

植物生态学报 ›› 2015, Vol. 39 ›› Issue (1): 104-109.DOI: 10.17521/cjpe.2015.0011
收稿日期:2014-05-29
接受日期:2014-10-21
出版日期:2015-01-10
发布日期:2015-01-22
通讯作者:
冯汉青
作者简介:# 共同第一作者
基金资助:
FENG Han-Qing*( ), GUAN Dong-Dong, JIAO Qing-Song, JIA Ling-Yun, SUN Kun
), GUAN Dong-Dong, JIAO Qing-Song, JIA Ling-Yun, SUN Kun
Received:2014-05-29
Accepted:2014-10-21
Online:2015-01-10
Published:2015-01-22
Contact:
Han-Qing FENG
About author:# Co-first authors
摘要:
为了进一步了解光照下植物呼吸作用的内在机理以及呼吸作用和光合作用的关系, 该文研究了在光照下菜豆(Phaseolus vulgaris)叶片抗氰呼吸与光合作用的关系。研究发现, 将黑暗下生长的菜豆幼苗叶片转到光照下10 h, 总呼吸、抗氰呼吸以及抗氰呼吸在总呼吸中的比例均逐步上升; 光照也导致了叶片叶绿体光合放氧和CO2固定的出现及其速率的增加, 但光合放氧和CO2固定速率的增加均滞后于抗氰呼吸的增加。将黑暗下生长的叶片转到光照下之前用抗氰呼吸的抑制剂水杨基氧肟酸(SHAM)处理叶片, 发现用SHAM处理并没有导致叶片在光照下光合放氧和CO2固定速率的明显变化, 这也提示了黑暗下生长的叶片转至光照的过程中, 抗氰呼吸和光合作用没有产生偶联。进一步研究发现, 在黑暗中对叶片施加短时间的光照能够增加抗氰呼吸在总呼吸中的比例, 但短时间的光照对叶片光合CO2固定速率没有影响。这些结果表明了光照对抗氰呼吸的诱导可以不依赖于光合作用, 光照可能是作为一种直接的信号去诱导抗氰呼吸。
冯汉青, 管东东, 焦青松, 贾凌云, 孙坤. 光照下菜豆叶片抗氰呼吸与光合作用关系的分析. 植物生态学报, 2015, 39(1): 104-109. DOI: 10.17521/cjpe.2015.0011
FENG Han-Qing,GUAN Dong-Dong,JIAO Qing-Song,JIA Ling-Yun,SUN Kun. Analysis of the relationship between cyanide-resistant respiration and photosynthesis under light in Phaseolus vulgaris leaves. Chinese Journal of Plant Ecology, 2015, 39(1): 104-109. DOI: 10.17521/cjpe.2015.0011
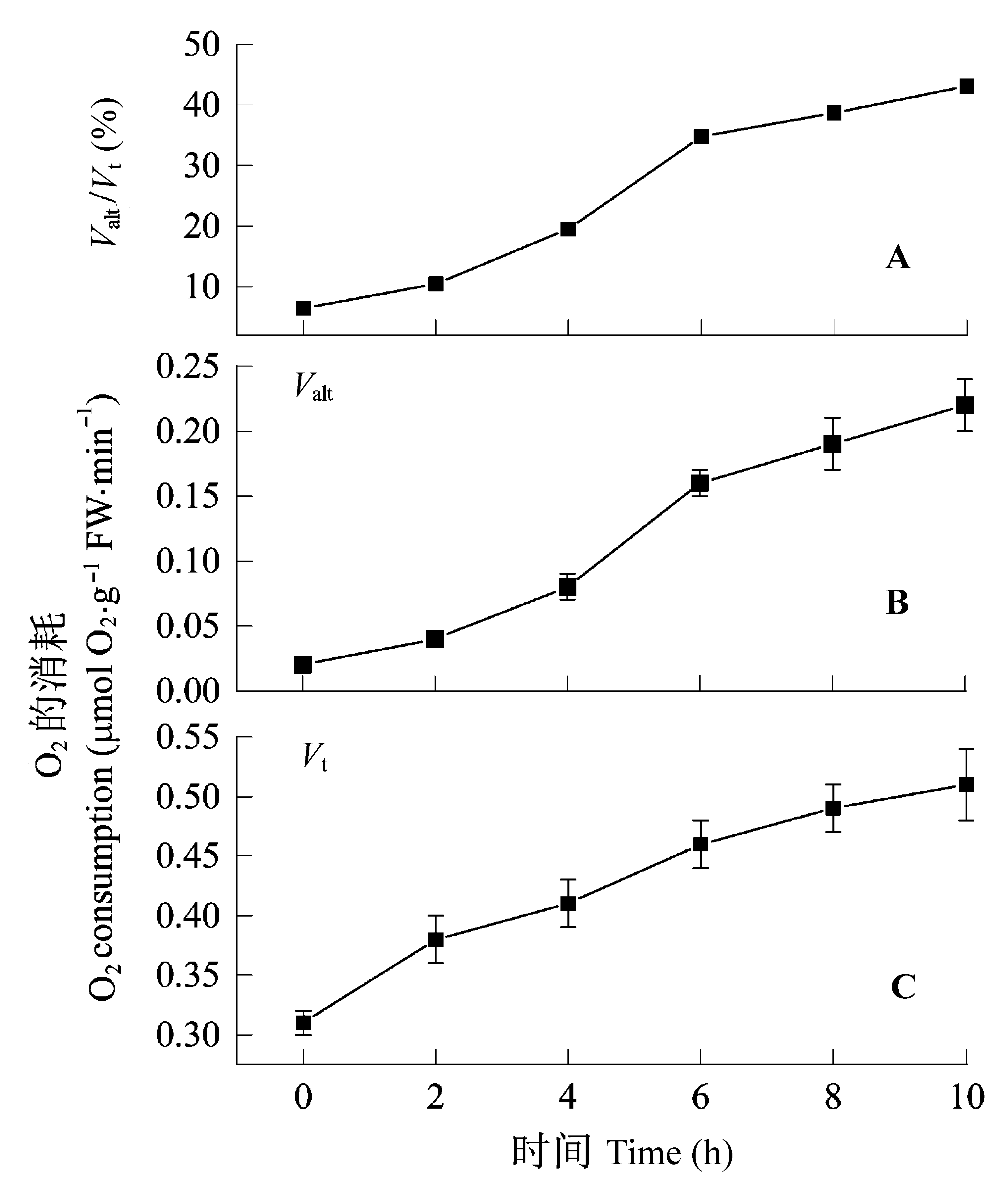
图1 黑暗生长的叶片转至光照下总呼吸(Vt)、抗氰呼吸的容量(Valt)和抗氰呼吸的容量与总呼吸的比值(Valt/Vt)的变化。数值为4次独立实验的平均值±标准偏差。所示时间为叶片转至光照下的时间。Valt/Vt来自于Valt平均值和Vt的平均值之间的比值。
Fig. 1 Changes in total respiration (Vt), capacity of cyanide- resistant respiration (Valt) and the ratio of Valt to Vt in dark- grown leaves exposed to continuous light for 10 h. These are individual samples taken during four different experiments. Time indicates hours after starting illumination. Values are mean values ± SD of four independent experiments. The horizontal axis shows the time after exposing to light. Valt/Vt was computed based on the average values of Vt and Valt.
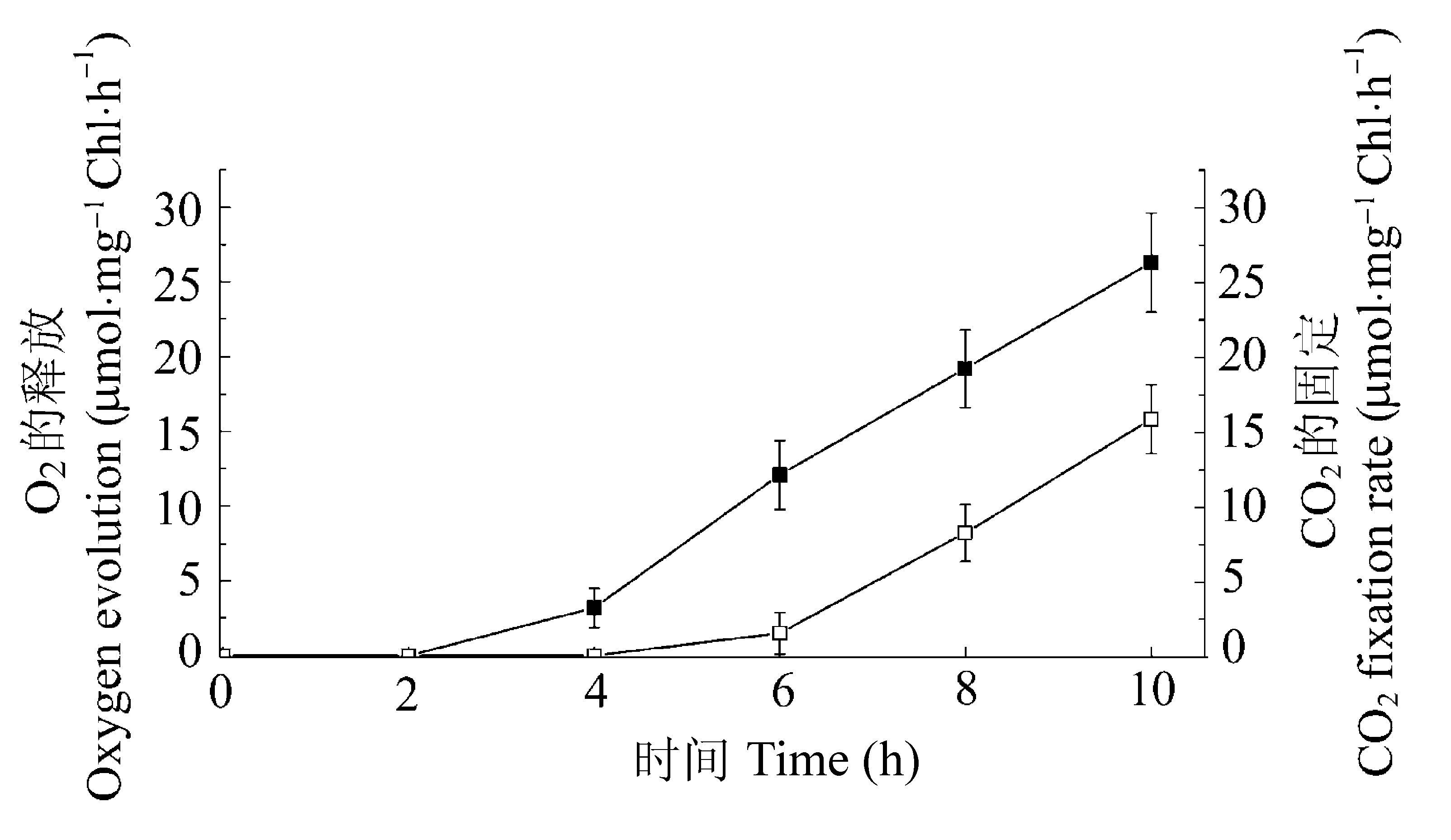
图3 黑暗生长的叶片转至光照下叶绿体CO2固定速率(□)和放氧速率(■)的变化。数值为4次独立实验的平均值±标准偏差。横坐标所示时间为叶片转至光照下的时间。
Fig. 3 Changes in oxygen evolution (■) and carbon dioxide fixation (□) in whole chloroplasts of the dark-grown leaves exposed to 10 h of continuous light. Values are mean values ± SD of four independent experiments. The horizontal axis shows the time after exposing to light.
| 光照的时间 Time of illumination (h) | SHAM对于光合作用的影响 Effects of SHAM on photosynthesis (% of control) | |
|---|---|---|
| 光合放氧 Oxygen evolution | 光合CO2固定 CO2 fixation | |
| 0 | 100a | 100a |
| 2 | 100a | 100a |
| 4 | 98 ± 5a | 100a |
| 6 | 101 ± 3a | 100a |
| 8 | 97 ± 4a | 96 ± 4a |
| 10 | 102 ± 4a | 95 ± 3a |
表1 黑暗中生长的叶片转至光照过程中水杨基氧肟酸(SHAM)对叶片光合CO2固定和放氧速率的影响
Table 1 The effects of salicylhydroxamic acid (SHAM) on photosynthetic oxygen evolution rate and carbon dioxide fixation rate when the dark-grown leaves were exposed to continuous light
| 光照的时间 Time of illumination (h) | SHAM对于光合作用的影响 Effects of SHAM on photosynthesis (% of control) | |
|---|---|---|
| 光合放氧 Oxygen evolution | 光合CO2固定 CO2 fixation | |
| 0 | 100a | 100a |
| 2 | 100a | 100a |
| 4 | 98 ± 5a | 100a |
| 6 | 101 ± 3a | 100a |
| 8 | 97 ± 4a | 96 ± 4a |
| 10 | 102 ± 4a | 95 ± 3a |
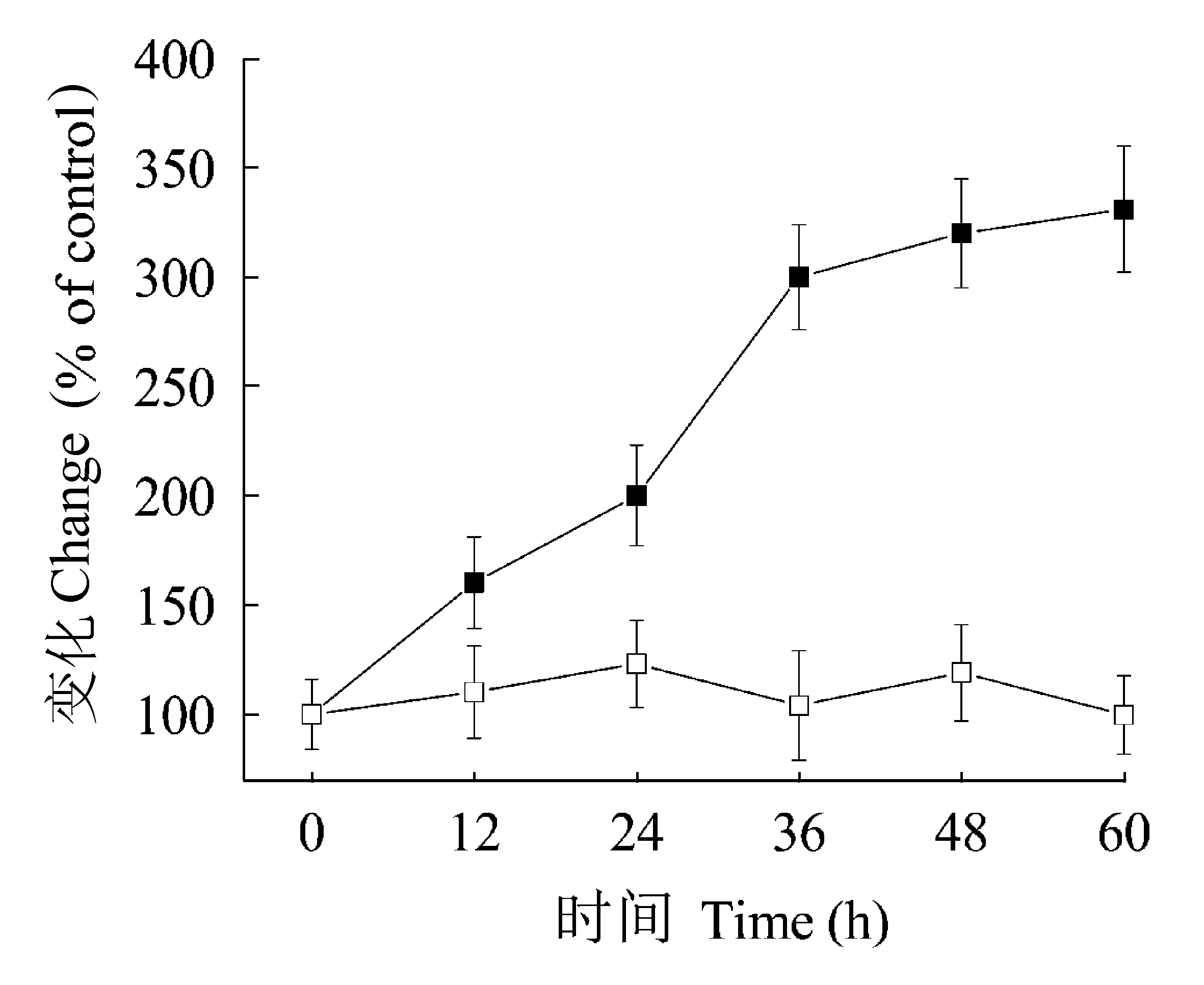
图4 短时间光照对抗氰呼吸途径与总呼吸比值(Valt/Vt, %) (■)以及对光合CO2固定速率(□)的影响。一周龄生长在12 h光照/12 h黑暗下的植物转至光黑暗中, 并每隔12 h给予10 min的短时间光照。以仍处于黑暗下的叶片作为对照(100%)。所示时间为叶片转至黑暗下的时间。数值为4次独立实验的平均值±标准偏差。
Fig. 4 Effect of short period of illumination on the ratio of cyanide-resistant respiration pathway to total respiration (Valt/Vt, %) (■) and photosynthetic CO2 fixation rate (□). One-week-old plants grown in 12 h light/12 h dark photoperiods were transferred to darkness and then received 10 min plus of light once every 12 h. Leaves in darkness were assigned the values of control (100%). Time indicates hours in darkness. Results are mean values ± SD of four independent experiments.
| 1 | Bartoli CG, Gomez F, Gergoff G, Guiamét JJ, Puntarulo S (2005). Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. Journal of Experimental Botany, 56, 1269-1276. |
| 2 | Bingham IJ, Farrar JF (1989). Activity and capacity of respiration pathways in barley roots deprived of inorganic nutrients. Plant Physiology and Biochemistry, 27, 847-854. |
| 3 | Bruick R, Mayfield SP (1999). Light-activated translation of chloroplast mRNAs. Trends in Plant Science, 4, 190-195. |
| 4 | Chivasa S, Carr JP (1998). Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. The Plant Cell, 10, 1489-1498. |
| 5 | Chivasa S, Murphy AM, Naylor M, Carr JP (1997). Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. The Plant Cell, 9, 547-557. |
| 6 | Escobar MA, Franklin KA, Svensson AS, Salter MG, Whitelam GC, Rasmusson AG (2004). Light regulation of the Arabidopsis respiratory chain. Multiple discrete photo- receptor responses contribute to induction of type II NAD(P)H dehydrogenase genes. Plant Physiology, 136, 2710-2721. |
| 7 | Finnegan PM, Whelan J, Millar AH, Zhang Q, Smith MK, Wiskich JT, Day DA (1997). Differential expression of the multigene family encoding the soybean mitochondrial alternative oxidase. Plant Physiology, 114, 455-466. |
| 8 | Gui MX, Wang XM, Huang WY (1991). The effect of light and exogenous gibberellic acid on respiration pathways during germination of tomato seeds. Physiologia Plantarum, 81, 403-407. |
| 9 | Igamberdiev AU, Bykova NV, Gardeström P (1997). Involve- ment of cyanide-resistant and rotenone-insensitive pathways of mito-chondrial electron transport during oxidation of glycine in higher plants. FEBS Letters, 412, 265-269. |
| 10 | Kim C, Meskauskiene R, Apel K, Laloi C (2008). No single way to understand singlet oxygen signalling in plants. EMBO Reports, 9, 435-439. |
| 11 | Liscum E, Hodgson DW, Campbell TJ (2003). Blue light signaling through the cryptochromes and phototropins. So that’s what the blues is all about. Plant Physiology, 133, 1429-1436. |
| 12 | Lurie S (1997). Stomatal opening and photosynthesis in greening leaves of Vicia faba L. Australian Journal of Plant Physiology, 4, 69-74. |
| 13 | Mackenzie S, McIntosh L (1999). Higher plant mitochondria. The Plant Cell, 11, 571-586. |
| 14 | Millenaar FF, Lambers H (2003). The Alternative oxidase: in vivo regulation and function. Plant Biology, 5, 2-15. |
| 15 | Obenland D, Diethelm R, Shibles R, Stewart C (1990). Relationship of alternative respiratory capacity and alternative oxidase amount during soybean seedling growth. Plant & Cell Physiology, 31, 897-901. |
| 16 | Padmasree K, Padmavathi L, Raghavendra AS (2002). Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Critical Reviews in Biochemistry and Molecular Biology, 37, 71-119. |
| 17 | Padmasree K, Raghavendra AS (2001). Restriction of mitochondrial oxidative metabolism leads to suppression of photosynthetic carbon assimilation but not of photochemical electron transport in pea mesophyll protoplasts. Current Science, 81, 680-684. |
| 18 | Raghavendra AS, Padmasree K (2003). Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends in Plant Science, 8, 546-553. |
| 19 | Ribas-Carbo M, Robinson SA, Gonzàlez-Meler MA, Lennon AM, Giles L, Siedow JN, Berry JA (2000). Effects of light on respiration and oxygen isotope fractionation in soybean cotyledons. Plant, Cell & Environment, 23, 983-989. |
| 20 | Siedow JN, Day DA (2000). Respiration and photorespiration. In: Buchanan B, Gruissem W, Jones R eds. Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, USA. 676-728. |
| 21 | Svensson ÅS, Rasmusson AG (2001). Light-dependent gene expression for proteins in the respiratory chain of potato leaves. The Plant Journal, 28, 73-82. |
| 22 | Vanlerberghe GC, McIntosh L (1997). Alternative oxidase: from gene to function. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 703-734. |
| 23 | Whelan J, Smith MK, Meijer M, Yu JW, Badger MR, Price GD, Day DA (1995). Cloning of an additional cDNA for the alternative oxidase in tobacco. Plant Physiology, 107, 1469-1470. |
| 24 | Xu F, Yuan S, Lin HH (2011). Response of mitochondrial alternative oxidase (AOX) to light signals. Plant Signaling & Behavior, 6, 55-58. |
| 25 | Yoshida K, Terashima I, Noguchi K (2006). Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant & Cell Physiology, 47, 22-31. |
| 26 | Yoshida K, Terashima I, Noguchi, K (2007). Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant & Cell Physiology, 48, 606-614. |
| 27 | Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH (2010). Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant, Cell & Environment, 33, 2121-2131. |
| 28 | Zhang LT, Gao HY, Zhang ZS, Xue ZC, Meng QW (2012). Multiple effects of inhibition of mitochondrial alternative oxidase pathway on photosynthetic apparatus in Rumex K-1 leaves. Biologia Plantarum, 56, 365-368. |
| 29 | Zhang LT, Zhang ZS, Gao HY, Xue ZC, Yang C, Meng XL, Meng QW (2011). Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiologia Plantarum, 143, 396-407. |
| [1] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [2] | 蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用[J]. 植物生态学报, 2023, 47(7): 988-997. |
| [3] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [4] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [5] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [6] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [7] | 叶子飘, 于冯, 安婷, 王复标, 康华靖. 植物气孔导度对CO2响应模型的构建[J]. 植物生态学报, 2021, 45(4): 420-428. |
| [8] | 李景, 王欣, 王振华, 王斌, 王成章, 邓美凤, 刘玲莉. 臭氧和气溶胶复合污染对杨树叶片光合作用的影响[J]. 植物生态学报, 2020, 44(8): 854-863. |
| [9] | 李旭, 吴婷, 程严, 谭钠丹, 蒋芬, 刘世忠, 褚国伟, 孟泽, 刘菊秀. 南亚热带常绿阔叶林4个树种对增温的生理生态适应能力比较[J]. 植物生态学报, 2020, 44(12): 1203-1214. |
| [10] | 刘校铭, 杨晓芳, 王璇, 张守仁. 暖温带落叶阔叶林辽东栎和五角枫生长和光合生理生态特征对模拟氮沉降的响应[J]. 植物生态学报, 2019, 43(3): 197-207. |
| [11] | 李鑫豪, 闫慧娟, 卫腾宙, 周文君, 贾昕, 查天山. 油蒿资源利用效率在生长季的相对变化及对环境因子的响应[J]. 植物生态学报, 2019, 43(10): 889-898. |
| [12] | 张娜, 朱阳春, 李志强, 卢信, 范如芹, 刘丽珠, 童非, 陈静, 穆春生, 张振华. 淹水和干旱生境下铅对芦苇生长、生物量分配和光合作用的影响[J]. 植物生态学报, 2018, 42(2): 229-239. |
| [13] | 韩玲, 赵成章, 冯威, 徐婷, 郑慧玲, 段贝贝. 张掖湿地芨芨草叶脉密度和叶脉直径的权衡关系对3种生境的响应[J]. 植物生态学报, 2017, 41(8): 872-881. |
| [14] | 韩吉梅, 张旺锋, 熊栋梁, 张亚黎. 植物光合作用叶肉导度及主要限制因素研究进展[J]. 植物生态学报, 2017, 41(8): 914-924. |
| [15] | 蔡建国, 韦孟琪, 章毅, 魏云龙. 遮阴对绣球光合特性和叶绿素荧光参数的影响[J]. 植物生态学报, 2017, 41(5): 570-576. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19