

Chin J Plan Ecolo ›› 2015, Vol. 39 ›› Issue (1): 104-109.DOI: 10.17521/cjpe.2015.0011
• Orginal Article • Previous Articles Next Articles
FENG Han-Qing*( ), GUAN Dong-Dong, JIAO Qing-Song, JIA Ling-Yun, SUN Kun
), GUAN Dong-Dong, JIAO Qing-Song, JIA Ling-Yun, SUN Kun
Received:2014-05-29
Accepted:2014-10-21
Online:2015-01-10
Published:2015-01-22
Contact:
Han-Qing FENG
About author:# Co-first authors
FENG Han-Qing,GUAN Dong-Dong,JIAO Qing-Song,JIA Ling-Yun,SUN Kun. Analysis of the relationship between cyanide-resistant respiration and photosynthesis under light in Phaseolus vulgaris leaves[J]. Chin J Plan Ecolo, 2015, 39(1): 104-109.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2015.0011
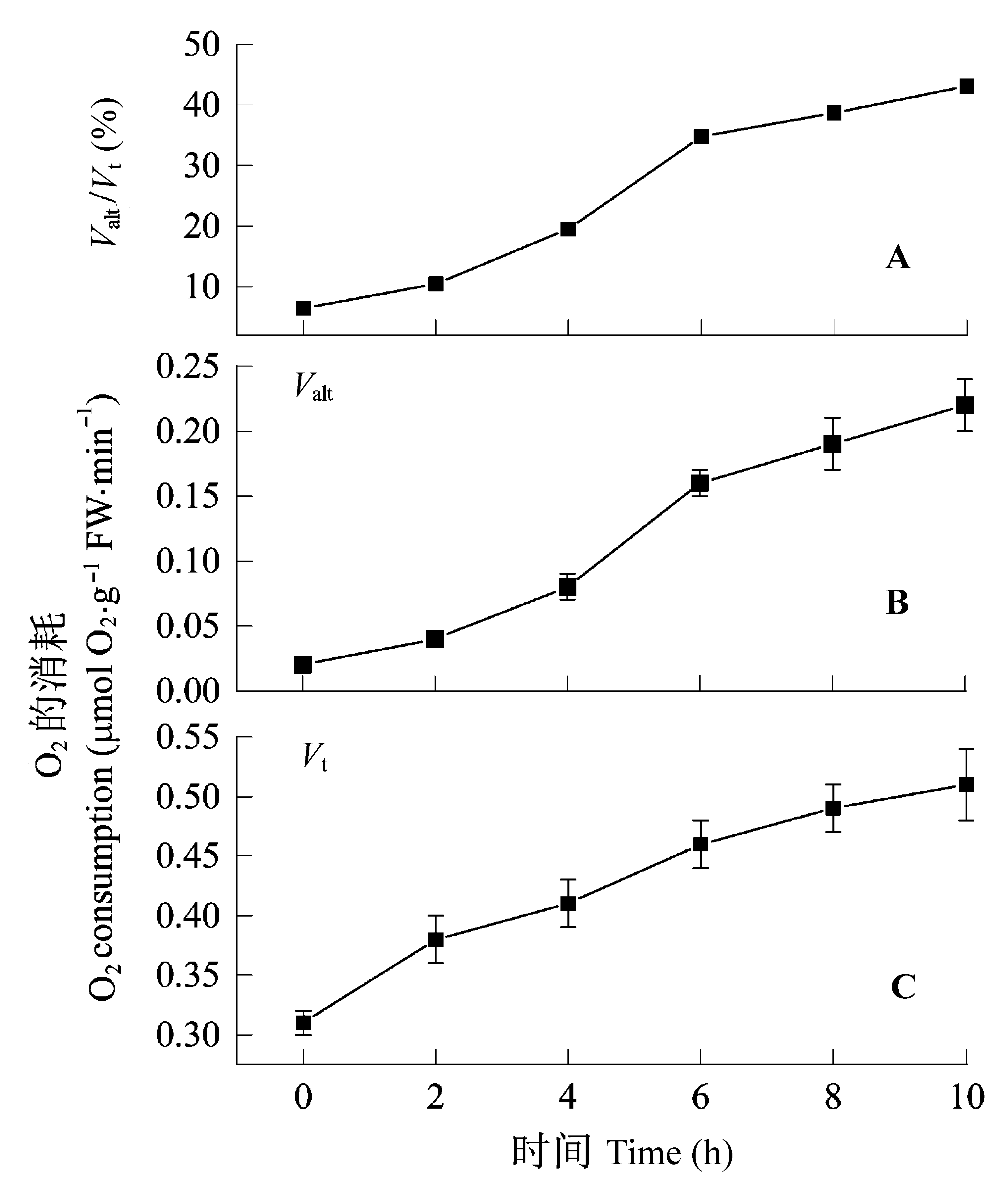
Fig. 1 Changes in total respiration (Vt), capacity of cyanide- resistant respiration (Valt) and the ratio of Valt to Vt in dark- grown leaves exposed to continuous light for 10 h. These are individual samples taken during four different experiments. Time indicates hours after starting illumination. Values are mean values ± SD of four independent experiments. The horizontal axis shows the time after exposing to light. Valt/Vt was computed based on the average values of Vt and Valt.
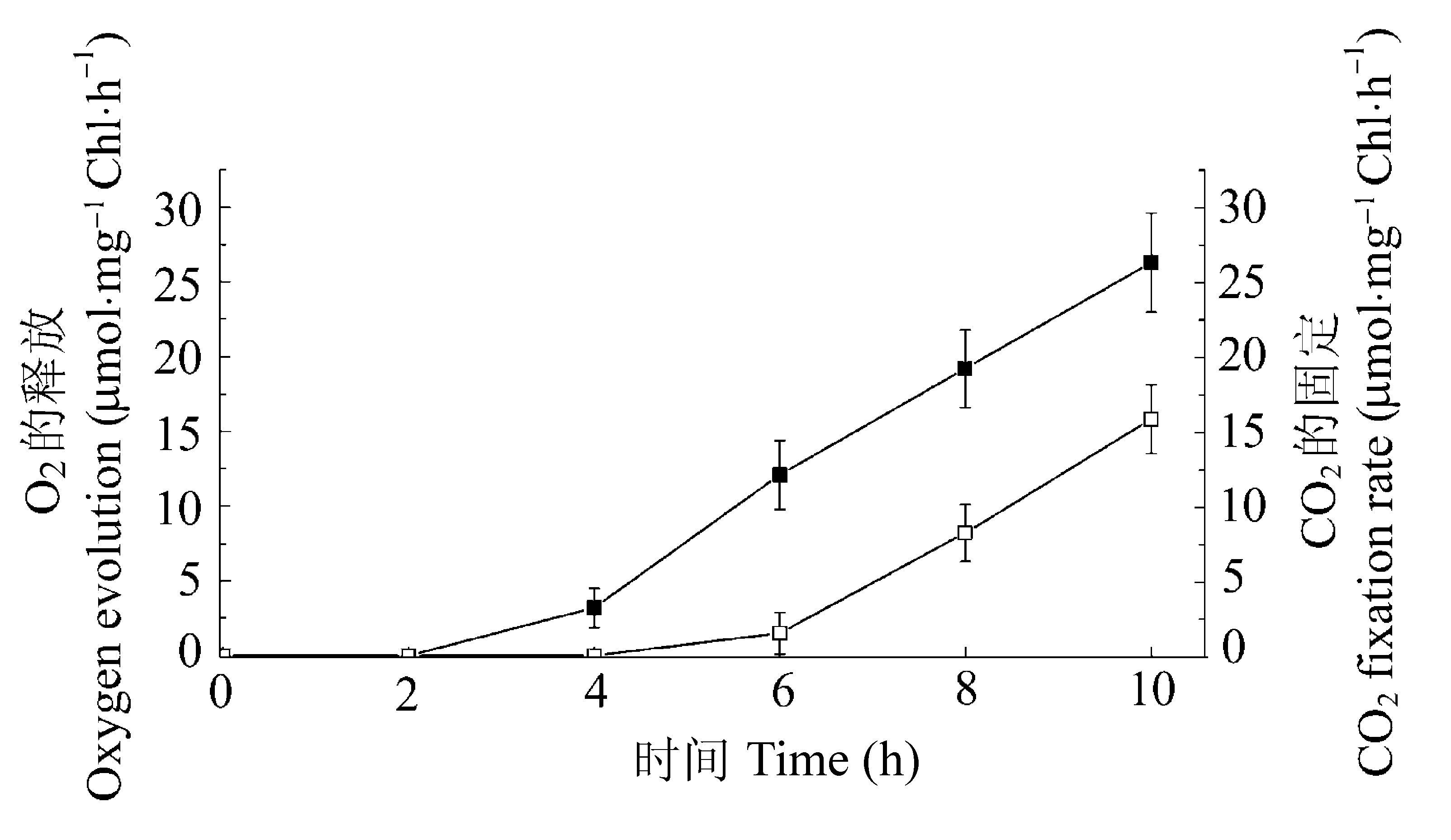
Fig. 3 Changes in oxygen evolution (■) and carbon dioxide fixation (□) in whole chloroplasts of the dark-grown leaves exposed to 10 h of continuous light. Values are mean values ± SD of four independent experiments. The horizontal axis shows the time after exposing to light.
| 光照的时间 Time of illumination (h) | SHAM对于光合作用的影响 Effects of SHAM on photosynthesis (% of control) | |
|---|---|---|
| 光合放氧 Oxygen evolution | 光合CO2固定 CO2 fixation | |
| 0 | 100a | 100a |
| 2 | 100a | 100a |
| 4 | 98 ± 5a | 100a |
| 6 | 101 ± 3a | 100a |
| 8 | 97 ± 4a | 96 ± 4a |
| 10 | 102 ± 4a | 95 ± 3a |
Table 1 The effects of salicylhydroxamic acid (SHAM) on photosynthetic oxygen evolution rate and carbon dioxide fixation rate when the dark-grown leaves were exposed to continuous light
| 光照的时间 Time of illumination (h) | SHAM对于光合作用的影响 Effects of SHAM on photosynthesis (% of control) | |
|---|---|---|
| 光合放氧 Oxygen evolution | 光合CO2固定 CO2 fixation | |
| 0 | 100a | 100a |
| 2 | 100a | 100a |
| 4 | 98 ± 5a | 100a |
| 6 | 101 ± 3a | 100a |
| 8 | 97 ± 4a | 96 ± 4a |
| 10 | 102 ± 4a | 95 ± 3a |
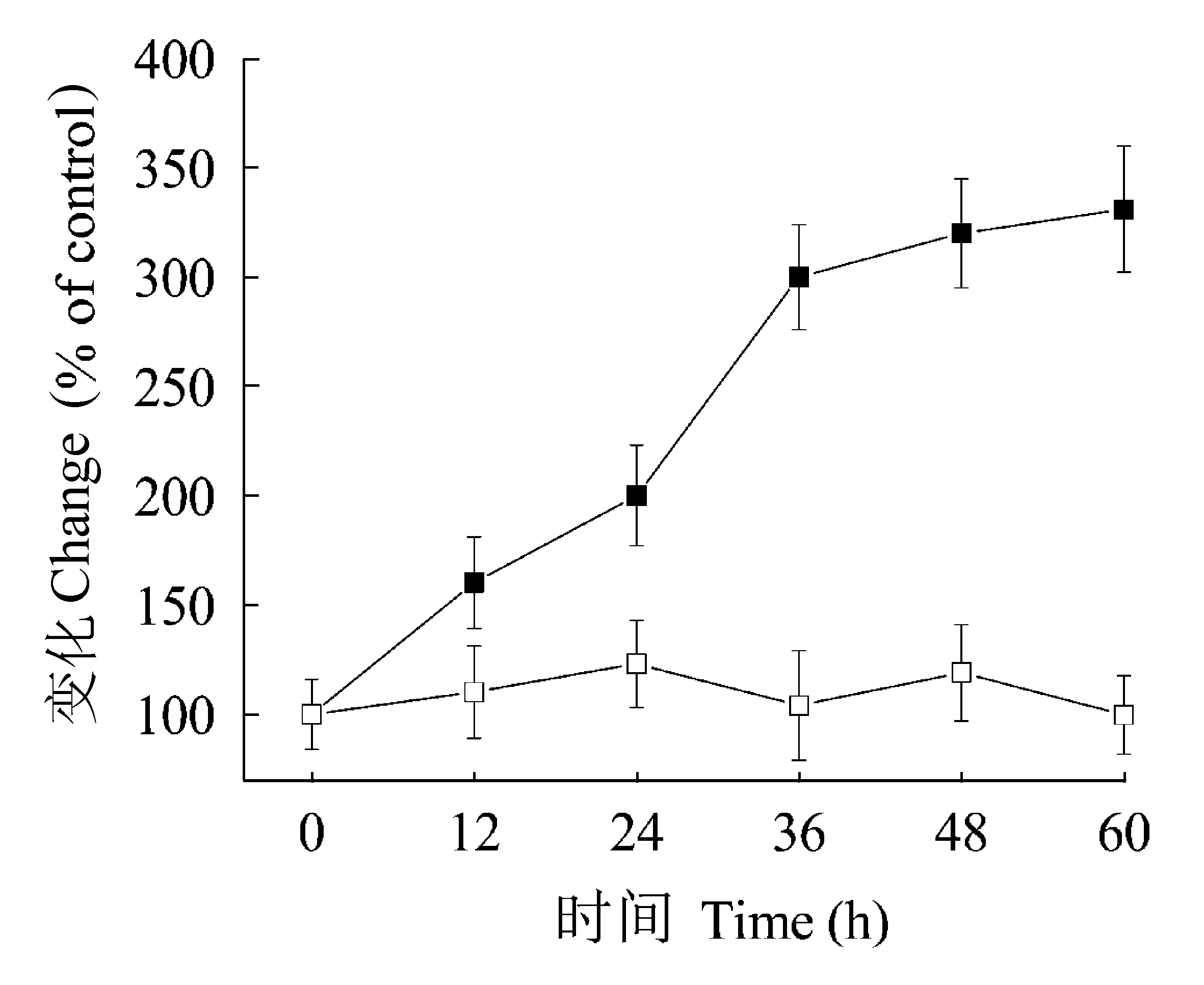
Fig. 4 Effect of short period of illumination on the ratio of cyanide-resistant respiration pathway to total respiration (Valt/Vt, %) (■) and photosynthetic CO2 fixation rate (□). One-week-old plants grown in 12 h light/12 h dark photoperiods were transferred to darkness and then received 10 min plus of light once every 12 h. Leaves in darkness were assigned the values of control (100%). Time indicates hours in darkness. Results are mean values ± SD of four independent experiments.
| 1 | Bartoli CG, Gomez F, Gergoff G, Guiamét JJ, Puntarulo S (2005). Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. Journal of Experimental Botany, 56, 1269-1276. |
| 2 | Bingham IJ, Farrar JF (1989). Activity and capacity of respiration pathways in barley roots deprived of inorganic nutrients. Plant Physiology and Biochemistry, 27, 847-854. |
| 3 | Bruick R, Mayfield SP (1999). Light-activated translation of chloroplast mRNAs. Trends in Plant Science, 4, 190-195. |
| 4 | Chivasa S, Carr JP (1998). Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. The Plant Cell, 10, 1489-1498. |
| 5 | Chivasa S, Murphy AM, Naylor M, Carr JP (1997). Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. The Plant Cell, 9, 547-557. |
| 6 | Escobar MA, Franklin KA, Svensson AS, Salter MG, Whitelam GC, Rasmusson AG (2004). Light regulation of the Arabidopsis respiratory chain. Multiple discrete photo- receptor responses contribute to induction of type II NAD(P)H dehydrogenase genes. Plant Physiology, 136, 2710-2721. |
| 7 | Finnegan PM, Whelan J, Millar AH, Zhang Q, Smith MK, Wiskich JT, Day DA (1997). Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiology, 114, 455-466. |
| 8 | Gui MX, Wang XM, Huang WY (1991). The effect of light and exogenous gibberellic acid on respiration pathways during germination of tomato seeds. Physiologia Plantarum, 81, 403-407. |
| 9 | Igamberdiev AU, Bykova NV, Gardeström P (1997). Involve- ment of cyanide-resistant and rotenone-insensitive pathways of mito-chondrial electron transport during oxidation of glycine in higher plants. FEBS Letters, 412, 265-269. |
| 10 | Kim C, Meskauskiene R, Apel K, Laloi C (2008). No single way to understand singlet oxygen signalling in plants. EMBO Reports, 9, 435-439. |
| 11 | Liscum E, Hodgson DW, Campbell TJ (2003). Blue light signaling through the cryptochromes and phototropins. So that’s what the blues is all about. Plant Physiology, 133, 1429-1436. |
| 12 | Lurie S (1997). Stomatal opening and photosynthesis in greening leaves of Vicia faba L. Australian Journal of Plant Physiology, 4, 69-74. |
| 13 | Mackenzie S, McIntosh L (1999). Higher plant mitochondria. The Plant Cell, 11, 571-586. |
| 14 | Millenaar FF, Lambers H (2003). The Alternative oxidase: in vivo regulation and function. Plant Biology, 5, 2-15. |
| 15 | Obenland D, Diethelm R, Shibles R, Stewart C (1990). Relationship of alternative respiratory capacity and alternative oxidase amount during soybean seedling growth. Plant & Cell Physiology, 31, 897-901. |
| 16 | Padmasree K, Padmavathi L, Raghavendra AS (2002). Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Critical Reviews in Biochemistry and Molecular Biology, 37, 71-119. |
| 17 | Padmasree K, Raghavendra AS (2001). Restriction of mitochondrial oxidative metabolism leads to suppression of photosynthetic carbon assimilation but not of photochemical electron transport in pea mesophyll protoplasts. Current Science, 81, 680-684. |
| 18 | Raghavendra AS, Padmasree K (2003). Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends in Plant Science, 8, 546-553. |
| 19 | Ribas-Carbo M, Robinson SA, Gonzàlez-Meler MA, Lennon AM, Giles L, Siedow JN, Berry JA (2000). Effects of light on respiration and oxygen isotope fractionation in soybean cotyledons. Plant, Cell & Environment, 23, 983-989. |
| 20 | Siedow JN, Day DA (2000). Respiration and photorespiration. In: Buchanan B, Gruissem W, Jones R eds. Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, USA. 676-728. |
| 21 | Svensson ÅS, Rasmusson AG (2001). Light-dependent gene expression for proteins in the respiratory chain of potato leaves. The Plant Journal, 28, 73-82. |
| 22 | Vanlerberghe GC, McIntosh L (1997). Alternative oxidase: from gene to function. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 703-734. |
| 23 | Whelan J, Smith MK, Meijer M, Yu JW, Badger MR, Price GD, Day DA (1995). Cloning of an additional cDNA for the alternative oxidase in tobacco. Plant Physiology, 107, 1469-1470. |
| 24 | Xu F, Yuan S, Lin HH (2011). Response of mitochondrial alternative oxidase (AOX) to light signals. Plant Signaling & Behavior, 6, 55-58. |
| 25 | Yoshida K, Terashima I, Noguchi K (2006). Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant & Cell Physiology, 47, 22-31. |
| 26 | Yoshida K, Terashima I, Noguchi, K (2007). Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant & Cell Physiology, 48, 606-614. |
| 27 | Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH (2010). Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant, Cell & Environment, 33, 2121-2131. |
| 28 | Zhang LT, Gao HY, Zhang ZS, Xue ZC, Meng QW (2012). Multiple effects of inhibition of mitochondrial alternative oxidase pathway on photosynthetic apparatus in Rumex K-1 leaves. Biologia Plantarum, 56, 365-368. |
| 29 | Zhang LT, Zhang ZS, Gao HY, Xue ZC, Yang C, Meng XL, Meng QW (2011). Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiologia Plantarum, 143, 396-407. |
| [1] | LI Wei-Bin, ZHANG Hong-Xia, ZHANG Yu-Shu, CHEN Ni-Na. Influence of diurnal asymmetric warming on carbon sink capacity in a broadleaf Korean pine forest in Changbai Mountains, China [J]. Chin J Plant Ecol, 2023, 47(9): 1225-1233. |
| [2] | JIANG Hai-Gang, ZENG Yun-Hong, TANG Hua-Xin, LIU Wei, LI Jie-Lin, HE Guo-Hua, QIN Hai-Yan, WANG Li-Chao, Victor RESCO de DIOS, YAO Yin-An. Rhythmic regulation of carbon fixation and water dissipation in three mosses [J]. Chin J Plant Ecol, 2023, 47(7): 988-997. |
| [3] | LIU Hai-Yan, ZANG Sha-Sha, ZHANG Chun-Xia, ZUO Jin-Cheng, RUAN Zuo-Xi, WU Hong-Yan. Photochemical reaction of photosystem II in diatoms under phosphorus starvation and its response to high light intensity [J]. Chin J Plant Ecol, 2023, 47(12): 1718-1727. |
| [4] | YUAN Yuan, MU Yan-Mei, DENG Yu-Jie, LI Xin-Hao, JIANG Xiao-Yan, GAO Sheng-Jie, ZHA Tian- Shan, JIA Xin. Effects of land cover and phenology changes on the gross primary productivity in an Artemisia ordosica shrubland [J]. Chin J Plant Ecol, 2022, 46(2): 162-175. |
| [5] | WU Lin-Sheng, ZHANG Yong-Guang, ZHANG Zhao-Ying, ZHANG Xiao-Kang, WU Yun-Fei. Remote sensing of solar-induced chlorophyll fluorescence and its applications in terrestrial ecosystem monitoring [J]. Chin J Plant Ecol, 2022, 46(10): 1167-1199. |
| [6] | JIN Chuan, LI Xin-Hao, JIANG Yan, XU Ming-Ze, TIAN Yun, LIU Peng, JIA Xin, ZHA Tian- Shan. Relative changes and regulation of photosynthetic energy partitioning components in Artemisia ordosica during growing season [J]. Chin J Plant Ecol, 2021, 45(8): 870-879. |
| [7] | WU Hong-Min, SHUANG Sheng-Pu, ZHANG Jin-Yan, CUN Zhu, MENG Zhen-Gui, LI Long-Gen, SHA Ben-Cai, CHEN Jun-Wen. Photodamage to photosystem in a typically shade-tolerant species Panax notoginseng exposed to a sudden increase in light intensity [J]. Chin J Plant Ecol, 2021, 45(4): 404-419. |
| [8] | YE Zi-Piao, YU Feng, AN Ting, WANG Fu-Biao, KANG Hua-Jing. Investigation on CO2-response model of stomatal conductance for plants [J]. Chin J Plant Ecol, 2021, 45(4): 420-428. |
| [9] | LI Jing, WANG Xin, WANG Zhen-Hua, WANG Bin, WANG Cheng-Zhang, DENG Mei-Feng, LIU Ling-Li. Effects of ozone and aerosol pollution on photosynthesis of poplar leaves [J]. Chin J Plant Ecol, 2020, 44(8): 854-863. |
| [10] | LI Xu, WU Ting, CHENG Yan, TAN Na-Dan, JIANG Fen, LIU Shi-Zhong, CHU Guo-Wei, MENG Ze, LIU Ju-Xiu. Ecophysiological adaptability of four tree species in the southern subtropical evergreen broad-leaved forest to warming [J]. Chin J Plant Ecol, 2020, 44(12): 1203-1214. |
| [11] | LIU Xiao-Ming, YANG Xiao-Fang, WANG Xuan, ZHANG Shou-Ren. Effects of simulated nitrogen deposition on growth and photosynthetic characteristics of Quercus wutaishanica and Acer pictum subsp. mono in a warm-temperate deciduous broad- leaved forest [J]. Chin J Plant Ecol, 2019, 43(3): 197-207. |
| [12] | LI Xin-Hao, YAN Hui-Juan, WEI Teng-Zhou, ZHOU Wen-Jun, JIA Xin, ZHA Tian-Shan. Relative changes of resource use efficiencies and their responses to environmental factors in Artemisia ordosica during growing season [J]. Chin J Plant Ecol, 2019, 43(10): 889-898. |
| [13] | CHENG Han-Ting, LI Qin-Fen, LIU Jing-Kun, YAN Ting-Liang, ZHANG Qiao-Yan, WANG Jin-Chuang. Seasonal changes of photosynthetic characteristics of Alpinia oxyphylla growing under Hevea brasiliensis [J]. Chin J Plant Ecol, 2018, 42(5): 585-594. |
| [14] | ZHANG Na, ZHU Yang-Chun, LI Zhi-Qiang, LU Xin, FAN Ru-Qin, LIU Li-ZhuTONG , Fei, CHEN Jing, MU Chun-Sheng, ZHANG Zhen-Hua. Effect of Pb pollution on the growth, biomass allocation and photosynthesis of Phragmites australis in flood and drought environment [J]. Chin J Plant Ecol, 2018, 42(2): 229-239. |
| [15] | Ji-Mei HAN, Wang-Feng ZHANG, Dong-Liang XIONG, Jaume FLEXAS, Ya-Li ZHANG. Mesophyll conductance and its limiting factors in plant leaves [J]. Chin J Plan Ecolo, 2017, 41(8): 914-924. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn