

植物生态学报 ›› 2008, Vol. 32 ›› Issue (2): 512-520.DOI: 10.3773/j.issn.1005-264x.2008.02.031
所属专题: 生物多样性
• 综述 • 上一篇
收稿日期:2006-05-30
接受日期:2006-10-11
出版日期:2008-03-30
发布日期:2008-03-30
通讯作者:
魏宇昆,高玉葆
作者简介:E-mail: ykwei@sippe.ac.cn本文的构思和写作得到了天津师范大学马成仓教授的帮助,特此致谢
基金资助:Received:2006-05-30
Accepted:2006-10-11
Online:2008-03-30
Published:2008-03-30
Contact:
WEI Yu-Kun,GAO Yu-Bao
摘要:
近百年的禾草内生真菌研究历经了由浅入深的过程,从最初的家畜中毒事件认识到是一种共生内生真菌存在的缘故,到如今利用分子生物学技术揭示其共生机制,人类发现这类植物内生真菌并非想象中的对生态系统无足轻重。Epichloı及其无性型Neotyphodium与禾本科植物是系统发生的互利共生关系,尤其是Neotyphodium可提高宿主抵抗环境胁迫的能力和抵御动物的取食,增强植物的竞争力。禾草内生真菌有3种生活史:有性生活史、无性生活史和兼性生活史,后者表明真菌在不同的宿主及环境下既能营有性生殖也可营无性繁殖,是一种更灵活而有效的生活史对策。对内生真菌分子系统学、生活史以及与宿主禾草协同进化的研究发现,Neotyphodium起源于禾草致病真菌Epichloı的某些种,或是Epichloı与Neotyphodium的种间杂交后代。植物和内生真菌各异的生活史策略,真菌的种间杂交,两者的协同进化亦或种群间基因流的差异,都促成了共生体多样化的基因组合(Genetic combination),也是其共生关系多样化的根源。内生真菌对宿主的有益作用只在特定基因型真菌、宿主和一定环境条件下才起作用,自然生态系统的共生关系要比农业系统复杂得多,是一个从互利共生至寄生关系的连续系统。未来对于更多共生体的遗传背景和基因与环境相互作用的阐明将有助于对禾草内生真菌共生关系本质更加深入的认识。
魏宇昆, 高玉葆. 禾草内生真菌的遗传多样性及其共生关系. 植物生态学报, 2008, 32(2): 512-520. DOI: 10.3773/j.issn.1005-264x.2008.02.031
WEI Yu-Kun, GAO Yu-Bao. REVIEW OF THE DIVERSITY OF ENDOPHYTE GENETICS AND SYMBIOTIC INTERACTIONS WITH GRASSES. Chinese Journal of Plant Ecology, 2008, 32(2): 512-520. DOI: 10.3773/j.issn.1005-264x.2008.02.031
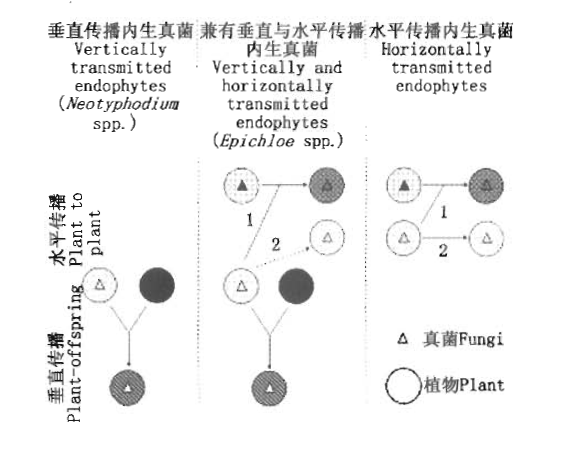
图1 共生体不同遗传组合的产生途径(Saikkonen et al. 2004b) 1: 表示减数分裂孢子传播途径Denote meiotic spores 2: 表示有丝分裂孢子传播途径Denote mitotic spores 图中三角和圆的不同颜色分别表示内生真菌和植物的不同遗传背景 The different color in figures denote different genetic background
Fig.1 Different consequences of vertical versus horizontal transmission to the genetic interplay between the endophytic fungus and the host plant (Saikkonen et al., 2004b)
| 栽培牧草 Cultivar grass | 野生禾草 Native grass |
|---|---|
| 引进种,经人工选育、栽培,基因型单一 Introduced species, selective breeding and cultivation, limited genetic diversity | 野生种,基因型多样 Wild species with numerous genetic types |
| 生物碱种类多、含量高、稳定(农业、草坪系统的营养成分含量高而稳定) Large number of alkaloid types with high and steady concentrations (high nutrient contents in agricultural and turfgrass systems) | 生物碱种类少,含量变化较大(自然生态系统土壤成分不稳定) Small number of alkaloid types, but varied a lot in concentrations (soil properties varied in natural ecosystems) |
| 多数内生真菌-禾草-植食动物关系研究均是引入种而非本地种,无脊椎动物也不是专性取食者 Most studies of endophyte-grass-herbivore interactions were focused on introduced species rather than native species, and the invertebrates involved were not specific predators | 自然植物群落中,大多数食草无脊椎动物均是专性的,只以一种或很少的宿主植物为食,可能对生物碱等有毒物质不敏感,内生真菌及其毒性物质对宿主的抗取食能力贡献不大 Most invertebrate herbivores were specific in natural plant communities, feeding on one or just a few plant species, not sensitive to alkaloid or other toxic compounds. There was little effect of resistance from the endophytes |
| 取食是首要的选择压力,是以生物碱为基础的防御性共生 Grazing was the primary selective pressure. The protective symbiosis was related with alkaloid production | 取食压力很小或不存在,甚至对植物适合度是正效应 Grazing pressure was little or did not exist, or perhaps there was a positive effect upon plant fitness |
| 内生真菌增加了植物的适合度,植物种群侵染率随时间增加 Endophyte infection enhanced the fitness of the host plants. The infection rate increased with time | 种群侵染率变化范围更广,变化幅度更大 Infection rate varied a lot among plant populations |
| 共生关系的空间变化不显著 The symbiosis did not vary significantly in spatial axis | 种群内共生关系的空间变化显著,侵染种群和非侵染种群常镶嵌分布 The plant-endophyte symbiosis varied significantly in space, and there was a kind of reciprocal distribution in the endophyte-infected and endophyte-free populations |
表1 栽培牧草和野生禾草与内生真菌共生关系的比较
Table 1 Comparison of the symbiotic consequences of cultivar versus natural grass
| 栽培牧草 Cultivar grass | 野生禾草 Native grass |
|---|---|
| 引进种,经人工选育、栽培,基因型单一 Introduced species, selective breeding and cultivation, limited genetic diversity | 野生种,基因型多样 Wild species with numerous genetic types |
| 生物碱种类多、含量高、稳定(农业、草坪系统的营养成分含量高而稳定) Large number of alkaloid types with high and steady concentrations (high nutrient contents in agricultural and turfgrass systems) | 生物碱种类少,含量变化较大(自然生态系统土壤成分不稳定) Small number of alkaloid types, but varied a lot in concentrations (soil properties varied in natural ecosystems) |
| 多数内生真菌-禾草-植食动物关系研究均是引入种而非本地种,无脊椎动物也不是专性取食者 Most studies of endophyte-grass-herbivore interactions were focused on introduced species rather than native species, and the invertebrates involved were not specific predators | 自然植物群落中,大多数食草无脊椎动物均是专性的,只以一种或很少的宿主植物为食,可能对生物碱等有毒物质不敏感,内生真菌及其毒性物质对宿主的抗取食能力贡献不大 Most invertebrate herbivores were specific in natural plant communities, feeding on one or just a few plant species, not sensitive to alkaloid or other toxic compounds. There was little effect of resistance from the endophytes |
| 取食是首要的选择压力,是以生物碱为基础的防御性共生 Grazing was the primary selective pressure. The protective symbiosis was related with alkaloid production | 取食压力很小或不存在,甚至对植物适合度是正效应 Grazing pressure was little or did not exist, or perhaps there was a positive effect upon plant fitness |
| 内生真菌增加了植物的适合度,植物种群侵染率随时间增加 Endophyte infection enhanced the fitness of the host plants. The infection rate increased with time | 种群侵染率变化范围更广,变化幅度更大 Infection rate varied a lot among plant populations |
| 共生关系的空间变化不显著 The symbiosis did not vary significantly in spatial axis | 种群内共生关系的空间变化显著,侵染种群和非侵染种群常镶嵌分布 The plant-endophyte symbiosis varied significantly in space, and there was a kind of reciprocal distribution in the endophyte-infected and endophyte-free populations |
| [1] | Ahlholm JU, Helander M, Henriksson J, Metzler M, Saikkonen K (2002a). Environmental conditions and host genotype direct genetic diversity of Venturia ditricha, a fungal endophyte of birch trees. Evolution, 56, 1566-1573. |
| [2] | Ahlholm JU, Helander M, Lehtmäki S, Wäli P, Saikkonen K (2002b). Vertically transmitted endophytes: effects of environmental conditions. Oikos, 99, 173-183. |
| [3] | Arnold AE, Meijia LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA (2003). Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America, 26, 15649-15654. |
| [4] | Bazzaz FA, Grace J (1997). Plant Resource Allocation. Academic Press, San Diego, CA. |
| [5] | Bell G, Koufopanou V (1986). The cost of reproduction. In: Dawkins R, Ridley M eds. Oxford Surveys in Evolutionary Biology. Oxford University Press, Oxford. 83-113. |
| [6] | Bernard EC, Gwinn KD, Pless CD, Williver CD (1997). Soil invertebrate species diversity and abundance in endophyte-infected tall fescue pastures. In: Bacon CW, Hill NS eds. Neotyphodium/Grass Interactions. Plenum, New York. 125-135. |
| [7] | Braverman SW (1986). Disease resistance in cool-season forage range and turf grasses. Ⅱ. Botanical Review, 52, 1-113. |
| [8] | Breen JP (1994). Acremonium endophyte interactions with enhanced plant resistance to insects. Annual Review of Entomology, 39, 401-423. |
| [9] |
Brem D, Leuchtmann A (2001). Epichloı grass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum. Oecologia, 126, 522-530.
DOI URL PMID |
| [10] | Bruel GW, Kaiser WJ, Klein RE (1994). An endophyte of Achnatherum inebrians, an intoxicating grass of northwest China. Mycologia, 86, 773-776. |
| [11] | Carroll GC (1988). Fungal endophytes in stems and leaves: from latent patent pathogen to mutualistic symbiont. Ecology, 69, 2-9. |
| [12] | Cheplick GP, Clay K, Marks S (1989). Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytologist, 111, 89-97. |
| [13] | Cheplick GP, Perera A, Koulouris K (2000). Effect of drought on the growth of Lolium perenne genotypes with and without fungal endophytes. Functional Ecology, 14, 657-667. |
| [14] | Clay K (1988). Fungal endophytes of grasses: a defensive mutualism between plants and fungi. Ecology, 69, 10-16. |
| [15] | Clay K (1990). Fungal endophytes of grasses. Annual Review of Ecology and Systematics, 21, 255-297. |
| [16] | Clay K (1994). The potential role of endophytes in ecosystems. In: Bacon CW, White JF eds. Biotechnology of Endophytic Fungi of Grasses. CRC Press, Boca Raton, FL, 73-86. |
| [17] | Clay K (1997a). Fungal endophytes, herbivores and the structure of grassland communities. In: Gange AC, Brown VK eds. Multitrophic Interactions in Terrestrial Systems. Blankwell, Oxford. 151-169. |
| [18] | Clay K (1997b). Consequences of endophyte-infected grasses on plant diversity. In: Bacon CW, Hill NS eds. Neotyphodium/Grass Interactions. Plenum Press, New York. 93-108. |
| [19] | Clay K (1997). Fungal endophyte infection and the population biology of grasses. In: Cheplick GP ed. The Population Biology of Grasses. Cambridge University Press, Cambridge, UK. 255-285. |
| [20] | Clay K, Holah J (1999). Fungal endophyte symbiosis and plant diversity in successional fields. Science, 285, 1742-1744. |
| [21] | Clay K, Schardl C (2002). Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. The American Naturalist, 160, 99-127. |
| [22] | Clement SL, Elberson LR, Youssef NN, Davitt CM, Doss RP (2001). Incidence and diversity of Neotyphodium fungal endophytes in tall fescue from Morocco, Tunisia, and Sardinia. Crop Science, 41, 570-576. |
| [23] | Faeth SH (2002). Are endophytic fungi defensive plant mutualists? Oikos, 98, 25-36. |
| [24] | Faeth SH, Bultman TL (2002). Endophytic fungi and interactions among host plants, herbivores and natural enemies. In: Tscharntke T, Hawkins BA eds. Multitrophic Level Interactions. Cambridge University Press, Cambridge, UK. 89-123. |
| [25] | Faeth SH, Fagan WF (2002). Fungal endophytes: common host plant symbionts but uncommon mutualists. Integrative and Comparative Biology, 42, 360-368. |
| [26] | Faeth SH, Sullivan TJ (2003). Mutualistic asexual endophytes in a native grass are usually parasitic. The American Naturalist, 161, 310-325. |
| [27] | Faeth SH, Helander ML, Saikkonen KT (2004). Asexual Neotyphodium endophytes in a native grass reduce competitive abilities. Ecology Letters, 7, 304-313. |
| [28] | Futuyma DJ, Moreno G (1988). The evolution of ecological specialization. Annual Review of Ecology and Systematics, 19, 207-233. |
| [29] | Gentile A, Rossi MS, Cabral D, Craven KD, Schardl CL (2005). Origin, divergence, and phylogeny of Epichloı endophytes of native Argentine grasses. Molecular Phylogenetics and Evolution, 35, 196-208. |
| [30] | Hamilton JG, Zangerl AR, DeLuca EH, Berenbaum MR (2001). The carbon-nutrient balance hypothesis: its rise and fall. Ecology Letters, 4, 86-95. |
| [31] | Hesse U, Shøberlein W, Wittenmayer L, Førster K, Warnstorff K, Diepenbrock W, Merbach W (2005). Influence of water supply and endophyte infection (Neotyphodium spp.)on vegetative and reproductive growth of two Lolium perenne L. genotypes. European Journal of Agronomy, 22, 45-54. |
| [32] | Jensen AMD, Roulund N (2004). Occurrence of Neotyphodium endophytes in permanent grassland with perennial ryegrass (Lolium perenne) in Denmark. Agriculture, Ecosystems and Environment, 104, 419-427. |
| [33] | Leuchtmann A, Clay K (1997). The population biology of grass endophytes. In: Carroll GC, Tudzynski P eds. The Mycota. Ⅴ. Plant Relationships, Part B. Springer-Verlag, Berlin. 185-204. |
| [34] | Lewis GC, Ravel C, Naffaa W, Astier C, Charmet G (1997). Occurrence of Acremonium endophytes in wild populations of Lolium spp. in European countries and a relationship between level of infection and climate in France. Annals of Applied Biology, 130, 227-238. |
| [35] | Liang Y (梁宇), Gao YB (高玉葆), Chen SP (陈世苹), Ren AZ (任安芝) (2001). Effects of endophyte infection on photosynthesis, transpiration and water use efficiency of Lolium perenne L. under drought stress. Acta Phytoecologica Sinica (植物生态学报), 25, 537-543. (in Chinese with English abstract) |
| [36] | Malinowski DP, Leuchtmann A, Schmidt D, Nosberger J (1997). Symbiosis with Neotyphodium uncinatum increases the competitive ability of meadow fescue (Festuca pratensis). Agronomy Journal, 89, 833-839. |
| [37] | Malinowski DP, Belesky DP (2000). Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science, 40, 923-940. |
| [38] | Malinowski DP, Belesky DP, Lewis GC (2005). Abiotic stresses in endophytic grasses. In: Roberts CA, West CP, Spiers DE eds. Neotyphodium in Cool-Season Grasses. Blackwell, Oxford, UK. 187-199. |
| [39] | Marks S, Clay K, Cheplick GP (1991). Effects of fungal endophytes on interspecific and intraspecific competition in the grasses Festuca arundinacea and Lolium perenne. Journal of Applied Ecology, 28, 194-204. |
| [40] | Marquis RJ (1992). The selective impact of herbivores. In: Fritz RS, Simms EL eds. Plant Resistance to Herbivores and Pathogens: Ecology, Evolution, and Genetics. Chicago University Press, Chicago, 301-325. |
| [41] | Maschinki J, Whitham TG (1989). The continuum of plant responses to herbivory: the influence of plant association, nutrient availability and timing. The American Naturalist, 134, 1-19. |
| [42] | Moon CD, Scott B, Schardl CL, Christensen MJ (2000). Evolutionary origins of Epichloı enodophytes from annual ryegrasses. Mycologia, 92, 1103-1118. |
| [43] | Moon CD, Miles CO, Järlfors U, Schardl CL (2002). The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the southern hemisphere. Mycologia, 94, 694-711. |
| [44] | Morse LJ, Day TA, Faeth SH (2002). Effect of Neotyphodium endophyte infection on growth and leaf gas exchange of Arizona fescue under contrasting water availability regimes. Environmental and Experimental Botany, 48, 257-268. |
| [45] | Müller CB, Krauss J (2005). Symbiosis between grasses and asexual fungal endophytes. Current Opinion in Plant Biology, 8, 450-456. |
| [46] |
Omacini M, Chaneton EJ, Ghersa CM, Müller CB (2001). Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature, 409, 78-81.
URL PMID |
| [47] | Omacini M, Chaneton EJ, Ghersa CM (2005). A hierarchical framework for understanding the ecosystem consequences of endophyte-grass symbioses. In: Roberts CA, West CP, Spiers DE eds. Neotyphodium in Cool-Season Grasses. Blackwell, Oxford, UK. 141-162. |
| [48] |
Petrini O, Sieber TH, Toti L, Viret O (1992). Ecology, metabolite production, and substrate utilization in endophytic fungi. Natural Toxins, 1, 185-196.
URL PMID |
| [49] | Rausher MD (1992). Natural selection and the evolution of plant-insect interactions. In: Roitberg BD, Isman MB eds. Insect Chemical Ecology: an Evolutionary Approach. Chapman & Hall, New York. 20-88. |
| [50] | Ravel C, Michalakis Y, Charmet G (1997). The effect of imperfect transmission on the frequency of mutualistic seed-borne endophytes in natural populations of grasses. Oikos, 80, 18-24. |
| [51] | Ren AZ (任安芝), Gao YB (高玉葆), Gao WS (高文生) (2002a). Effects of endophyte of infection on seed germination, seeding growth and osmotic stress resistance of perennial ryegrass (Lolium perenne L.). Acta Phytoecologica Sinica (植物生态学报), 26, 420-426. (in Chinese with English abstract) |
| [52] | Ren AZ (任安芝), Gao YB (高玉葆), Li X (李侠) (2002b). Effect of fungal endophyte infection on some physiological characters of Lolium perenne under drought conditions. Chinese Journal of Applied and Environmental Biology (应用与环境生物学报), 8, 535-539. (in Chinese with English abstract) |
| [53] | Reznick D (1985). Cost of reproduction: an evaluation of the empirical evidence. Oikos, 44, 257-267. |
| [54] | Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998). Fungal endophytes: a continuum of interactions with host plants. Annual Review of Ecology and Systematics, 29, 319-343. |
| [55] | Saikkonen K (2000). Kentucky-31, far from home. Science, 287, 1887a. |
| [56] | Saikkonen K, Ahlholm J, Helander M, Lehtimäki S, Niemeläinen O (2000). Endophytic fungi in wild and cultivated grasses in Finland. Ecography, 23, 360-366. |
| [57] | Saikkonen K, Ion D, Gyllenberg M (2002). The persistence of fungal endophytes in structured grass metapopulations. Proceedings of the Royal Society of London B: Biological Sciences, 269, 1397-1403. |
| [58] | Saikkonen K, Helander M, Faeth SH (2004a). Fungal endophytes: hitch-hikers of the green world. In: Gillings M,Holmes A eds. Plant Microbiology. BIOS Science Publishers Limited, Oxford, UK. 79-97. |
| [59] |
Saikkonen K, Wäli P, Helander M, Faeth SH (2004b). Evolution of endophyte-plant symbioses. Trends in Plant Science, 9, 275-280.
DOI URL PMID |
| [60] | Schardl CL, Leuchtmann A, Tsai HF, Collett MA, Watt DM, Scott DB (1994). Origin of a fungal symbiont of perennial ryegrass by interspecific hybridization of a mutualist with the ryegrass choke pathogen, Epichloı typhina. Genetics, 136, 1307-1317. |
| [61] |
Schardl CL (1996). Epichloı species: fungal symbionts of grasses. Annual Review of Phytopathology, 34, 109-130.
DOI URL PMID |
| [62] | Schardl CL (2001). Epichloı festucae and related mutualistic symbionts of grasses. Fungal Genetics and Biology, 33, 69-82. |
| [63] | Siegel MR, Jarlfors U, Latch GCM, Johnson MC (1987). Ultrastructrue of Acremonium coenophialum, Acremonium lolii and Epichloı typhina endophytes in host and nonhost Festuca and Lolium species of grasses. Canadian Journal of Botany, 65, 2357-2367. |
| [64] |
Siegel MR, Latch GCM, Bush LP, Fannin FF, Rowan DD, Tapper BA, Bacon CW, Johnson MC (1990). Fungal endophyte-infected grasses: alkaloid accumulation and aphid response. Journal of Chemical Ecology, 16, 3301-3315.
URL PMID |
| [65] | Siegel MR, Bush LP (1997). Toxin production in grass/endophyte association. In: Carroll GC, Tudzynski P eds. The Mycota. Ⅴ. Plant Relationships, Part B. Springer-Verlag, Berlin, 185-208. |
| [66] | Stearns SC (1989). Trade-offs in life-history evolution. Functional Ecology, 3, 259-268. |
| [67] | Strong DR, Lawton JH, Southwood R (1984). Insects on Plants. Community Patterns and Mechanisms. Blackwell Scientific, Oxford, England, 313. |
| [68] |
Sullivan TJ, Faeth SH (2004). Gene flow in the endophyte Neotyphodium and implications for coevolution with Fectuca arizonica. Molecular Ecology, 13, 649-656.
URL PMID |
| [69] | Tsai HF, Liu JS, Staben C, Christensen MJ, Latch GCM, Siegel MR, Schardl CL (1994). Evolutionary diversification of fungal endophytes of tall fescue grass by hybridization with Epichloı species. Proceedings of the National Academy of Sciences of the United States of America, 91, 2542-2546. |
| [70] | Vinton MA, Kathol ES, Vogel KP, Hopkins AA (2001). Endophytic fungi in Canada wild rye in natural grasslands. Journal of Range Management, 54, 390-395. |
| [71] | Wei YK, Gao YB, Xu H, Su D, Zhang X, Wang YH, Lin F, Chen L, Nie LY, Ren AZ (2006). Occurrence of endophytes in grasses native to northern China. Grass and Forage Science, 61, 422-429. |
| [72] | Wei YK, Gao YB, Zhang X, Su D, Wang YH, Xu H, Lin F, Ren AZ, Chen L, Nie LY (2007). Distribution and diversity of Epichloı/Neotyphodium fungal endophytes from different populations of Achnatherum sibiricum (Poaceae) in the Inner Mongolia Steppe, China. Fungal Diversity, 24, 329-345. |
| [73] | Wei YK (魏宇昆), Gao YB (高玉葆), Li C (李川), Xu H (许华), Ren AZ (任安芝) (2006). Genetic diversity of Neotyphodium endophytes isolated from Achnatherum sibiricum populations in mid- and eastern Inner Mongolia Steppe, China. Journal of Plant Ecology (Chinese Version) (formerly Acta Phytoecologica Sinica) (植物生态学报), 30, 640-649. (in Chinese with English abstract) |
| [74] | White JF (1988). Endophyte-host associations in forage grasses. Ⅺ. A proposal concerning origin and evolution. Mycologia, 80, 442-446. |
| [75] | White JF, Morgan-Jones G, Morrow AC (1993). Taxonomy, life cycle, reproduction and detection of Acremonium endophytes. Agriculture, Ecosystems and Environment, 44, 13-37. |
| [76] | White JF, Sullivan RF, Balady GA, Gianfagna TJ, Yue Q, Meyer WA, Cabral D (2001). A fungal endosymbiont of the grass Brumus setifolius: distribution in some Andean populations, identification, and examination of beneficial properties. Symbiosis, 31, 241-257. |
| [77] | Wilkinson HH, Schardl CL (1997). The evolution of mutualism in grass-endophyte associations. In: Bacon CW, Hill NS eds. Neotyphodium/Grass Interactions. Plenum Press, New York. 13-26. |
| [78] | Zabalgogeazcoa I, de Aldana BRV, Criado BG, Ciudad AG (1999). The infection of Festuca rubra by the fungal endophyte Epichloı festuca in Mediterranean permanent grasslands. Grass and Forage Science, 54, 91-95. |
| [1] | 李冠军, 陈珑, 余雯静, 苏亲桂, 吴承祯, 苏军, 李键. 固体培养内生真菌对土壤盐胁迫下木麻黄幼苗渗透调节和抗氧化系统的影响[J]. 植物生态学报, 2023, 47(6): 804-821. |
| [2] | 陈天翌, 娄安如. 青藏高原东侧白桦种群的遗传多样性与遗传结构[J]. 植物生态学报, 2022, 46(5): 561-568. |
| [3] | 石新建, 张靖歆, 秦天姿, 刘金铭, 高玉葆, 任安芝. 内生真菌感染对宿主羽茅及邻生植物抗病性的影响[J]. 植物生态学报, 2021, 45(8): 860-869. |
| [4] | 秦天姿, 任安芝, 樊晓雯, 高玉葆. 内生真菌种类和母本基因型对内生真菌-禾草共生体叶形状和叶面积的影响[J]. 植物生态学报, 2020, 44(6): 654-660. |
| [5] | 张新新, 王茜, 胡颖, 周玮, 陈晓阳, 胡新生. 植物边缘种群遗传多样性研究进展[J]. 植物生态学报, 2019, 43(5): 383-395. |
| [6] | 吴曼, 李娟娟, 刘金铭, 任安芝, 高玉葆. 刈割干扰和养分添加条件下Epichloë内生真菌感染对羽茅所在群落多样性和生产力的影响[J]. 植物生态学报, 2019, 43(2): 85-93. |
| [7] | 李春杰, 姚祥, 南志标. 醉马草内生真菌共生体研究进展[J]. 植物生态学报, 2018, 42(8): 793-805. |
| [8] | 张俪文, 韩广轩. 植物遗传多样性与生态系统功能关系的研究进展[J]. 植物生态学报, 2018, 42(10): 977-989. |
| [9] | 孙茜, 薛子可, 解琳琳, 贺学礼, 赵丽莉. 沙冬青及其伴生植物深色有隔内生真菌物种多样性[J]. 植物生态学报, 2017, 41(7): 729-737. |
| [10] | 乔鲜果, 郭柯, 赵利清, 刘长成, 赵海卫, 侯东杰, 高趁光. 中国石生针茅草原的分布、群落特征和分类[J]. 植物生态学报, 2017, 41(2): 231-237. |
| [11] | 牛毅, 高远, 李隔萍, 任安芝, 高玉葆. 内生真菌对羽茅抗病性的影响[J]. 植物生态学报, 2016, 40(9): 925-932. |
| [12] | 孙茜, 贺超, 贺学礼, 赵丽莉. 沙冬青与伴生植物深色有隔内生真菌定殖规律及其与土壤因子的相关性[J]. 植物生态学报, 2015, 39(9): 878-889. |
| [13] | 李秀璋, 姚祥, 李春杰, 南志标. 禾草内生真菌作为生防因子的潜力分析[J]. 植物生态学报, 2015, 39(6): 621-634. |
| [14] | 刘慧, 陈薇, 周勇, 李夏, 任安芝, 高玉葆. 内生真菌和丛枝菌根真菌对羊草生长的影响[J]. 植物生态学报, 2015, 39(5): 477-485. |
| [15] | 王锦楠, 陈进福, 陈武生, 周新洋, 许东, 李际红, 亓晓. 柴达木地区野生黑果枸杞种群遗传多样性的AFLP分析[J]. 植物生态学报, 2015, 39(10): 1003-1011. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
