

植物生态学报 ›› 2008, Vol. 32 ›› Issue (2): 413-423.DOI: 10.3773/j.issn.1005-264x.2008.02.020
收稿日期:2007-06-01
接受日期:2007-09-29
出版日期:2008-06-01
发布日期:2008-03-30
通讯作者:
赵平
作者简介:E-mail: zhaoping@scib.ac.cn
ZHAO Ping( ), SUN Gu-Chou, ZENG Xiao-Ping
), SUN Gu-Chou, ZENG Xiao-Ping
Received:2007-06-01
Accepted:2007-09-29
Online:2008-06-01
Published:2008-03-30
Contact:
ZHAO Ping
Supported by:摘要:
利用光合作用测定系统(Li-COR 6400和叶室荧光仪),测定了亚热带阔叶树种的光合速率和荧光参数,分析了38 ℃适度高温对叶片光合作用和吸收光能分配的影响。测试树种包括华南亚热带地区常见的阳生性树种木荷(Schima superba)、耐荫树种黄果厚壳桂(Cryptocarya concinna)和中生性树种红锥(Castanopsis hystrix)。适度高温处理均引起所有树种的光合能力下降,而且木荷和红锥下降的程度比黄果厚壳桂明显。与25 ℃的对照温度相比,适度高温处理的木荷叶片用于光化学反应所消耗的光能下降,红锥和黄果厚壳桂也有相似的反应,表明适度高温限制叶片用于光化学反应的吸收光能。无论哪个树种,38 ℃适度高温处理的植物,叶片总吸收光能中额外多余的那部分和处于非活化状态PSⅡ所吸收的那部分光能都增加,而且黄果厚壳桂比木荷和红锥显著,因此,亚热带阔叶森林的树种对适度高温的响应因种类而异。研究结果意味着将来气候变化导致温度的上升对演替后期树种黄果厚壳桂的光合过程的限制比演替早期的树种木荷和中生性树种红锥会更严重。
赵平, 孙谷畴, 曾小平. 适度高温下亚热带阔叶树种叶片的光合速率和吸收光能的分配. 植物生态学报, 2008, 32(2): 413-423. DOI: 10.3773/j.issn.1005-264x.2008.02.020
ZHAO Ping, SUN Gu-Chou, ZENG Xiao-Ping. PHOTOSYNTHETIC RATES AND PARTITIONING OF ABSORBED LIGHT ENERGY IN LEAVES OF SUBTROPICAL BROAD-LEAF TREES UNDER MODERATELY HIGH-TEMPERATURE. Chinese Journal of Plant Ecology, 2008, 32(2): 413-423. DOI: 10.3773/j.issn.1005-264x.2008.02.020
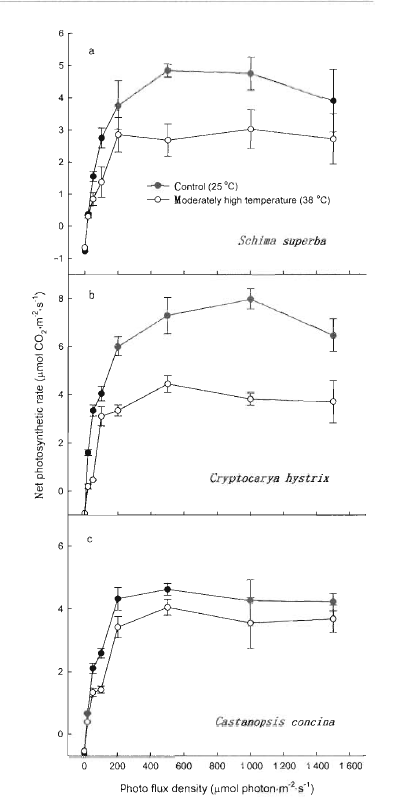
Fig.1 Light response curves of net photosynthetic rate in moderately high temperature exposed and in control leaves The curves were obtained under CO2 concentration of 365 μmol·mol-1 at 25 ℃
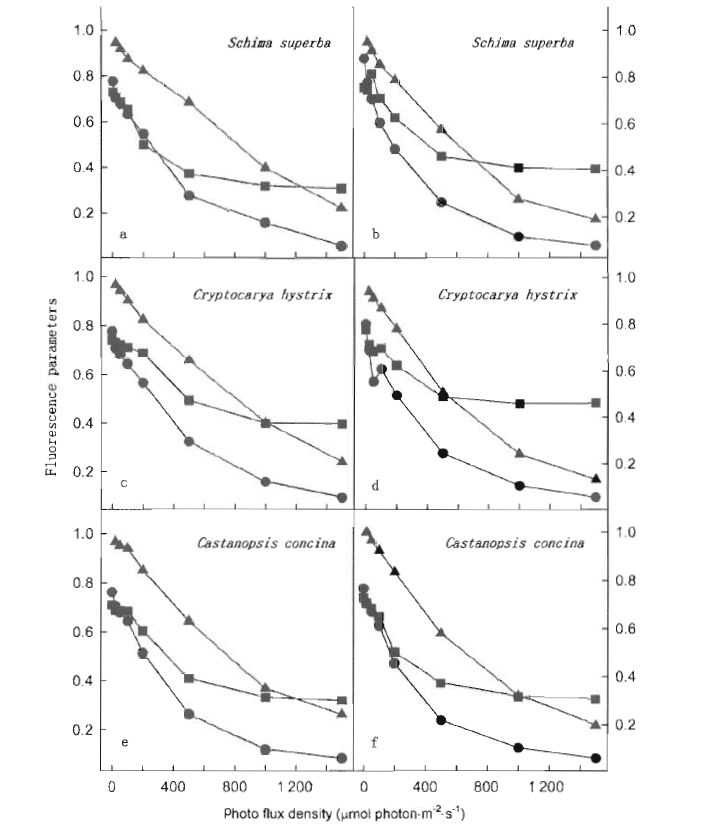
Fig.2 Light response of chlorophyll fluorescence parameters of ΔF/Fm' (circle), Fv'/Fm' (square) and qP (triangle) in leaves under ambient temperature (25 ℃) (a, c and e) and moderately high-temperature (38 ℃) exposition (b, d and f)
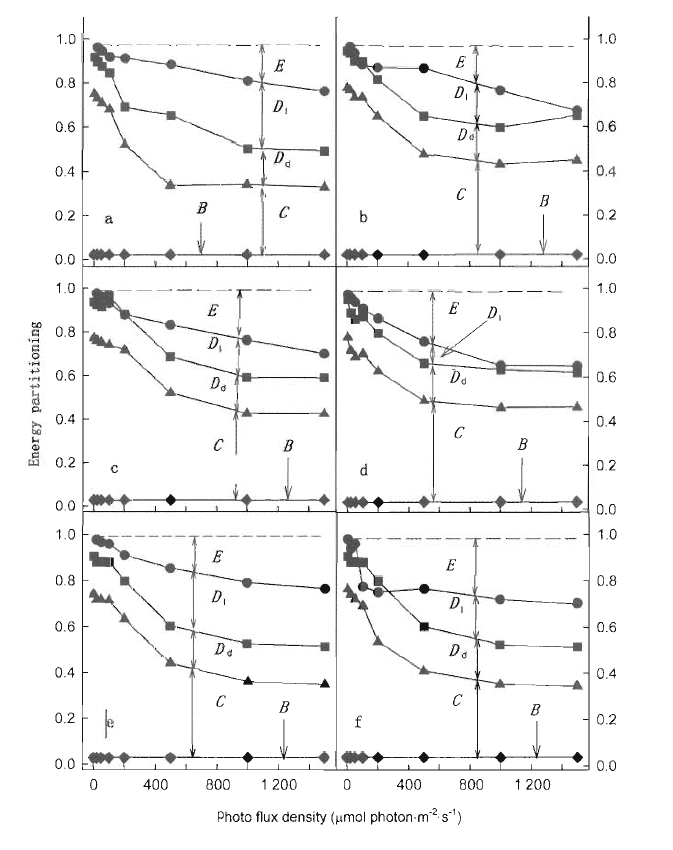
Fig.3 Partitioning of the absorbed light energy in control leaves (a, c and e) and moderately high-temperature acclimatized leaves (b, d and f) E (circle), Dl (square), Dd (triangle) and B (diamond) denote the allocation of absorbed energy to excess, heat dissipation in height, loss in dark, photochemical reaction a, b: Schima superba c, d: Castanopsis hystrix e, f: Cryptocarya concinna
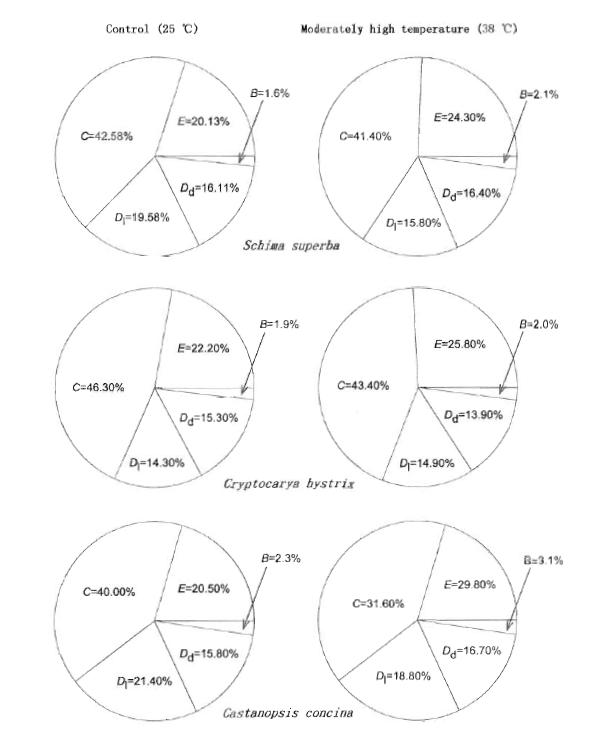
Fig.4 Several schematic representations displaying portion of total absorbed photon energy that was excessive (E), the energy lost in active PSⅡ in the light (Dl), the energy lost in active PS Ⅱ in the dark (Dd), the energy utilized by photochemical reaction in the leaves (C) and fraction of energy absorbed by inactive PSⅡ (B) The figure represents the related proportion of individual fraction of those five components of energy to amount of absorbed energy integrated over the entire regime of irradiance from 0 to 1 500 μmol photon·m-2·s-1
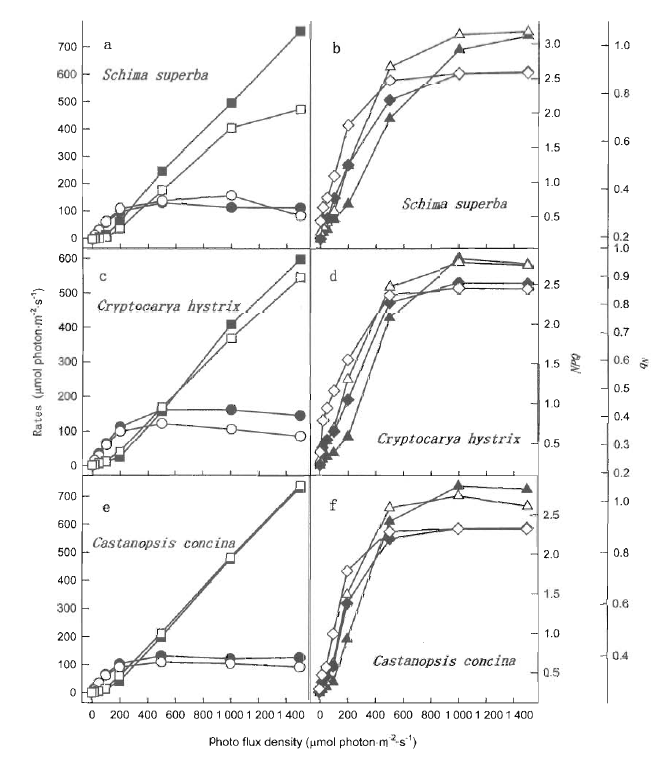
Fig.5 Photo flux density response of estimated rates of photochemical reaction and heat dissipation (a, c and e), NPQ and qN (b, d and f) in moderately high temperature exposed leaves (open symbols) and control leaves (closed symbols) of Schima superba, Castanopsis hystrix and Cryptocarya concimma The different symbols represent estimated rate of photochemical reaction (circles) and energy loss in active PSⅡ (squares), NPQ (triangles) and qN (diamonds)
| [1] | Aro EM, McCaffery S, Aderson JA (1993). Photoinhibition and DI protein degradation in pear acclimated to different growth irradiance. Plant Physiology, 103, 835-847. |
| [2] | Berry JA, Bjørkman O (1980). Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31, 491-543. |
| [3] |
Bjørkman O, Demmig B (1987). Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77k among vascular planta of diverse origins. Planta, 170, 489-504.
URL PMID |
| [4] | Crafts-Brandner SJ, Salvncci ME (2000). Rubisco activase constrain the photosynthetic potential of leaves at high temperature and CO2. Proceedings of the National Academy of Sciences of the United State of America, 97, 13430-13435. |
| [5] | Demmig-Adams B, Admas WW, Barker DH, Logan BA, Bowling DB, Verhoever AS (1996). Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiologia Plantarum, 98, 253-264. |
| [6] | Demmig-Adams B, Adams WW (1992). Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology, 43, 599-626. |
| [7] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [8] | Gilmore AM (1997). Mechanistic aspects of xanthophyl cycle-dependent photoprotection in higher plant chloroplast and leaves. Physiologia Plantarum, 99, 197-209. |
| [9] | Habaux M, Tardy F, Ravenel J, Chanu D, Purot P (1996). Thylakoid membrane stability to heat stress by flash spectroscopic measurement of the electrochromic shift in intact potato leaves: influence of xanthophylls content. Plant, Cell and Environment, 19, 1359-1368. |
| [10] | Haldimann P, Feller U (2004). Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1, 5-bisphosphate carboxylase/oxygenase. Plant, Cell and Environment, 27, 1169-1183. |
| [11] | Hikosaka K, Kato MC, Hirose T (2004). Photosynthetic rates and partitioning of absorbed light energy in photoinhibition leaves. Physiologia Plantarum, 121, 699-708. |
| [12] | Kato MC, Hikosaka K, Hirotsu N, Makino A, Hirose T (2003). The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms the rate of photo inactivation in photosystem Ⅱ. Plant Cell Physiology, 44, 318-325. |
| [13] | Kornyeyer D, Holaday AS, Logan BA (2004). Minimization of the photon energy absorbed by “closed” reaction centers of photosystem Ⅱ as a photoprotective strategy in higher plants. Photosynthetica, 42, 377-386. |
| [14] | Krause GH (1988). Photoinhibition of photosynthesis, an evaluation of damaging and protective mechanisms. Physiologia Plantarum, 74, 566-574. |
| [15] | Krall JP, Edwards GE (1992). Relationship between photosystem Ⅱ activity and CO2 fixation in leaves. Physiologia Plantarum, 86, 180-187. |
| [16] | Larcher W (2001). Ökophysiologie der Pflanzen. Verlag Eugen Ulmer GmbH, Stuttgart, Germany, 74-166. |
| [17] | Qin DH (秦大河) (2003). Fact, impact, adaptation and mitigation strategy of climate change. Bulletin of National Science Foundation of China (中国科学基金), 17, 1-3. (in Chinese with English abstract) |
| [18] | Osmond CB (1994). What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Bake NR, Rowyer JR eds. Photoinhibitin of Photosynthesis From Molecular Mechanism to the Field. Bioscientific Publishers, Oxford, 1-24. |
| [19] | Schreiber O, Bilger W, Nubauer C (1995). Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM eds. Ecophysiology of Photosynthesis. Springer-Verlag, Berlin, 49-70. |
| [20] | Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TE (2004). Thylakoid membrane responses to moderately high leaf temperature in Pima Cotton. Plant, Cell and Environment, 27, 725-735. |
| [21] | von Caemmerer S, Farquhar GD (1981). Some relationship between the biochemistry of photosynthesis and gas exchange of leaves. Planta, 153, 376-387. |
| [1] | 李伟斌, 张红霞, 张玉书, 陈妮娜. 昼夜不对称增温对长白山阔叶红松林碳汇能力的影响[J]. 植物生态学报, 2023, 47(9): 1225-1233. |
| [2] | 蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用[J]. 植物生态学报, 2023, 47(7): 988-997. |
| [3] | 刘海燕, 臧纱纱, 张春霞, 左进城, 阮祚禧, 吴红艳. 磷饥饿下硅藻光系统II光化学反应及其对高光强的响应[J]. 植物生态学报, 2023, 47(12): 1718-1727. |
| [4] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [5] | 靳川, 李鑫豪, 蒋燕, 徐铭泽, 田赟, 刘鹏, 贾昕, 查天山. 黑沙蒿光合能量分配组分在生长季的相对变化与调控机制[J]. 植物生态学报, 2021, 45(8): 870-879. |
| [6] | 武洪敏, 双升普, 张金燕, 寸竹, 孟珍贵, 李龙根, 沙本才, 陈军文. 短期生长环境光强骤增导致典型阴生植物三七光系统受损的机制[J]. 植物生态学报, 2021, 45(4): 404-419. |
| [7] | 叶子飘, 于冯, 安婷, 王复标, 康华靖. 植物气孔导度对CO2响应模型的构建[J]. 植物生态学报, 2021, 45(4): 420-428. |
| [8] | 李景, 王欣, 王振华, 王斌, 王成章, 邓美凤, 刘玲莉. 臭氧和气溶胶复合污染对杨树叶片光合作用的影响[J]. 植物生态学报, 2020, 44(8): 854-863. |
| [9] | 李旭, 吴婷, 程严, 谭钠丹, 蒋芬, 刘世忠, 褚国伟, 孟泽, 刘菊秀. 南亚热带常绿阔叶林4个树种对增温的生理生态适应能力比较[J]. 植物生态学报, 2020, 44(12): 1203-1214. |
| [10] | 刘校铭, 杨晓芳, 王璇, 张守仁. 暖温带落叶阔叶林辽东栎和五角枫生长和光合生理生态特征对模拟氮沉降的响应[J]. 植物生态学报, 2019, 43(3): 197-207. |
| [11] | 李鑫豪, 闫慧娟, 卫腾宙, 周文君, 贾昕, 查天山. 油蒿资源利用效率在生长季的相对变化及对环境因子的响应[J]. 植物生态学报, 2019, 43(10): 889-898. |
| [12] | 张娜, 朱阳春, 李志强, 卢信, 范如芹, 刘丽珠, 童非, 陈静, 穆春生, 张振华. 淹水和干旱生境下铅对芦苇生长、生物量分配和光合作用的影响[J]. 植物生态学报, 2018, 42(2): 229-239. |
| [13] | 韩吉梅, 张旺锋, 熊栋梁, 张亚黎. 植物光合作用叶肉导度及主要限制因素研究进展[J]. 植物生态学报, 2017, 41(8): 914-924. |
| [14] | 蔡建国, 韦孟琪, 章毅, 魏云龙. 遮阴对绣球光合特性和叶绿素荧光参数的影响[J]. 植物生态学报, 2017, 41(5): 570-576. |
| [15] | 陈良华, 赖娟, 胡相伟, 杨万勤, 张健, 王小军, 谭灵杰. 接种丛枝菌根真菌对受镉胁迫美洲黑杨雌、雄株光合生理的影响[J]. 植物生态学报, 2017, 41(4): 480-488. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19