

植物生态学报 ›› 2016, Vol. 40 ›› Issue (9): 925-932.DOI: 10.17521/cjpe.2015.0417
收稿日期:2015-11-22
接受日期:2016-04-22
出版日期:2016-09-10
发布日期:2016-09-29
通讯作者:
任安芝
基金资助:
Yi NIU, Yuan GAO, Ge-Ping LI, An-Zhi REN*( ), Yu-Bao GAO
), Yu-Bao GAO
Received:2015-11-22
Accepted:2016-04-22
Online:2016-09-10
Published:2016-09-29
Contact:
An-Zhi REN
摘要:
为探讨不同种类内生真菌对宿主植物羽茅(Achnatherum sibiricum)抗病性的影响, 以感染不同内生真菌的天然禾草羽茅为实验材料, 进行了体外纯培养的内生真菌、感染内生真菌的离体叶片和在体叶片对3种植物病原真菌的抑菌实验。结果表明: 体外纯培养条件下, 分离自羽茅的内生真菌Neotyphodium sibiricum、Neotyphodium gansuensis和Epichloë gansuensis对新月弯孢霉(Curvularia lunata)、根腐离蠕孢(Bipolaris sorokiniana)和枝孢霉(Cladosporium sp.)等3种病原真菌都具有抑制作用, 其中N. sibiricum的抑制作用最强, 对新月弯孢霉、根腐离蠕孢和枝孢霉的抑菌率分别为47.8%、40.1%、39.4%; 内生真菌培养滤液也可以有效抑制这3种病原真菌的孢子萌发, 其中N. gansuensis的抑制作用最强, 新月弯孢、根腐离蠕孢和枝孢霉的孢子萌发率分别为9.8%、8.7%、8.5%。对于离体叶片, N. sibiricum和N. gansuensis感染可以有效降低叶片受3种病原真菌侵染后的病斑数和孢子浓度, 其中N. sibiricum对根腐离蠕孢的抑制作用显著高于N. gansuensis, 而E. gansuensis只降低新月弯孢和枝孢霉侵染的病斑数以及枝孢霉侵染的孢子浓度。在体条件下, 内生真菌均可以显著降低病原真菌侵染羽茅后的病斑数、病斑长度和孢子浓度, 其中E. gansuensis的抑菌作用趋于最弱, 而N. sibiricum的抑菌作用趋于最强。
牛毅, 高远, 李隔萍, 任安芝, 高玉葆. 内生真菌对羽茅抗病性的影响. 植物生态学报, 2016, 40(9): 925-932. DOI: 10.17521/cjpe.2015.0417
Yi NIU, Yuan GAO, Ge-Ping LI, An-Zhi REN, Yu-Bao GAO. Effect of different species of endophytes on fungal disease resistance of Achnatherum sibiricum. Chinese Journal of Plant Ecology, 2016, 40(9): 925-932. DOI: 10.17521/cjpe.2015.0417
| 抑菌率 Inhibition rate (%) | 平均孢子萌发率 Average spore germination rate (%) | ||||
|---|---|---|---|---|---|
| F | p | F | p | ||
| E | 131.323 | 0.000 | 643.791 | 0.000 | |
| P | 0.837 | 0.441 | 12.453 | 0.000 | |
| E × P | 14.210 | 0.010 | 5.788 | 0.000 | |
表1 不同内生真菌对3种病原真菌抗性的双因素方差分析
Table 1 Two-way ANOVA for pathogens fungi resistance of different morphotypes of endophytes
| 抑菌率 Inhibition rate (%) | 平均孢子萌发率 Average spore germination rate (%) | ||||
|---|---|---|---|---|---|
| F | p | F | p | ||
| E | 131.323 | 0.000 | 643.791 | 0.000 | |
| P | 0.837 | 0.441 | 12.453 | 0.000 | |
| E × P | 14.210 | 0.010 | 5.788 | 0.000 | |
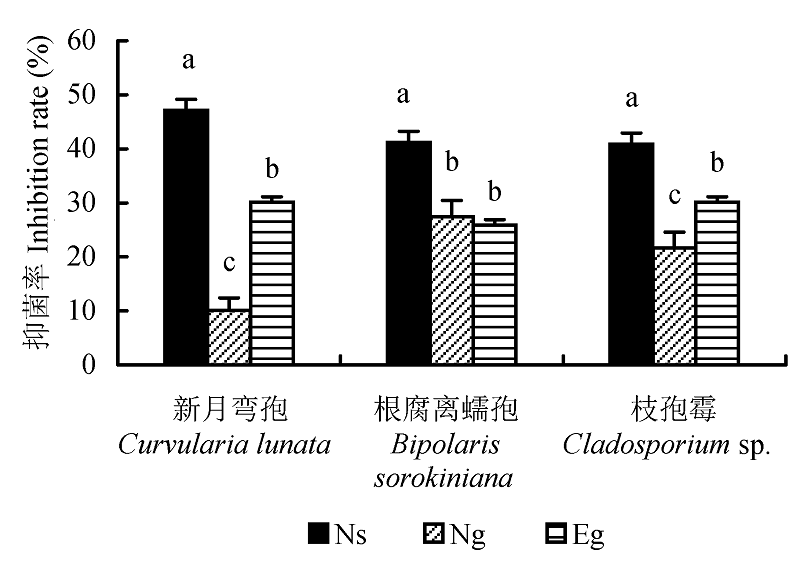
图1 不同内生真菌对3种病原真菌的平均抑菌率(平均值±标准误差)。不同小写字母表示差异显著(p < 0.05)。Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum。
Fig. 1 Mean inhibition rate of different morphotypes of endophytes on three species of pathogens fungi (mean ± SE). Different small letters indicate significant difference (p < 0.05). Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum.
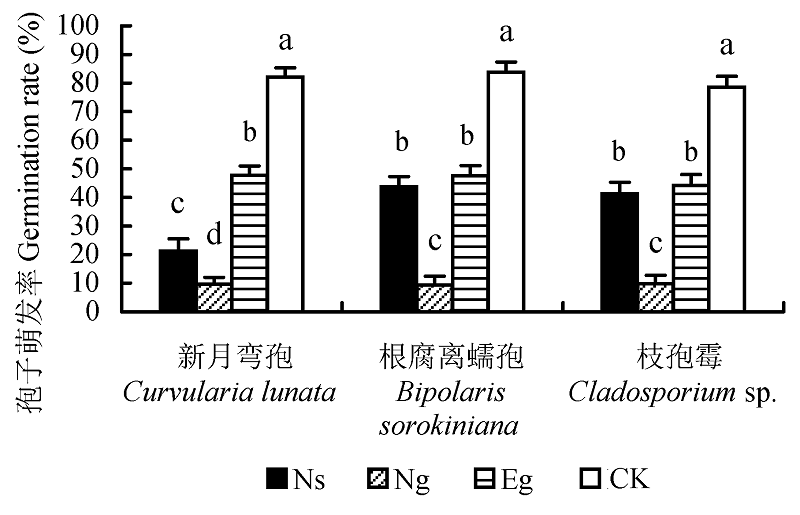
图2 不同内生真菌培养滤液对病原真菌孢子萌发率的影响(平均值±标准误差)。不同小写字母表示差异显著(p < 0.05)。CK, 对照; Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum。
Fig. 2 Effect of culture filtrate on germination rate of the pathogenic fungi spores (mean ± SE). CK, control; Eg, Epi- chloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum. Different small letters indicate significant difference (p < 0.05).
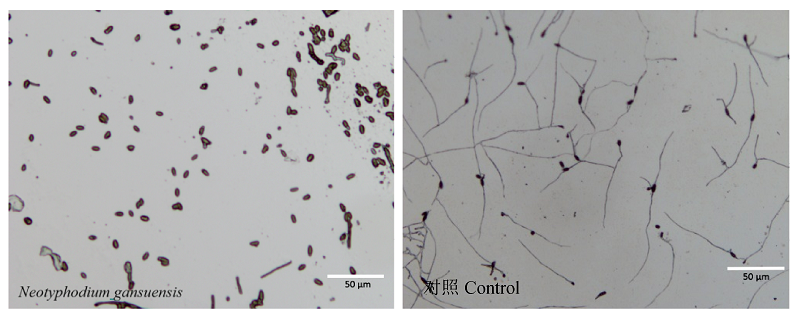
图3 Neotyphodium gansuensis培养滤液对根腐离蠕孢孢子萌发的影响。左图中根腐离蠕孢孢子的萌发受到了抑制, 发芽率低。
Fig. 3 Influence of culture filtrate of Neotyphodium gansuensis on spore germination of Bipolaris sorokiniana. Bipolaris sorokiniana spore germination is inhibited in the left picture, germination rate is low.
| 离体实验 Detached experiment | 在体实验 Intact experiment | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 病斑数 Mean number of lesions | 病斑长度 Mean length of lesions | 孢子浓度 Spore concentration of pathogens fungi | 病斑数 Mean number of lesions | 病斑长度 Mean length of lesions | 孢子浓度 Spore concentration of pathogens fungi | ||||||||||||
| F | P | F | P | F | P | F | P | F | P | F | P | ||||||
| E | 8.94 | 0.000 | 6.65 | 0.001 | 107.20 | 0.000 | 11.25 | 0.000 | 5.14 | 0.011 | 17.90 | 0.000 | |||||
| P | 109.50 | 0.000 | 485.30 | 0.000 | 677.50 | 0.000 | 20.90 | 0.000 | 37.57 | 0.000 | 66.43 | 0.000 | |||||
| E × P | 3.21 | 0.010 | 4.05 | 0.002 | 17.32 | 0.000 | 9.25 | 0.000 | 14.70 | 0.000 | 4.28 | 0.000 | |||||
表2 感染不同内生真菌的活体植株对3种病原真菌抗性的双因素方差分析
Table 2 Two-way ANOVA for three resistances of pathogenic fungi of intact plants infected different endophyte
| 离体实验 Detached experiment | 在体实验 Intact experiment | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 病斑数 Mean number of lesions | 病斑长度 Mean length of lesions | 孢子浓度 Spore concentration of pathogens fungi | 病斑数 Mean number of lesions | 病斑长度 Mean length of lesions | 孢子浓度 Spore concentration of pathogens fungi | ||||||||||||
| F | P | F | P | F | P | F | P | F | P | F | P | ||||||
| E | 8.94 | 0.000 | 6.65 | 0.001 | 107.20 | 0.000 | 11.25 | 0.000 | 5.14 | 0.011 | 17.90 | 0.000 | |||||
| P | 109.50 | 0.000 | 485.30 | 0.000 | 677.50 | 0.000 | 20.90 | 0.000 | 37.57 | 0.000 | 66.43 | 0.000 | |||||
| E × P | 3.21 | 0.010 | 4.05 | 0.002 | 17.32 | 0.000 | 9.25 | 0.000 | 14.70 | 0.000 | 4.28 | 0.000 | |||||
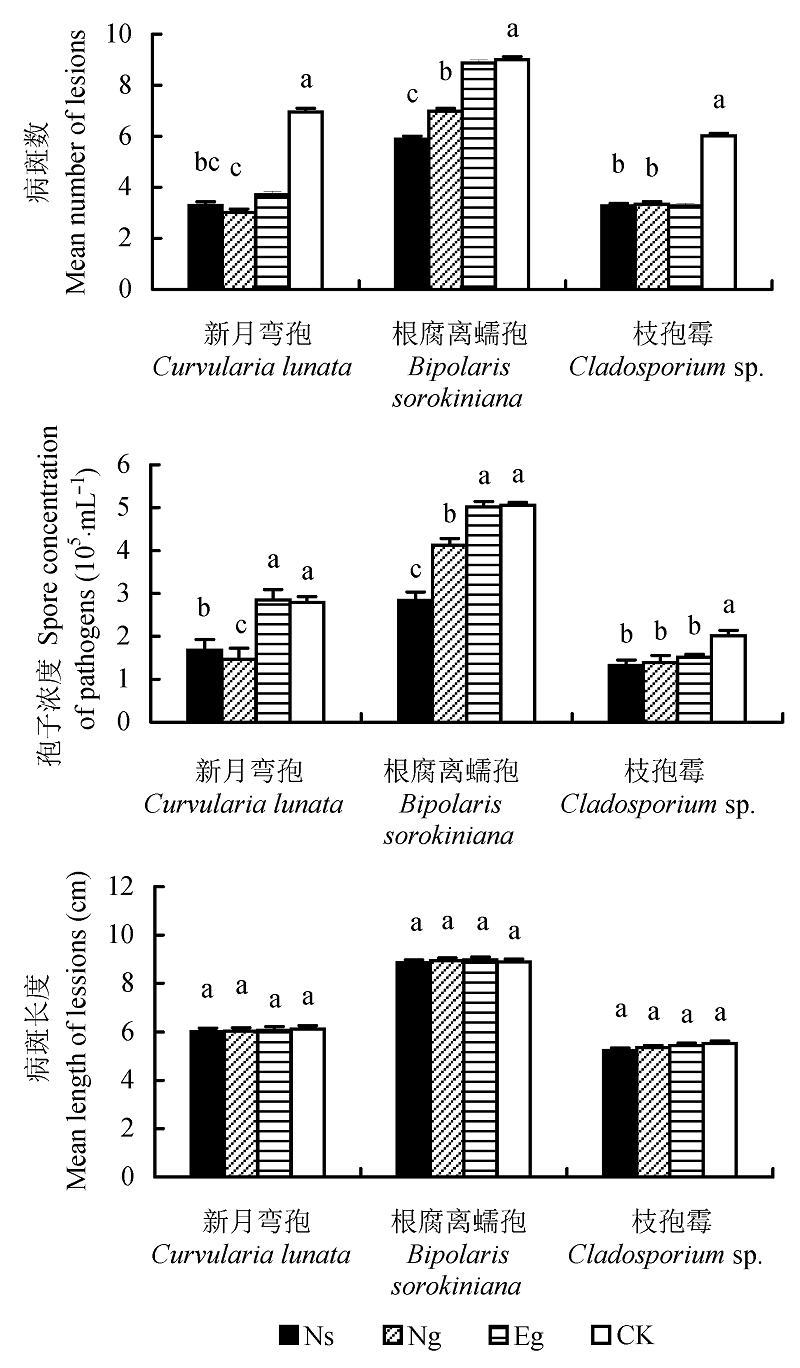
图4 感染不同内生真菌的羽茅离体叶片受病原真菌侵染后的病斑数、病斑长度以及病原真菌的孢子浓度(平均值±标准误差)。不同小写字母表示差异显著(p < 0.05)。CK, 对照; Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum。
Fig. 4 Mean number and length of lesions and spore concentration on detached leaves of Achnatherum sibiricum after inoculation with pathogens-fungi (mean ± SE). Different small letters indicate significant difference (p < 0.05). CK, control; Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum.
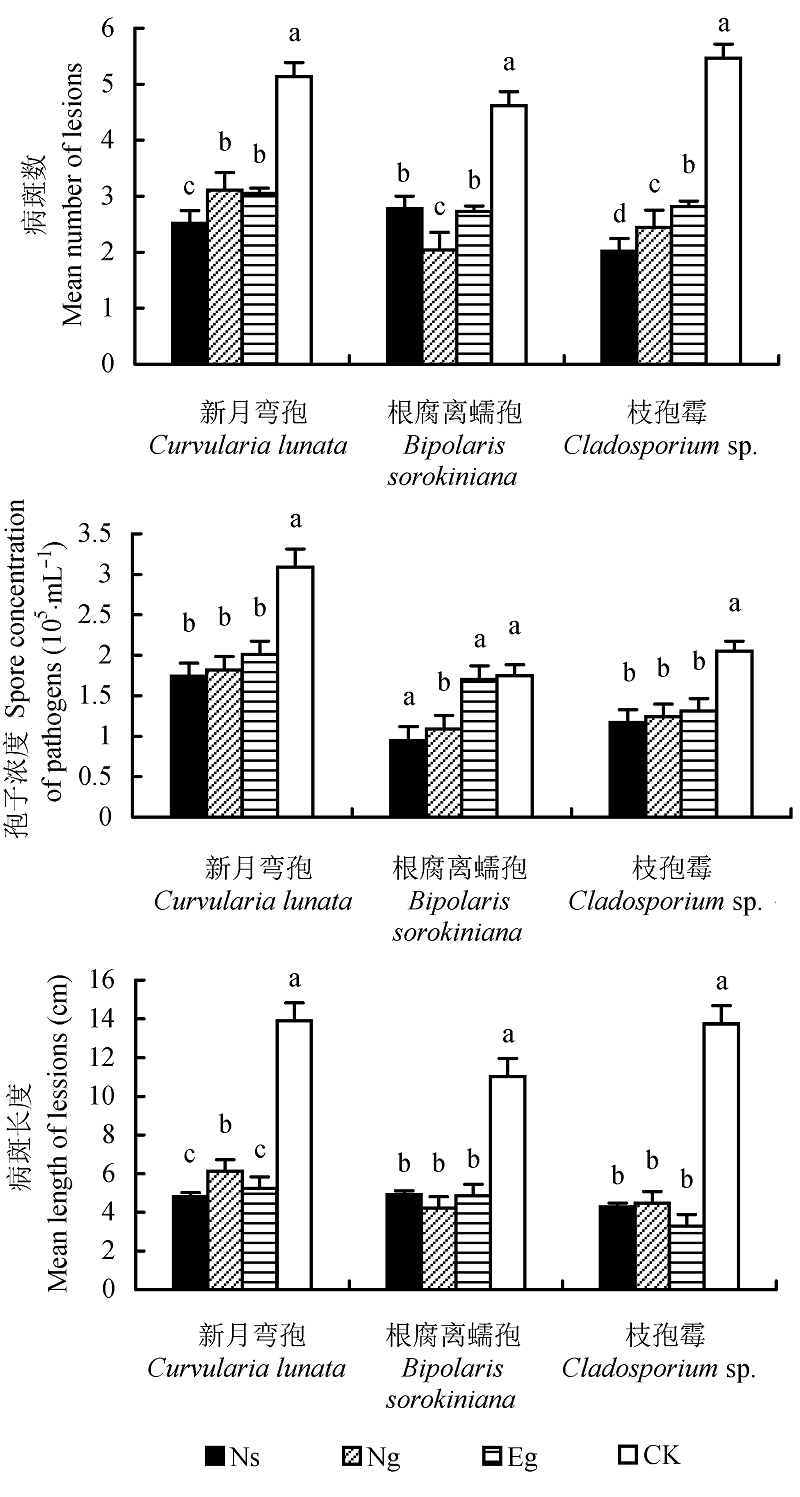
图5 感染不同内生真菌的羽茅在体叶片受病原真菌侵染后的病斑数、病斑长度以及病原真菌的孢子浓度(平均值±标准误差)。不同小写字母表示差异显著(p < 0.05)。CK, 对照; Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum。
Fig. 5 Mean number and length of lesions and spore concentra- tion on intact leaves of A. sibiricum after inoculation with pathogens-fungi (mean ± SE). Different small letters indicate significant difference (p < 0.05). CK, control; Eg, Epichloë gansuensis; Ng, Neotyphodium gansuensis; Ns, Neotyphodium sibiricum.
| 1 | Bonos SA, Wilson MM, Meyer WA, Funk CR (2005). Suppression of red thread in fine fescues through endophyte- mediated resistance.Applied Turfgrass Science, doi:10.1094/ATS-2005-0725-01-RS. |
| 2 | Brem D, Leuchtmann A (2001). Epichloëgrass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum.Oecologia, 126, 522-530. |
| 3 | Burpee LL, Bouton JH (1993). Effect of eradication of the endophyte Acremonium coenophialum on epidemics of Rhizoctonia blight in tall fescue.Plant Disease, 77, 157-159. |
| 4 | Carroll G (1988). Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology, 69, 2-9. |
| 5 | Cassandra LS, Thomas RG (2015). Endophytic association of the pine pathogen Fusarium circinatum with corn (Zea mays). Fungal Ecology, 13, 120-129. |
| 6 | Cheplick GP, Faeth SH (2009). Ecology and Evolution of the Grass-endophyte Symbiosis. Oxford University Press, Oxford, UK. |
| 7 | Christensen MJ (1996). Antifungal activity in grasses infected with Acre monium and Epichloë endophytes. Australasian Plant Pathology, 25(3), 186-191. |
| 8 | Clay K, Schardl C (2002). Evolutionary origins and ecological consequences of endophyte symbiosis with grasses.The American Naturalist, 160, 99-127. |
| 9 | Ewald PW (1987). Transmission modes and evolution of the parasitism-mutualism continuum .Annals of the New York Academy of Sciences, 503, 295-306. |
| 10 | Ewald PW (1994). Evolution of Infectious Disease. Oxford University Press, Oxford, UK. |
| 11 | Fletcher LR (1999). “Non-toxic” endophytes in ryegrass and their effects on livestock health and production. In: Woodfield DR, Matthew C eds. Ryegrass Endophyte: An Essential New Zealand Symbiosis. Grassland Research and Practice Series No. 7. New Zealand Grassland Association, Palmerston North. 133-139. |
| 12 | Iannone L, Pinget A, Nagabhyru P (2012). Beneficial effects of Neotyphodium tembladerae and Neotyphodium pampeanum on a wild forage grass.Grass and Forage Science, 67, 382-390. |
| 13 | Krauss J, Harri SA, Bush L, Husi R, Bigler L, Power SA, Muller C (2007). Effects of fertilizer, fungal endophytes and plant cultivar on the performance of insect herbivores and their natural enemies.Functional Ecology, 21, 107-116. |
| 14 | Leuchtmann A, Bacon CW, Schardl CL (2014). Nomenclatural realignment of Neotyphodium species with genus Epichloë.Mycologia, 106, 202-215. |
| 15 | Leuchtmann A, Oberhofer M (2013). The Epichloë endophytes associated with the woodland grass Hordelymus europaeus including four new taxa.Mycologia, 105, 1315-1324. |
| 16 | Leuchtmann A, Schmidt D, Bush LP (2000). Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloe/Neotyphodium endophytes.Journal of Chemical Ecology, 26, 1025-1036. |
| 17 | Li X, Han R, Ren AZ, Gao YB (2010). Using high-temperature treatment to construct endophyte-free Achnatherum sibiricum.Microbiology China, 37, 1395-1400. (in Chinese with English abstract)[李夏, 韩荣, 任安芝, 高玉葆 (2000). 高温处理构建不感染内生真菌羽茅种群的方法探讨. 微生物学通报, 37, 1395-1400.] |
| 18 | Liu XG, Gao KX, Gu JC, Du JL, Tang XG (1999). Testing on the antagonism of the dominant of endophytic fungi from Populus tomentosa, Chaetomium ND35 in the laboratory.Scientia Silvae Sinicae, 35, 57-62. (in Chinese with English abstract)[刘晓光, 高克祥, 谷建才, 杜建玲, 唐秀光 (1999). 毛白杨内生菌优势种毛壳ND35室内拮抗作用的研究. 林业科学, 35, 57-62.] |
| 19 | Nan ZB, Li CJ (2000). Neotyphodium in native grasses in China and observations on endophyte/host interaction. Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium. Soest, Germany. 41-50. |
| 20 | Niones JT, Takemoto D (2014). An isolate of Epichloë festucae, an endophytic fungus of temperate grasses, has growth inhibitory activity against selected grass pathogens.Journal of General Plant Pathology, 80, 337-347. |
| 21 | Perez LI, Gundel PE, Ghersa CM, Omacini M (2013). Family issues: Fungal endophyte protects host grass from the closely related pathogen Claviceps purpurea.Fungal Ecology, 6, 379-386. |
| 22 | Reddy MN, Faeth SH (2010). Damping-off of Festuca arizonica caused by Fusarium.American Journal of Plant Sciences, 1, 104-105. |
| 23 | Siegel MR, Latch GCM (1991). Expression of antifungal activity in agar culture by isolates of grass endophytes.Mycologia, 83, 529-537. |
| 24 | Sullivan TJ, Faeth SH (2004). Gene flow in the endophyte Neotyphodium and implications for coevolution with Festuca arizonica.Molecular Ecology, 13, 649-656. |
| 25 | Vignale MV, Astiz-Gasso MM, Novas MV, Iannone LJ (2013). Epichloidendophytes confer resistance to the smut Ustilago Bullata in the wild grass Bromus auleticus (Trin.).Biological Control, 67, 1-7. |
| 26 | Wali PR, Helander M, Nissinen O, Saikkonen K (2006). Susceptibility of endophyte-infected grasses to winter pathogens (snow molds).Canadian Journal of Botany, 84, 1043-1051. |
| 27 | Wang XY, Zhou Y, Ren AZ, Gao YB (2014). Effect of endophyte infection on fungal disease resistance of Leymuschinensis.Acta Ecologica Sinica, 34, 6789-6796. (in Chinese with English abstract)[王欣禹, 周勇, 任安芝, 高玉葆 (2014). 内生真菌感染对宿主羊草抗病性的影响. 生态学报, 34, 6789-6796.] |
| 28 | Xia C, Zhang X, Christensen MJ, Nan ZB, Li CJ (2015). Epichloë endophyte affects the ability of powdery mildew (Blumeria graminis) to colonise drunken horse grass (Achnatherum inebrians).Fungal Ecology, 16, 26-33. |
| 29 | Xie FX, Ren AZ, Wang YH, Lin F, Gao YB (2008). A comparative study of the inhibitive effect of fungal endophytes on turf grass fungus pathogens.Acta Ecologica Sinica, 28, 3913-3920. (in Chinese with English abstract)[谢凤行, 任安芝, 王银华, 林枫, 高玉葆 (2008). 内生真菌对草坪植物病原真菌抑制作用的比较. 生态学报, 28, 3913-3920.] |
| 30 | Zhang GM, Wang ZF, Liu YR, Zhang XG, Jiang JM (1995). Study on selectivity toxicity to tobacco of cultural filtrates from pathogen of tobacco black death.Journal of Shandong Agricultural University, 26, 131-136. (in Chinese with English abstract)[张广民, 王智发, 刘延荣, 张修国, 姜金明 (1995). 烟草低头黑病菌培养滤液对烟草毒性及作用特性的研究. 山东农业大学学报, 26, 131-136.] |
| [1] | 李冠军, 陈珑, 余雯静, 苏亲桂, 吴承祯, 苏军, 李键. 固体培养内生真菌对土壤盐胁迫下木麻黄幼苗渗透调节和抗氧化系统的影响[J]. 植物生态学报, 2023, 47(6): 804-821. |
| [2] | 石新建, 张靖歆, 秦天姿, 刘金铭, 高玉葆, 任安芝. 内生真菌感染对宿主羽茅及邻生植物抗病性的影响[J]. 植物生态学报, 2021, 45(8): 860-869. |
| [3] | 秦天姿, 任安芝, 樊晓雯, 高玉葆. 内生真菌种类和母本基因型对内生真菌-禾草共生体叶形状和叶面积的影响[J]. 植物生态学报, 2020, 44(6): 654-660. |
| [4] | 吴曼, 李娟娟, 刘金铭, 任安芝, 高玉葆. 刈割干扰和养分添加条件下Epichloë内生真菌感染对羽茅所在群落多样性和生产力的影响[J]. 植物生态学报, 2019, 43(2): 85-93. |
| [5] | 李春杰, 姚祥, 南志标. 醉马草内生真菌共生体研究进展[J]. 植物生态学报, 2018, 42(8): 793-805. |
| [6] | 孙茜, 薛子可, 解琳琳, 贺学礼, 赵丽莉. 沙冬青及其伴生植物深色有隔内生真菌物种多样性[J]. 植物生态学报, 2017, 41(7): 729-737. |
| [7] | 孙茜, 贺超, 贺学礼, 赵丽莉. 沙冬青与伴生植物深色有隔内生真菌定殖规律及其与土壤因子的相关性[J]. 植物生态学报, 2015, 39(9): 878-889. |
| [8] | 李秀璋, 姚祥, 李春杰, 南志标. 禾草内生真菌作为生防因子的潜力分析[J]. 植物生态学报, 2015, 39(6): 621-634. |
| [9] | 刘慧, 陈薇, 周勇, 李夏, 任安芝, 高玉葆. 内生真菌和丛枝菌根真菌对羊草生长的影响[J]. 植物生态学报, 2015, 39(5): 477-485. |
| [10] | 贾彤, 任安芝, 魏茂英, 尹立佳, 高玉葆. 不同传播方式的内生真菌感染对羽茅的生理生态影响[J]. 植物生态学报, 2015, 39(1): 72-80. |
| [11] | 闫姣,贺学礼,张亚娟,许伟,张娟,赵丽莉. 荒漠北沙柳根系丛枝菌根真菌和黑隔内生真菌定殖状况[J]. 植物生态学报, 2014, 38(9): 949-958. |
| [12] | 周勇, 郑璐雨, 朱敏杰, 李夏, 任安芝, 高玉葆. 内生真菌感染对禾草宿主生境土壤特性和微生物群落的影响[J]. 植物生态学报, 2014, 38(1): 54-61. |
| [13] | 魏宇昆, 高玉葆. 禾草内生真菌的遗传多样性及其共生关系[J]. 植物生态学报, 2008, 32(2): 512-520. |
| [14] | 魏宇昆, 高玉葆, 李川, 许华, 任安芝. 内蒙古中东部草原羽茅内生真菌的遗传多样性[J]. 植物生态学报, 2006, 30(4): 640-649. |
| [15] | 梁宇, 陈世苹, 高玉葆, 任安芝. 内生真菌感染对干旱胁迫下黑麦草生长的影响[J]. 植物生态学报, 2002, 26(5): 621-626. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19