

植物生态学报 ›› 2024, Vol. 48 ›› Issue (4): 523-533.DOI: 10.17521/cjpe.2022.0346 cstr: 32100.14.cjpe.2022.0346
王袼1,*, 胡姝娅2, 李阳2, 陈晓鹏1, 李红玉1, 董宽虎1, 何念鹏3, 王常慧1,2,**
收稿日期:2022-08-25
接受日期:2023-02-20
出版日期:2024-04-20
发布日期:2024-05-11
通讯作者:
**
作者简介:wg18335963261@163.com
基金资助:WANG Ge1,*, HU Shu-Ya2, LI Yang2, CHEN Xiao-Peng1, LI Hong-Yu1, DONG Kuan-Hu1, HE Nian-Peng3, WANG Chang-Hui1,2,**
Received:2022-08-25
Accepted:2023-02-20
Online:2024-04-20
Published:2024-05-11
Contact:
**
Supported by:摘要:
土壤中的无机氮是维持陆地生态系统生产力的主要限制因子, 主要通过有机氮的矿化产生。土壤氮可利用性的高低取决于土壤微生物、地上植被类型以及土壤理化性质等。土壤微生物对环境变化极为敏感, 尤其是温度与微生物生长繁殖密切相关。因此, 在较大空间范围内研究微生物调控氮矿化速率的温度敏感性(Q10)对于预测全球气候变化对陆地生态系统生产力的影响至关重要。该研究以中国三大高原(内蒙古高原、黄土高原和青藏高原)的不同类型草原为研究对象, 通过野外采集土壤样品, 室内不同温度培养测定三大高原不同类型草地土壤的净氮矿化速率, 计算其Q10, 并分析其与土壤微生物及土壤理化性质参数的相关性。结果表明: (1)黄土高原不同类型草地土壤氮矿化速率的Q10显著高于内蒙古高原和青藏高原; (2)在黄土高原和内蒙古高原, 草甸草原和典型草原土壤净氮矿化速率的Q10显著高于荒漠草原, 而青藏高原高寒草甸草原土壤净氮矿化速率的Q10显著低于高寒典型草原和高寒荒漠草原; (3)三大高原草地土壤微生物生物量碳含量与土壤净氮矿化速率的Q10显著相关; (4) Q10的空间格局受微生物、土壤质地以及底物的共同调控。该研究结果为中国不同类型草原土壤氮循环对全球变化响应的研究提供了重要数据, 对于陆地生态系统氮循环模型的完善具有一定的科学价值。
王袼, 胡姝娅, 李阳, 陈晓鹏, 李红玉, 董宽虎, 何念鹏, 王常慧. 不同类型草原土壤净氮矿化速率的温度敏感性. 植物生态学报, 2024, 48(4): 523-533. DOI: 10.17521/cjpe.2022.0346
WANG Ge, HU Shu-Ya, LI Yang, CHEN Xiao-Peng, LI Hong-Yu, DONG Kuan-Hu, HE Nian-Peng, WANG Chang-Hui. Temperature sensitivity of soil net nitrogen mineralization rates across different grassland types. Chinese Journal of Plant Ecology, 2024, 48(4): 523-533. DOI: 10.17521/cjpe.2022.0346
| 采样点 Sample site | 草原类型 Steppe type | 纬度 Latitude (° N) | 经度 Longitude (° E) | 海拔 Altitude (m) | 年平均气温 Mean annual air temperature (℃) | 年降水量 Mean annual precipitation (mm) | 土壤pH Soil pH | 优势物种 Dominant species |
|---|---|---|---|---|---|---|---|---|
| 黄土高原 Loess Plateau | 山地草甸草原 Mountain meadow steppe | 35.99-36.29 | 111.64-113.36 | 843.67 ± 7.82c | 10.82 ± 0.16a | 573.73 ± 2.58a | 8.05 ± 0.02a | 白羊草、兴安胡枝子、薹草 Bothriochloa ischaemum, Lespedeza davurica, Carex spp. |
| 山地典型草原 Mountain typical steppe | 36.07-37.58 | 107.19-110.18 | 1 288.00 ± 37.47b | 8.23 ± 0.37b | 466.37 ± 9.60b | 8.09 ± 0.02a | 白羊草、兴安胡枝子、铁杆蒿 Bothriochloa ischaemum, Lespedeza davurica, Artemisia gmelinii | |
| 山地荒漠草原 Mountain desert steppe | 37.42-37.46 | 104.44-105.78 | 1 461.67 ± 37.90a | 7.05 ± 0.82c | 256.58 ± 9.53c | 8.23 ± 0.13a | 红砂、糙隐子草、阿尔泰狗娃花 Reaumuria songarica, Cleistogenes squarrosa, Aster altaicus | |
| 内蒙古高原 Nei Mongol Plateau | 草甸草原 Meadow steppe | 44.52-45.11 | 120.33-123.51 | 357.67 ± 45.83c | 4.87 ± 0.18a | 405.78 ± 6.29a | 8.18 ± 0.15a | 羊草、狗尾草、芦苇 Leymus chinensis, Setaria viridis, Phragmites australis |
| 典型草原 Typical steppe | 43.55-44.77 | 116.52-118.36 | 1 111.00 ± 18.46a | 0.96 ± 0.12c | 362.39 ± 6.19b | 7.59 ± 0.05b | 羊草、针茅、薹草 Leymus chinensis, Stipa capillata, Carex spp. | |
| 荒漠草原 Desert steppe | 43.63-44.01 | 112.15-114.89 | 1 026.00 ± 12.44b | 2.09 ± 0.31b | 220.88 ± 9.68c | 7.05 ± 0.02c | 猪毛菜、蒙古韭、蒺藜 Kali collinum, Allium mongolicum, Tribulus terrestris | |
| 青藏高原 Qingzang Plateau | 高寒草甸草原 Alpine meadow steppe | 31.46-32.48 | 92.01-95.45 | 4 400.00 ± 44.15b | -1.82 ± 0.41a | 552.67 ± 10.35a | 6.92 ± 0.07b | 薹草、高山嵩草、钉柱委陵菜 Carex spp., Carex parvula, Potentilla saundersiana |
| 高寒典型草原 Alpine typical steppe | 31.38-31.92 | 85.84-90.74 | 4 678.25 ± 27.10a | -4.04 ± 0.29c | 433.99 ± 10.66b | 8.16 ± 0.03a | 紫花针茅、薹草、针茅 Stipa purpurea, Carex spp., Stipa capillata | |
| 高寒荒漠草原 Alpine desert steppe | 32.30-32.48 | 80.15-83.34 | 4 488.00 ± 23.65b | -2.89 ± 0.24b | 266.75 ± 11.28c | 8.23 ± 0.05a | 针茅、薹草、戈壁针茅 Stipa capillata, Carex spp., Stipa tianschanica var. gobica |
表1 不同类型草原采样点地理位置、植被、土壤基本信息
Table 1 Geographical location, vegetation and soil basic information of sampling sites across different grassland types
| 采样点 Sample site | 草原类型 Steppe type | 纬度 Latitude (° N) | 经度 Longitude (° E) | 海拔 Altitude (m) | 年平均气温 Mean annual air temperature (℃) | 年降水量 Mean annual precipitation (mm) | 土壤pH Soil pH | 优势物种 Dominant species |
|---|---|---|---|---|---|---|---|---|
| 黄土高原 Loess Plateau | 山地草甸草原 Mountain meadow steppe | 35.99-36.29 | 111.64-113.36 | 843.67 ± 7.82c | 10.82 ± 0.16a | 573.73 ± 2.58a | 8.05 ± 0.02a | 白羊草、兴安胡枝子、薹草 Bothriochloa ischaemum, Lespedeza davurica, Carex spp. |
| 山地典型草原 Mountain typical steppe | 36.07-37.58 | 107.19-110.18 | 1 288.00 ± 37.47b | 8.23 ± 0.37b | 466.37 ± 9.60b | 8.09 ± 0.02a | 白羊草、兴安胡枝子、铁杆蒿 Bothriochloa ischaemum, Lespedeza davurica, Artemisia gmelinii | |
| 山地荒漠草原 Mountain desert steppe | 37.42-37.46 | 104.44-105.78 | 1 461.67 ± 37.90a | 7.05 ± 0.82c | 256.58 ± 9.53c | 8.23 ± 0.13a | 红砂、糙隐子草、阿尔泰狗娃花 Reaumuria songarica, Cleistogenes squarrosa, Aster altaicus | |
| 内蒙古高原 Nei Mongol Plateau | 草甸草原 Meadow steppe | 44.52-45.11 | 120.33-123.51 | 357.67 ± 45.83c | 4.87 ± 0.18a | 405.78 ± 6.29a | 8.18 ± 0.15a | 羊草、狗尾草、芦苇 Leymus chinensis, Setaria viridis, Phragmites australis |
| 典型草原 Typical steppe | 43.55-44.77 | 116.52-118.36 | 1 111.00 ± 18.46a | 0.96 ± 0.12c | 362.39 ± 6.19b | 7.59 ± 0.05b | 羊草、针茅、薹草 Leymus chinensis, Stipa capillata, Carex spp. | |
| 荒漠草原 Desert steppe | 43.63-44.01 | 112.15-114.89 | 1 026.00 ± 12.44b | 2.09 ± 0.31b | 220.88 ± 9.68c | 7.05 ± 0.02c | 猪毛菜、蒙古韭、蒺藜 Kali collinum, Allium mongolicum, Tribulus terrestris | |
| 青藏高原 Qingzang Plateau | 高寒草甸草原 Alpine meadow steppe | 31.46-32.48 | 92.01-95.45 | 4 400.00 ± 44.15b | -1.82 ± 0.41a | 552.67 ± 10.35a | 6.92 ± 0.07b | 薹草、高山嵩草、钉柱委陵菜 Carex spp., Carex parvula, Potentilla saundersiana |
| 高寒典型草原 Alpine typical steppe | 31.38-31.92 | 85.84-90.74 | 4 678.25 ± 27.10a | -4.04 ± 0.29c | 433.99 ± 10.66b | 8.16 ± 0.03a | 紫花针茅、薹草、针茅 Stipa purpurea, Carex spp., Stipa capillata | |
| 高寒荒漠草原 Alpine desert steppe | 32.30-32.48 | 80.15-83.34 | 4 488.00 ± 23.65b | -2.89 ± 0.24b | 266.75 ± 11.28c | 8.23 ± 0.05a | 针茅、薹草、戈壁针茅 Stipa capillata, Carex spp., Stipa tianschanica var. gobica |
| 扩增基因 Amplification gene | 引物 Primer | 引物序列(5′-3′) Primer sequence (5′-3′) | 片段长度 Fragment length | 扩增条件 Amplification procedure |
|---|---|---|---|---|
| 氨氧化古菌-氨单加氧酶基因 AOA-amoA | Arch-amoAF | STAATGGTCTGGCTTAGACG | 635 bp | 94 ℃预变性3 min; 94 ℃变性30 s, 55 ℃退火30 s, 72 ℃延伸1 min, 共30个循环; 72 ℃延伸10 min Pre-denaturation at 94 °C for 3 min; follow by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min; extension at 72 °C for 10 min |
| Arch-amoAR | GCGGCCATCCATCTGTATGT | |||
| 氨氧化细菌-氨单加氧酶基因 AOB-amoA | AmoA-1F | GGGGTTTCTACTGGTGGT | 491 bp | |
| AmoA-2R | CCCCTCKGSAAAGCCTTCTTC |
表2 荧光定量PCR所用引物及扩增条件
Table 2 Primer sequences and reaction conditions used for fluorescence quantitative PCR
| 扩增基因 Amplification gene | 引物 Primer | 引物序列(5′-3′) Primer sequence (5′-3′) | 片段长度 Fragment length | 扩增条件 Amplification procedure |
|---|---|---|---|---|
| 氨氧化古菌-氨单加氧酶基因 AOA-amoA | Arch-amoAF | STAATGGTCTGGCTTAGACG | 635 bp | 94 ℃预变性3 min; 94 ℃变性30 s, 55 ℃退火30 s, 72 ℃延伸1 min, 共30个循环; 72 ℃延伸10 min Pre-denaturation at 94 °C for 3 min; follow by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 1 min; extension at 72 °C for 10 min |
| Arch-amoAR | GCGGCCATCCATCTGTATGT | |||
| 氨氧化细菌-氨单加氧酶基因 AOB-amoA | AmoA-1F | GGGGTTTCTACTGGTGGT | 491 bp | |
| AmoA-2R | CCCCTCKGSAAAGCCTTCTTC |
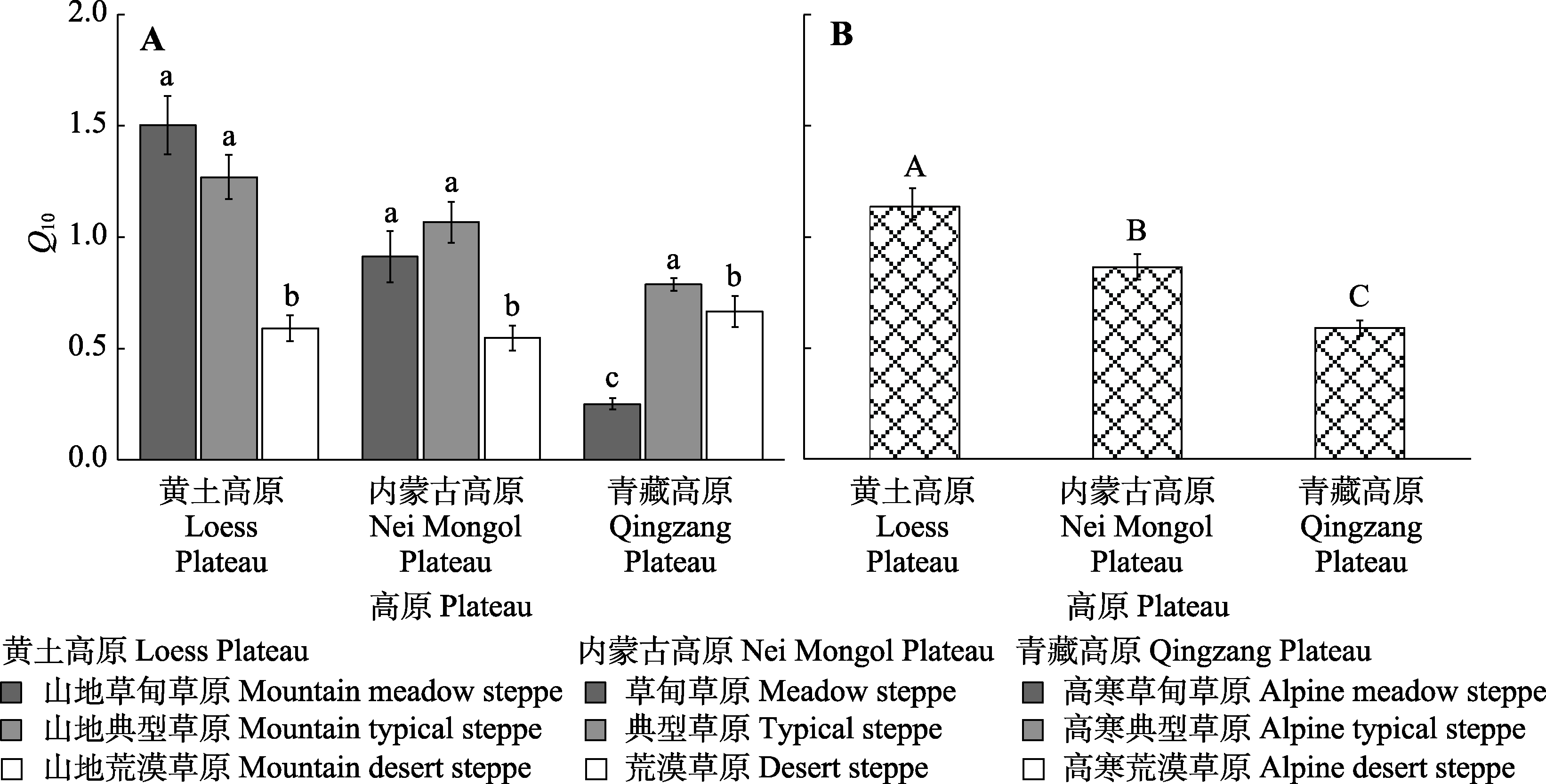
图2 不同高原的不同草地类型(A)和不同高原(B)土壤净氮矿化速率的温度敏感性(Q10, 平均值±标准误)。不同小写字母表示同一高原不同草地类型间差异显著(p < 0.05); 不同大写字母表示不同高原间差异显著(p < 0.05)。
Fig. 2 Temperature sensitivity of soil net nitrogen mineralization (Q10, mean ± SE) of different steppe types (A) and different plateau (B). Different lowercase letters indicate significant difference between different steppe types on the same plateau (p < 0.05); different uppercase letters indicate significant difference between different plateaus (p < 0.05).
| 因子 Factor | Q10 | 微生物生物量 碳含量 MBC content | 微生物生物量 氮含量 MBN content | 微生物生物量 碳氮含量比 MBC:MBN | 氨氧化古菌丰度 AOA abundance | 氨氧化细菌丰度 AOB abundance | 氨氧化细菌与 古菌丰度比 AOA:AOB |
|---|---|---|---|---|---|---|---|
| 样带 Transect (T) | 32.82** | 59.60** | 44.31 | 30.13** | 58.20** | 48.71** | 36.46** |
| 草地类型 Steppe type (S) | 21.96** | 63.05** | 14 910.07** | 0.82 | 81.76** | 18.64** | 12.37** |
| T × S | 14.79** | 3.64** | 2 246.00** | 3.62** | 17.33** | 6.26** | 4.87** |
表3 不同高原和草地类型对土壤净氮矿化速率的温度敏感性(Q10)、微生物特征的主效应及其交互效应的方差分析(F值)
Table 3 Results (F-value) of two-way ANOVA on the effect of different plateau and different steppe types and their interactions on temperature sensitivity of soil net nitrogen mineralization rates (Q10) and soil microorganism
| 因子 Factor | Q10 | 微生物生物量 碳含量 MBC content | 微生物生物量 氮含量 MBN content | 微生物生物量 碳氮含量比 MBC:MBN | 氨氧化古菌丰度 AOA abundance | 氨氧化细菌丰度 AOB abundance | 氨氧化细菌与 古菌丰度比 AOA:AOB |
|---|---|---|---|---|---|---|---|
| 样带 Transect (T) | 32.82** | 59.60** | 44.31 | 30.13** | 58.20** | 48.71** | 36.46** |
| 草地类型 Steppe type (S) | 21.96** | 63.05** | 14 910.07** | 0.82 | 81.76** | 18.64** | 12.37** |
| T × S | 14.79** | 3.64** | 2 246.00** | 3.62** | 17.33** | 6.26** | 4.87** |
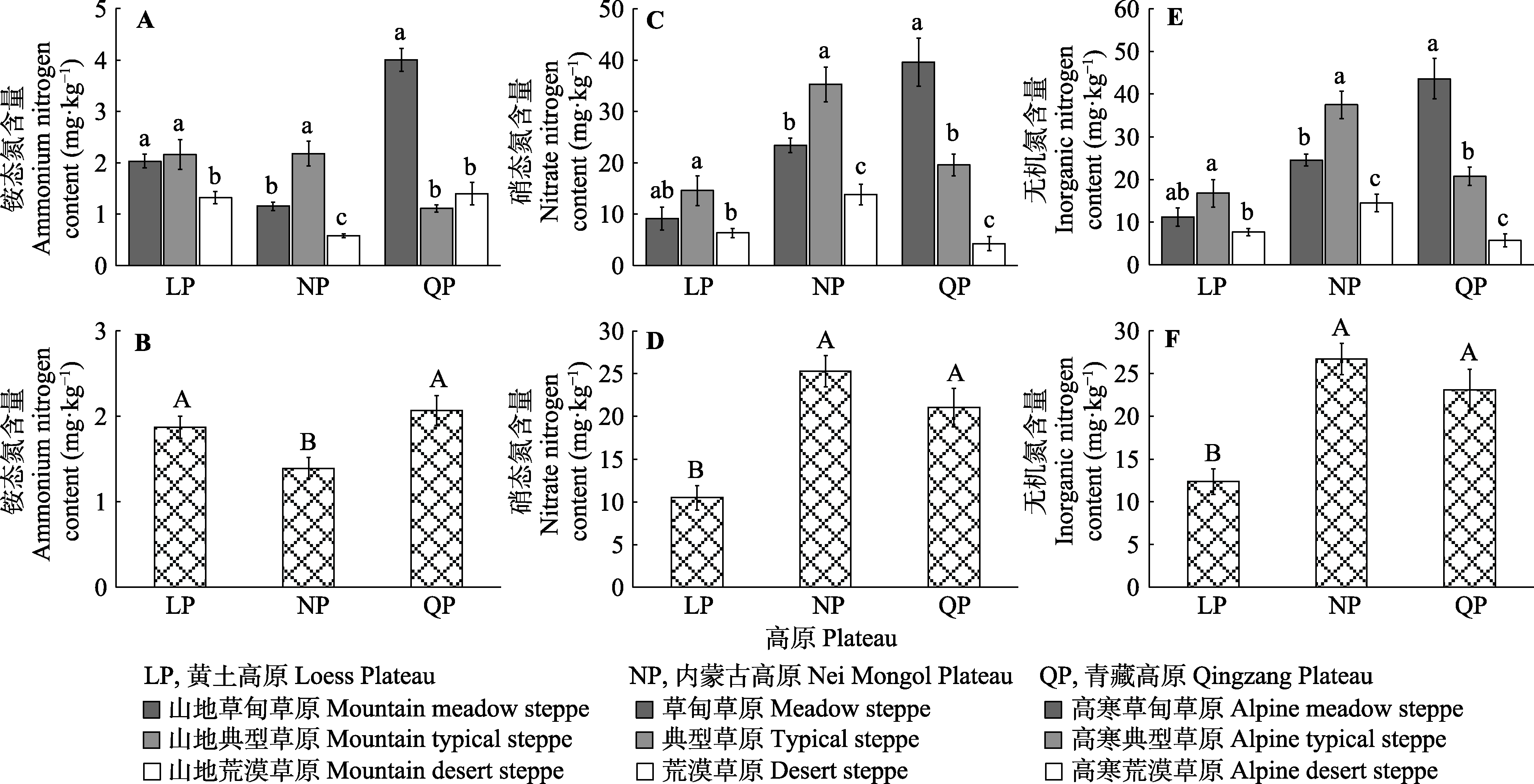
图3 不同高原和草地类型的铵态氮(A、B)、硝态氮(C、D)和无机氮(E、F)含量(平均值±标准误)。不同小写字母表示同一高原不同草地类型间差异显著(p < 0.05), 不同大写字母表示不同高原间差异显著(p < 0.05)。
Fig. 3 Contents of ammonium (A, B), nitrate (C, D) and inorganic nitrogen (E, F) in different plateau and steppe types (mean ± SE). Different lowercase letters indicate significant difference between different steppe types on the same plateau (p < 0.05); different uppercase letters indicate significant difference between different plateaus (p < 0.05).
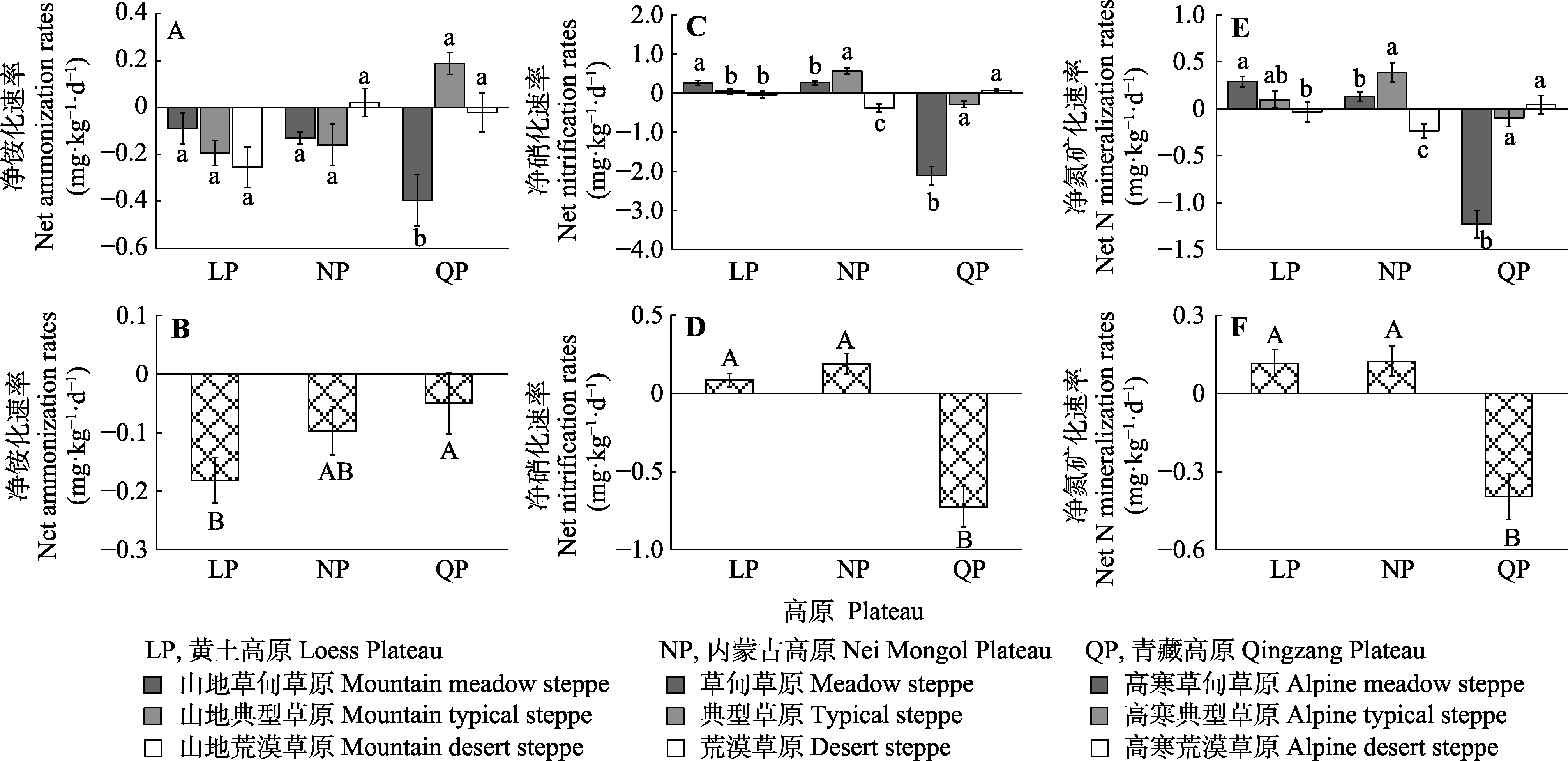
图4 不同高原和草地类型的净铵化(A、B)、净硝化(C、D)、净氮矿化(E、F)速率(平均值±标准误)。不同小写字母表示同一高原不同草地类型间差异显著(p < 0.05), 不同大写字母表示不同高原间差异显著(p < 0.05)。
Fig. 4 Rate of net ammonization (A, B), net nitrification (C, D) and net nitrogen (N) mineralization (E, F) of different plateaus and steppe types (mean ± SE). Different lowercase letters indicate significant difference between different steppe types on the same plateau (p < 0.05); different uppercase letters indicate significant difference between different plateaus (p < 0.05).
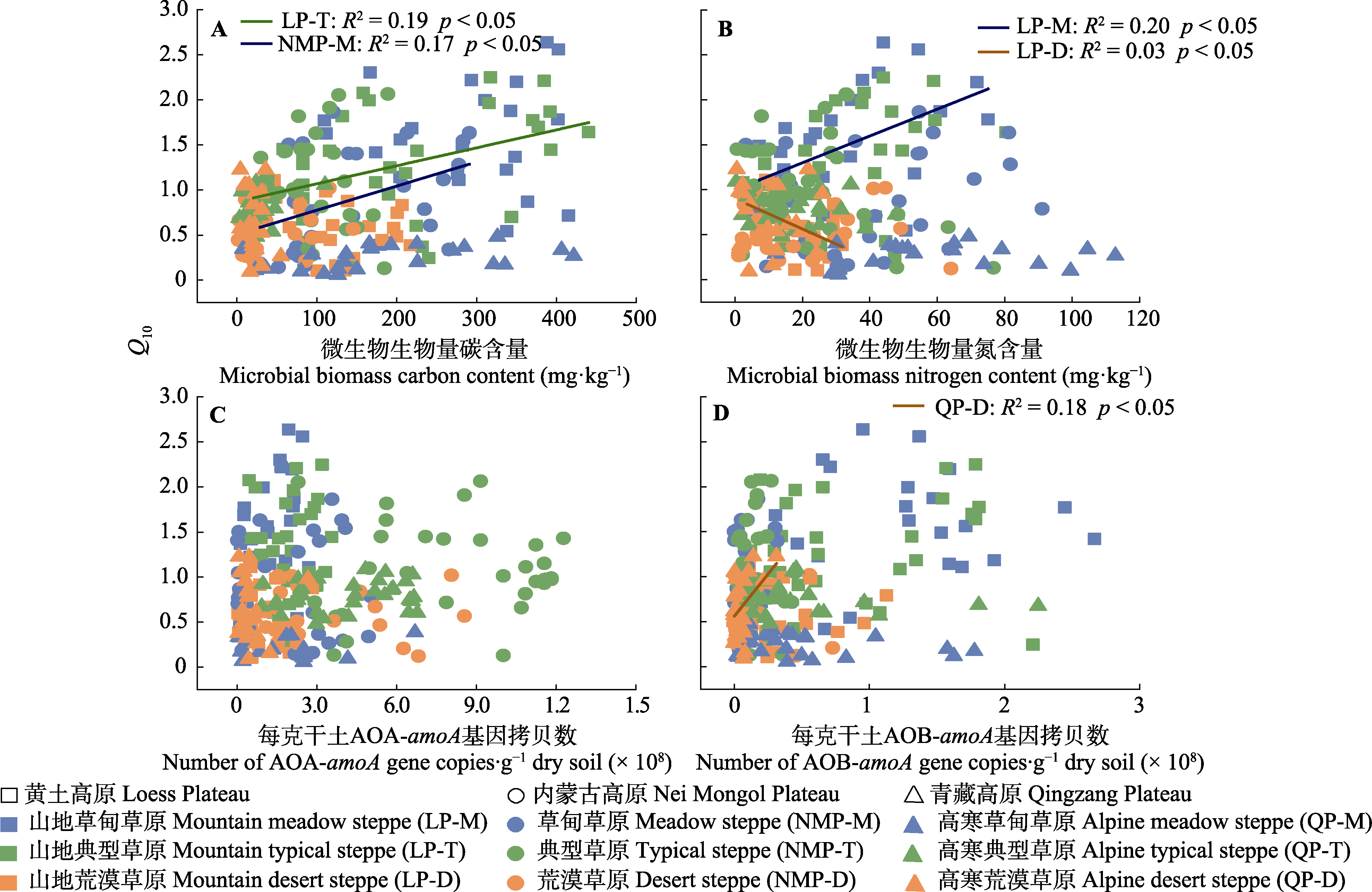
图5 土壤净氮矿化速率的温度敏感性(Q10)与微生物生物量碳含量(A)、微生物生物量氮含量(B)、氨氧化古菌(AOA)丰度(C)、氨氧化细菌(AOB)丰度(D)的线性相关分析。
Fig. 5 Linear correlation analysis between temperature sensitivity (Q10) of soil net nitrogen mineralization rates and microbial biomass carbon content (A), microbial biomass nitrogen content (B), ammonium-oxidation archaea (AOA) abundance (C) and ammonium-oxidation bacterial (AOB) abundance (D).
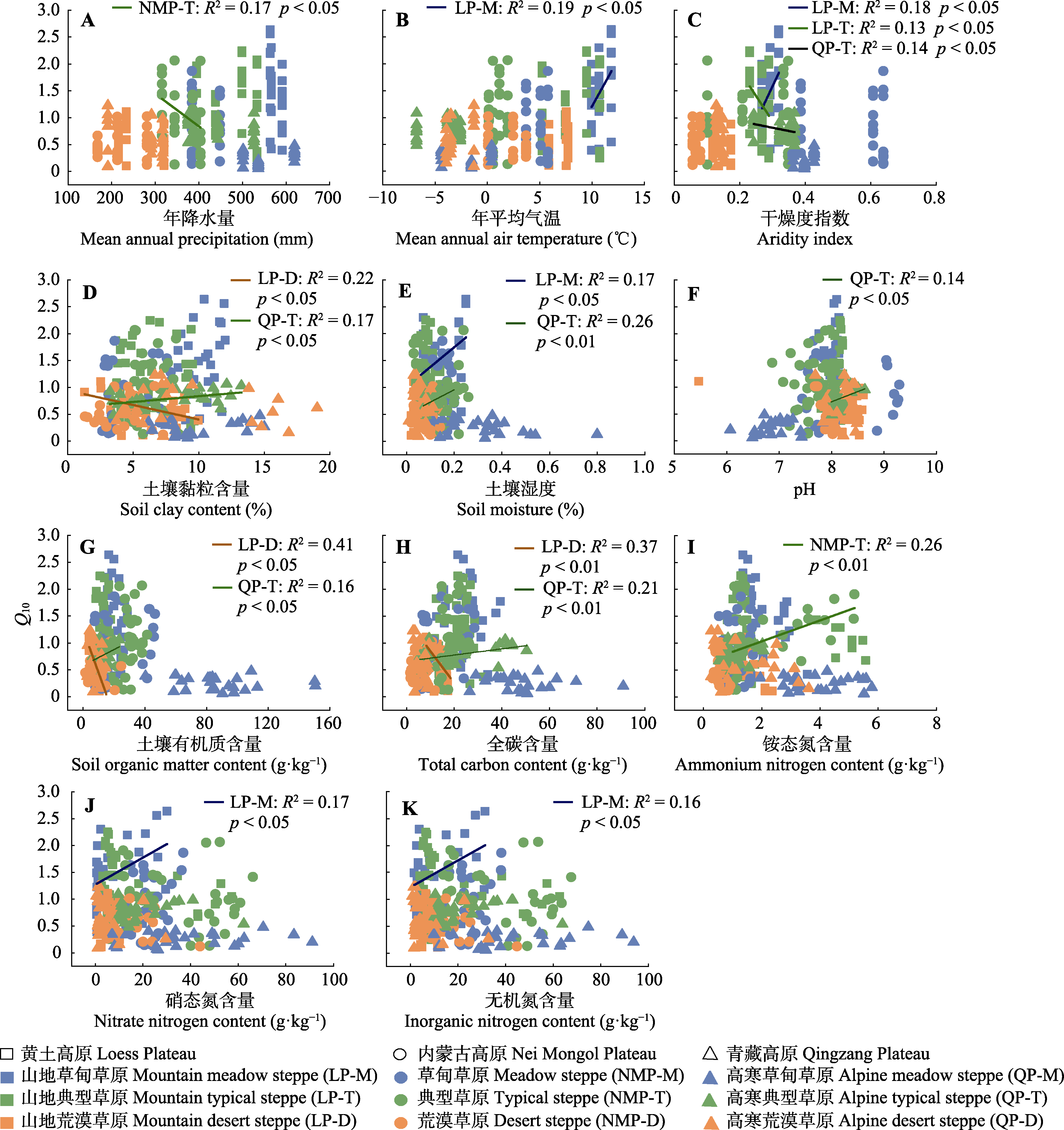
图6 土壤净氮矿化速率的温度敏感性(Q10)与年降水量(A)、年平均气温(B)、干燥度指数(C)、土壤黏粒含量(D)、土壤含水量(E)、pH (F)、土壤有机质(G)、全碳(H)、铵态氮(I)、硝态氮(J)、无机氮(K)含量的线性相关分析。
Fig. 6 Linear correlation analysis between temperature sensitivity (Q10) of soil net nitrogen mineralization rates and mean annual precipitation (A), mean annual air temperature (B), aridity index (C), soil clay content (D), soil moisture content (E), pH (F), soil organic matter content (G), total carbon content (H), ammonium nitrogen content (I), nitrate nitrogen content content (J) and inorganic nitrogen content (K).
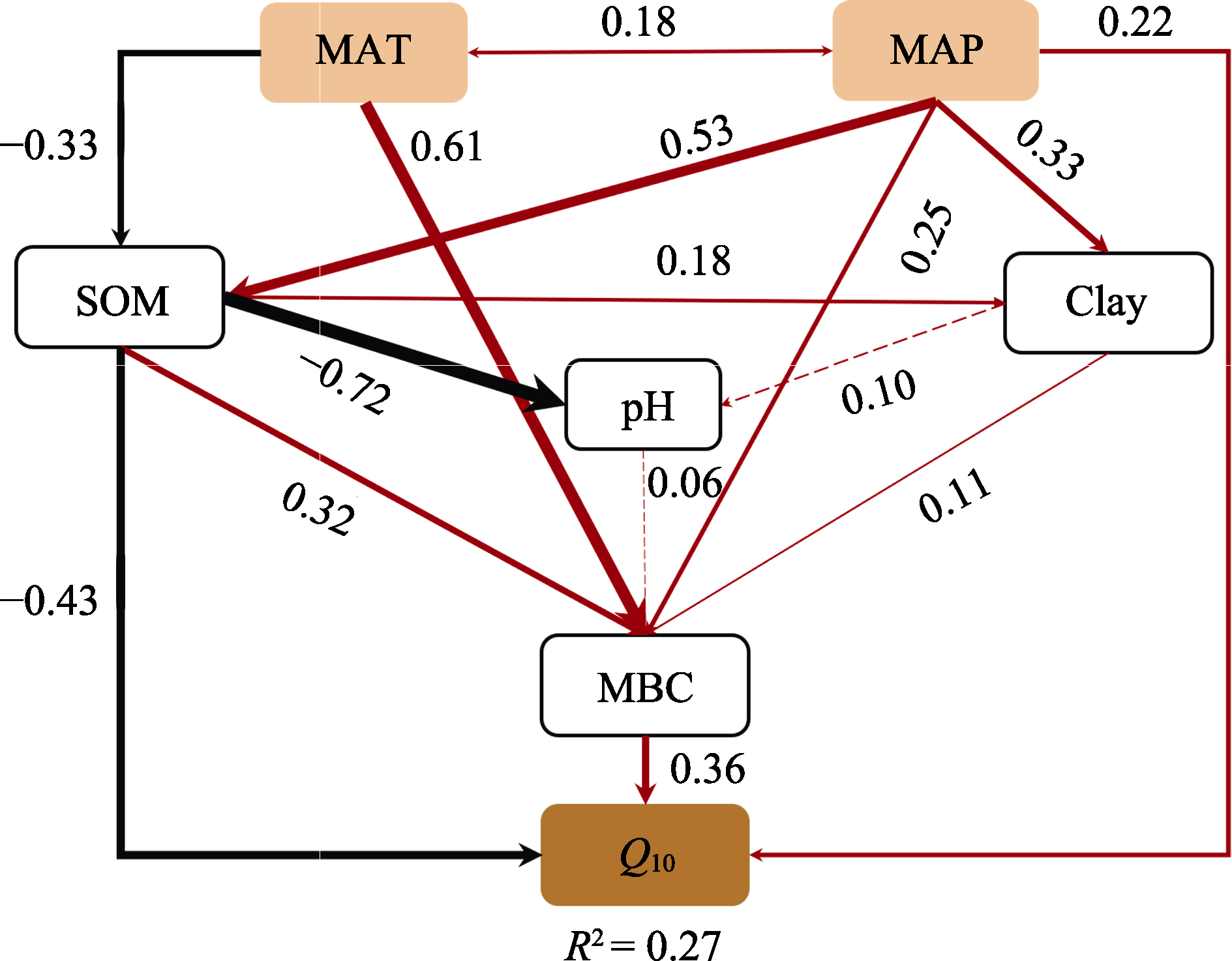
图7 年平均气温(MAT)、年降水量(MAP)、土壤有机质含量(SOM)、土壤黏粒含量(Clay)、土壤pH、微生物生物量碳含量(MBC)对草原土壤净氮矿化速率温度敏感性(Q10)影响的结构方程模型。红色和黑色实线分别表示正相关和负相关关系, 虚线表示相关性不显著。线段粗细分别代表相关性的显著程度, 箭头相邻的数字表示关系的标准化路径系数。χ2 = 8.581, p = 0.199, df = 6, 近似均方根误差(RMSEA) < 0.05。
Fig. 7 Structural equation model of effects of mean annual air temperature (MAT), mean annual precipitation (MAP), soil organic matter content (SOM), soil clay content (Clay), soil pH and microbial biomass carbon content (MBC) on temperature sensitivity (Q10) of soil net nitrogen mineralization rate. The solid red and black lines indicate positive and negative effects, respectively, the dashed line indicates that the correlation is not significant. Arrow line thickness indicates the strength of the causal relationship. Numbers adjacent to arrows represented standardized path coefficients of the relationships. χ2 = 8.581, p = 0.199, df = 6, root mean square error of approximation (RMSEA) < 0.05.
| [1] | Bosatta E, Ågren GI (1999). Soil organic matter quality interpreted thermodynamically. Soil Biology & Biochemistry, 31, 1889-1891. |
| [2] | Clark DR, McKew BA, Dong LF, Leung G, Dumbrell AJ, Stott A, Grant H, Nedwell DB, Trimmer M, Whitby C (2020). Mineralization and nitrification: archaea dominate ammonia-oxidising communities in grassland soils. Soil Biology & Biochemistry, 143, 107725. DOI: 10.1016/j.soilbio.2020.107725. |
| [3] | Dai Z, Yu M, Chen H, Zhao H, Huang Y, Su W, Xia F, Chang S, Brookes PC, Dahlgren RA, Xu J (2020). Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Global Change Biology, 26, 5267-5276. |
| [4] | Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009). Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geoscience, 2, 621-624. |
| [5] | Du E, Terrer C, Pellegrini AFA, Ahlström A, van Lissa CJ, Zhao X, Xia N, Wu X, Jackson RB, (2020). Global patterns of terrestrial nitrogen and phosphorus limitation. Nature Geoscience, 13, 221-226. |
| [6] | Guo YL, Mao WQ, Wang HY, Zhang WY (2021). Climate change characteristics of the boundary layer height with dryness and wetness and their relationship in extreme arid areas. Climatic and Environmental Research, 26, 532-540. |
| [郭燕玲, 毛文茜, 王泓宇, 张文煜 (2021). 极端干旱区边界层高度与干湿的气候变化特征及相互关系分析. 气候与环境研究, 26, 532-540.] | |
| [7] | Hassink J, Bouwman LA, Zwart KB, Bloem J, Brussaard L (1993). Relationships between soil texture, physical protection of organic matter, soil biota, and C and N mineralization in grassland soils. Geoderma, 57, 105-128. |
| [8] | He JZ, Zhang LM (2009). Advances in ammonia-oxidizing microorganisms and global nitrogen cycle. Acta Ecologica Sinica, 29, 406-415. |
| [贺纪正, 张丽梅 (2009). 氨氧化微生物生态学与氮循环研究进展. 生态学报, 29, 406-415.] | |
| [9] | Hu HW, Chen D, He JZ (2015). Microbial regulation of terrestrial nitrous oxide formation: understanding the biological pathways for prediction of emission rates. FEMS Microbiology Reviews, 39, 729-749. |
| [10] | Hu S, Wang C, Risch AC, Liu Y, Li Y, Li L, Xu X, He N, Han X, Huang J (2022). Hydrothermal conditions determine soil potential net N mineralization rates in arid and semi-arid grasslands. Functional Ecology, 36, 2626-2635. |
| [11] | Kirschbaum MUF (1995). The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biology & Biochemistry, 27, 753-760. |
| [12] | Lambers H, Chapin III FS, Pons TL (2008). Plant Physiological Ecology. 2nd ed. Springer, New York. |
| [13] |
Li ZL, Tian DS, Wang BX, Wang JS, Wang S, Chen HYH, Xu XF, Wang CH, He NP, Niu SL (2019). Microbes drive global soil nitrogen mineralization and availability. Global Change Biology, 25, 1078-1088.
DOI PMID |
| [14] | Lin YX, Hu HW, Ye GP, Fan JB, Ding WX, He ZY, Zheng Y, He JZ (2021). Ammonia-oxidizing bacteria play an important role in nitrification of acidic soils: a meta-analysis. Geoderma, 404, 115395. DOI: 10.1016/j.geoderma.2021.115395. |
| [15] |
Lipson DA (2007). Relationships between temperature responses and bacterial community structure along seasonal and altitudinal gradients. FEMS Microbiology Ecology, 59, 418-427.
PMID |
| [16] | Liu Y, He NP, Wen XF, Yu GR, Gao Y, Jia YL (2016). Patterns and regulating mechanisms of soil nitrogen mineralization and temperature sensitivity in Chinese terrestrial ecosystems. Agriculture, Ecosystems & Environment, 215, 40-46. |
| [17] |
Liu Y, Wang C, He N, Wen X, Gao Y, Li S, Niu S, Butterbach- Bahl K, Luo Y, Yu G (2017). A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: latitudinal patterns and mechanisms. Global Change Biology, 23, 455-464.
DOI PMID |
| [18] | Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B, Keiblinger KM, Zechmeister-Boltenstern S, Richter A (2014). Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nature Communications, 5, 3694. DOI: 10.1038/ncomms4694. |
| [19] | Pan QM, Xue JG, Tao J, Xu MY, Zhang WH (2018). Current status of grassland degradation and measures for grassland restoration in northern China. Chinese Science Bulletin, 63, 1642-1650. |
| [潘庆民, 薛建国, 陶金, 徐明月, 张文浩 (2018). 中国北方草原退化现状与恢复技术. 科学通报, 63, 1642-1650.] | |
| [20] | Ramond JB, Jordaan K, Díez B, Heinzelmann SM, Cowan DA (2022). Microbial biogeochemical cycling of nitrogen in arid ecosystems. Microbiology and Molecular Biology Reviews, 86, e00109-21. DOI: 10.1128/mmbr.00109-21. |
| [21] | Rütting T, Schleusner P, Hink L, Prosser JI (2021). The contribution of ammonia-oxidizing archaea and bacteria to gross nitrification under different substrate availability. Soil Biology & Biochemistry, 160, 108353. DOI: 10.1016/j.soilbio.2021.108353. |
| [22] | Sanaullah M, Rumpel C, Charrier X, Chabbi A (2012). How does drought stress influence the decomposition of plant litter with contrasting quality in a grassland ecosystem? Plant and Soil, 352, 277-288. |
| [23] | Schleuss PM, Widdig M, Biederman LA, Borer ET, Crawley MJ, Kirkman KP, Seabloom EW, Wragg PD, Spohn M (2021). Microbial substrate stoichiometry governs nutrient effects on nitrogen cycling in grassland soils. Soil Biology & Biochemistry, 155, 108168, DOI: 10.1016/j.soilbio.2021.108168. |
| [24] | Verhamme DT, Prosser JI, Nicol GW (2011). Ammonia concentration determines differential growth of ammonia- oxidising archaea and bacteria in soil microcosms. The ISME Journal, 5, 1067-1071. |
| [25] | Wang CH, Wan SQ, Xing XR, Zhang L, Han XG (2006). Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biology & Biochemistry, 38, 1101-1110. |
| [26] | Wang WJ, Chalk PM, Chen D, Smith CJ (2001). Nitrogen mineralisation, immobilisation and loss, and their role in determining differences in net nitrogen production during waterlogged and aerobic incubation of soils. Soil Biology & Biochemistry, 33, 1305-1315. |
| [27] | Wu JG, Chang W, Ai L, Chang XX (2007). The soil nitrogen mineralization under four typical ecosystem in Qilian Mountains. Ecology and Environment, 16, 1000-1006. |
| [吴建国, 苌伟, 艾丽, 常学向 (2007). 祁连山中部四种典型生态系统土壤氮矿化的研究. 生态环境, 16, 1000-1006.] | |
| [28] | Wu Q, Yue K, Ma Y, Heděnec P, Cai Y, Chen J, Zhang H, Shao J, Chang S, Li Y (2022). Contrasting effects of altered precipitation regimes on soil nitrogen cycling at the global scale. Global Change Biology, 28, 6679-6695. |
| [29] | Xu YQ, Li LH, Wang QB, Chen QS, Cheng WX (2007). The pattern between nitrogen mineralization and grazing intensities in an Inner Mongolian typical steppe. Plant and Soil, 300, 289-300. |
| [30] | Yahdjian L, Gherardi L, Sala OE (2011). Nitrogen limitation in arid-subhumid ecosystems: a meta-analysis of fertilization studies. Journal of Arid Environments, 75, 675-680. |
| [31] | Yang JQ, Diao HJ, Hu SY, Wang CH (2021). Effects of nitrogen addition at different levels on soil microorganisms in saline-alkaline grassland of northern China. Chinese Journal of Plant Ecology, 45, 780-789. |
|
[杨建强, 刁华杰, 胡姝娅, 王常慧 (2021). 不同水平氮添加对盐渍化草地土壤微生物特征的影响. 植物生态学报, 45, 780-789.]
DOI |
|
| [32] | Zhao N, Zhang HX, Wang RM, Yang MY, Zhang Y, Zhao XN, Yu GR, He NP (2014). Effect of grazing intensity on temperature sensitivity of soil nitrogen mineralization in Zoigё alpine meadow. Acta Ecologica Sinica, 34, 4234-4241. |
| [赵宁, 张洪轩, 王若梦, 杨满业, 张艳, 赵小宁, 于贵瑞, 何念鹏 (2014). 放牧对若尔盖高寒草甸土壤氮矿化及其温度敏感性的影响. 生态学报, 34, 4234-4241.] | |
| [33] | Zhu JX, Wang QF, He NP, Wang RM, Dai JZ (2013). Soil nitrogen mineralization and associated temperature sensitivity of different Inner Mongolian grasslands. Acta Ecologica Sinica, 33, 6320-6327. |
| [朱剑兴, 王秋凤, 何念鹏, 王若梦, 代景忠 (2013). 内蒙古不同类型草地土壤氮矿化及其温度敏感性. 生态学报, 33, 6320-6327.] |
| [1] | 罗娜娜, 盛茂银, 王霖娇, 石庆龙, 何宇. 长期植被恢复对中国西南喀斯特石漠化土壤活性有机碳组分含量和酶活性的影响[J]. 植物生态学报, 2023, 47(6): 867-881. |
| [2] | 杨建强, 刁华杰, 胡姝娅, 王常慧. 不同水平氮添加对盐渍化草地土壤微生物特征的影响[J]. 植物生态学报, 2021, 45(7): 780-789. |
| [3] | 王毅, 孙建, 叶冲冲, 曾涛. 气候因子通过土壤微生物生物量氮促进青藏高原高寒草地地上生态系统功能[J]. 植物生态学报, 2021, 45(5): 434-443. |
| [4] | 李品, 木勒德尔•吐尔汗拜, 田地, 冯兆忠. 全球森林土壤微生物生物量碳氮磷化学计量的季节动态[J]. 植物生态学报, 2019, 43(6): 532-542. |
| [5] | 王祥, 朱亚琼, 郑伟, 关正翾, 盛建东. 昭苏山地草甸4种典型土地利用方式下的土壤呼吸特征[J]. 植物生态学报, 2018, 42(3): 382-396. |
| [6] | 陈宝明, 韦慧杰, 陈伟彬, 朱政财, 原亚茹, 张永隆, 蓝志刚. 外来入侵植物对土壤氮转化主要过程及相关微生物的影响[J]. 植物生态学报, 2018, 42(11): 1071-1081. |
| [7] | 王薪琪, 韩轶, 王传宽. 帽儿山不同林龄落叶阔叶林土壤微生物生物量及其季节动态[J]. 植物生态学报, 2017, 41(6): 597-609. |
| [8] | 李铭, 朱利川, 张全发, 程晓莉. 不同土地利用类型对丹江口库区土壤氮矿化的影响[J]. 植物生态学报, 2012, 36(6): 530-538. |
| [9] | 张文丽, 刘菊, 王建柱, 陈芳清. 三峡库区不同林龄人工橘林土壤异养呼吸及其温度敏感性[J]. 植物生态学报, 2010, 34(11): 1265-1273. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19