and female Fraxinus mandschurica to different habitats [J]. Chin J Plant Ecol, 2023, 47(9): 1287-1297. |
| [3] | LI Wei-Bin, ZHANG Hong-Xia, ZHANG Yu-Shu, CHEN Ni-Na. Influence of diurnal asymmetric warming on carbon sink capacity in a broadleaf Korean pine forest in Changbai Mountains, China [J]. Chin J Plant Ecol, 2023, 47(9): 1225-1233. |
| [4] | FENG Shan-Shan, HUANG Chun-Hui, TANG Meng-Yun, JIANG Wei-Xin, BAI Tian-Dao. Geographical variation of needles phenotypic and anatomic traits between populations of Pinus yunnanensis var. tenuifolia and its environmental interpretation [J]. Chin J Plant Ecol, 2023, 47(8): 1116-1130. |
| [5] | JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses [J]. Chin J Plant Ecol, 2023, 47(7): 988-997. |
| [6] | FENG Ke, LIU Dong-Mei, ZHANG Qi, AN Jing, HE Shuang-Hui. Effect of tourism disturbance on soil microbial diversity and community structure in a Pinus tabuliformis forest [J]. Chin J Plant Ecol, 2023, 47(4): 584-596. |
| [7] | SHI Dang, GUO Chuan-Chao, JIANG Nan-Lin, TANG Ying-Ying, ZHENG Feng, WANG Jin, LIAO Kang, LIU Li-Qiang. Characteristics and spatial distribution pattern of natural regeneration young plants of Prunus armeniaca in Xinjiang, China [J]. Chin J Plant Ecol, 2023, 47(4): 515-529. |
| [8] | WANG Jing-Jing, WANG Jia-Hao, HUANG Zhi-Yun, Vanessa Chiamaka OKECHUKW, HU Die, QI Shan-Shan, DAI Zhi-Cong, DU Dao-Lin. Effects of endophytic nitrogen-fixing bacteria on the growth strategy of an invasive plant Sphagneticola trilobata under different nitrogen levels [J]. Chin J Plant Ecol, 2023, 47(2): 195-205. |
| [9] | LIU Hai-Yan, ZANG Sha-Sha, ZHANG Chun-Xia, ZUO Jin-Cheng, RUAN Zuo-Xi, WU Hong-Yan. Photochemical reaction of photosystem II in diatoms under phosphorus starvation and its response to high light intensity [J]. Chin J Plant Ecol, 2023, 47(12): 1718-1727. |
| [10] | YU Qiu-Wu, YANG Jing, SHEN Guo-Chun. Relationship between canopy structure and species composition of an evergreen broadleaf forest in Tiantong region, Zhejiang, China [J]. Chin J Plant Ecol, 2022, 46(5): 529-538. |
| [11] | YUAN Yuan, MU Yan-Mei, DENG Yu-Jie, LI Xin-Hao, JIANG Xiao-Yan, GAO Sheng-Jie, ZHA Tian- Shan, JIA Xin. Effects of land cover and phenology changes on the gross primary productivity in an Artemisia ordosica shrubland [J]. Chin J Plant Ecol, 2022, 46(2): 162-175. |
| [12] | MENG Qing-Jing, FAN Wei-Guo. Calcium-tolerance type and adaptability to high-calcium habitats of Rosa roxburghii [J]. Chin J Plant Ecol, 2022, 46(12): 1562-1572. |
| [13] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [14] | JIN Chuan, LI Xin-Hao, JIANG Yan, XU Ming-Ze, TIAN Yun, LIU Peng, JIA Xin, ZHA Tian- Shan. Relative changes and regulation of photosynthetic energy partitioning components in Artemisia ordosica during growing season [J]. Chin J Plant Ecol, 2021, 45(8): 870-879. |
| [15] | YE Zi-Piao, YU Feng, AN Ting, WANG Fu-Biao, KANG Hua-Jing. Investigation on CO2-response model of stomatal conductance for plants [J]. Chin J Plant Ecol, 2021, 45(4): 420-428. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn