

植物生态学报 ›› 2009, Vol. 33 ›› Issue (5): 984-992.DOI: 10.3773/j.issn.1005-264x.2009.05.018
郝海平1,2, 姜闯道1, 石雷1, 唐宇丹1,*( ), 姚涓1, 李志强3
), 姚涓1, 李志强3
收稿日期:2008-12-23
修回日期:2009-06-21
出版日期:2009-12-23
发布日期:2009-09-30
通讯作者:
唐宇丹
作者简介:*(tangyudan@ibcas.ac.cn)基金资助:
HAO Hai-Ping1,2, JIANG Chuang-Dao1, SHI Lei1, TANG Yu-Dan1,*( ), YAO Juan1, LI Zhi-Qiang3
), YAO Juan1, LI Zhi-Qiang3
Received:2008-12-23
Revised:2009-06-21
Online:2009-12-23
Published:2009-09-30
Contact:
TANG Yu-Dan
摘要:
以光核桃(Prunus mira)幼苗为材料, 通过控制根系温度研究了根系温度变化与叶片脱落酸(ABA)的关系及其对光合机构热稳定性的影响。结果表明: 1)环境高温(37和40 ℃)胁迫下保持根系温度适宜((25±2) ℃)时, 幼苗叶片相对含水量(Relative water content, RWC)下降较少, 但叶片ABA含量低, 超氧化物歧化酶(SOD)、抗坏血酸过氧化物酶(APX)、过氧化物酶(POD)和过氧化氢酶(CAT)活性低, 过氧化氢(H2O2)含量和膜质过氧化水平(丙二醛(Malondialdehyde, MDA)浓度)提高, 最大光化学效率(Fv/Fm)下降程度较大; 2)而同等环境高温(37和40 ℃)条件下根系温度逐步升高时, 幼苗叶片RWC降低, 叶片ABA含量增加, SOD、APX、POD、CAT活性高, H2O2含量高, MDA生成量低, Fv/Fm降低程度较小。与37 ℃相比, 40 ℃处理条件下各生理指标变化趋势相似, 但差异加大。因此认为: 高温胁迫条件下, 根系温度适宜时RWC高, 但导致光合机构伤害较重; 根系感受高温胁迫能够增加叶片ABA含量, 有助于保护光合机构、提高光合机构的抗热性。
郝海平, 姜闯道, 石雷, 唐宇丹, 姚涓, 李志强. 根系温度对光核桃幼苗光合机构热稳定性的影响. 植物生态学报, 2009, 33(5): 984-992. DOI: 10.3773/j.issn.1005-264x.2009.05.018
HAO Hai-Ping, JIANG Chuang-Dao, SHI Lei, TANG Yu-Dan, YAO Juan, LI Zhi-Qiang. EFFECTS OF ROOT TEMPERATURE ON THERMOSTABILITY OF PHOTOSYNTHETIC APPARATUS IN PRUNUS MIRA SEEDLINGS. Chinese Journal of Plant Ecology, 2009, 33(5): 984-992. DOI: 10.3773/j.issn.1005-264x.2009.05.018
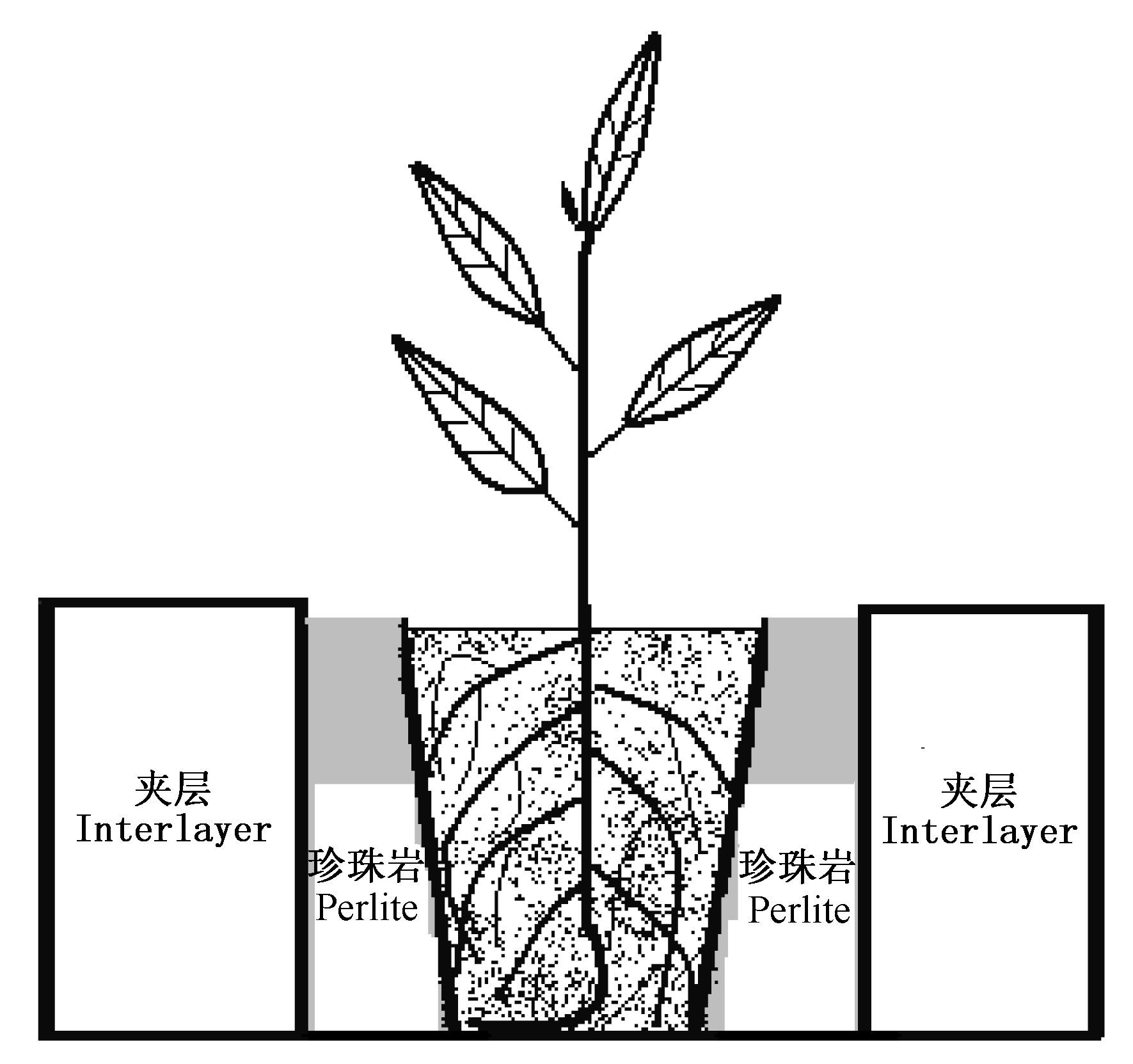
图1 控温装置示意图 根据试验处理, 夹层填充冰袋或干燥珍珠岩 According to the experimental design, ice bag or dry perlite was filled in the temperature control interlayer space
Fig. 1 Sketch map of temperature control container
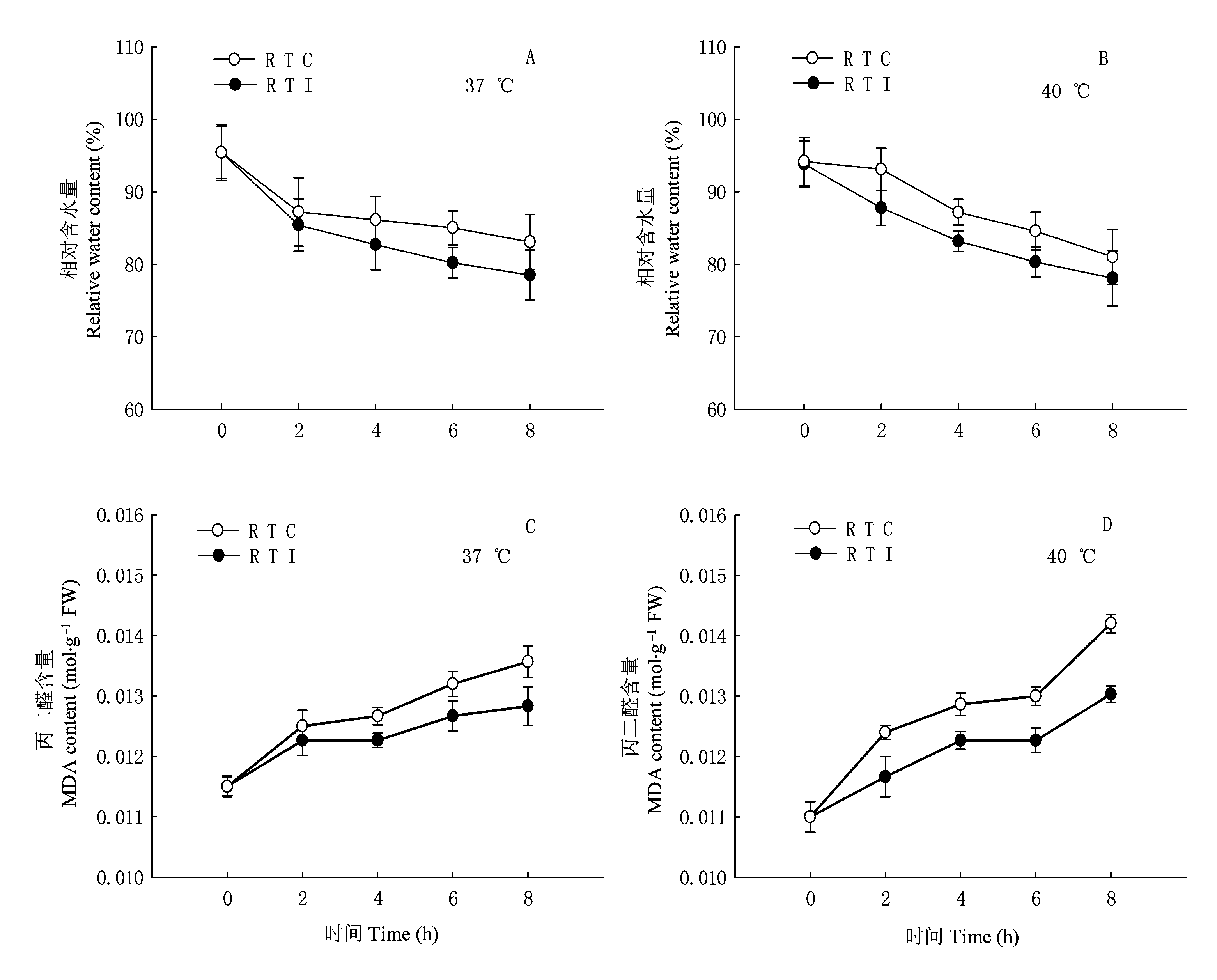
图2 环境高温(37或40 ℃)条件下光核桃幼苗的根系温度变化对叶片相对含水量(A、B)和丙二醛(MDA)含量 (C、D)的影响(平均值±标准误差, n=3) RTC: 根系控温 Constant root temperature RTI: 根系逐步升温 Root temperature increasing gradually RTC、RTI: 同表1 See Table 1
Fig. 2 Effects of root temperature of Prunus mira seedlings on leaf relative water content (A, B) and Malondialdehyde (MDA) content (C, D) in the leaves of P. mira at high air temperature of 37 or 40 ℃ (mean±SE, n=3)
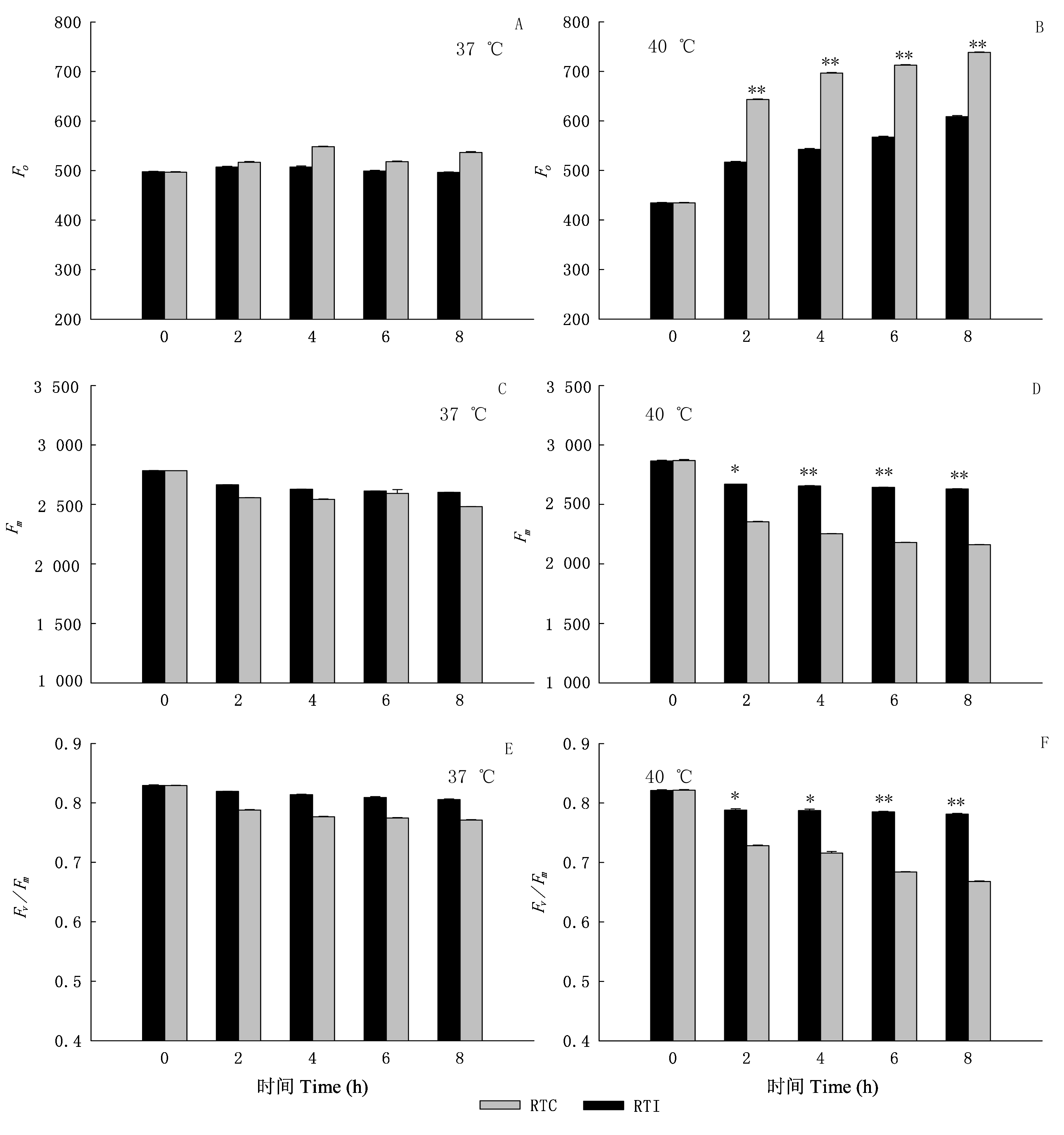
图3 环境高温(37或40 ℃)条件下光核桃幼苗的根系温度对叶片初始荧光(Fo)(A, B)、最大荧光产量(Fm)(C, D)、光系统Ⅱ最大光化学效率(Fv/Fm) (E, F)的影响(平均值±标准误差, n=6) 单星号表示Duncan’s检验中差异达到0.05显著水平, 双星号表示差异达到0.01显著水平 Values within the same columns with single or double asterisk are significantly different at 0.05 or 0.01 probability level using Duncan’s multiple range test RTC、RTI: 同表1 See Table 1
Fig. 3 Effects of root temperature of Prunus mira seedlings on the initial fluorescence (Fo) (A, B), maximal fluorescence (Fm) (C, D) and maximum quantum yield of PSⅡ photochemistry (Fv/Fm) (E, F) in the leaves of P. mira at 37 ℃ or 40 ℃ (mean±SE, n=6)
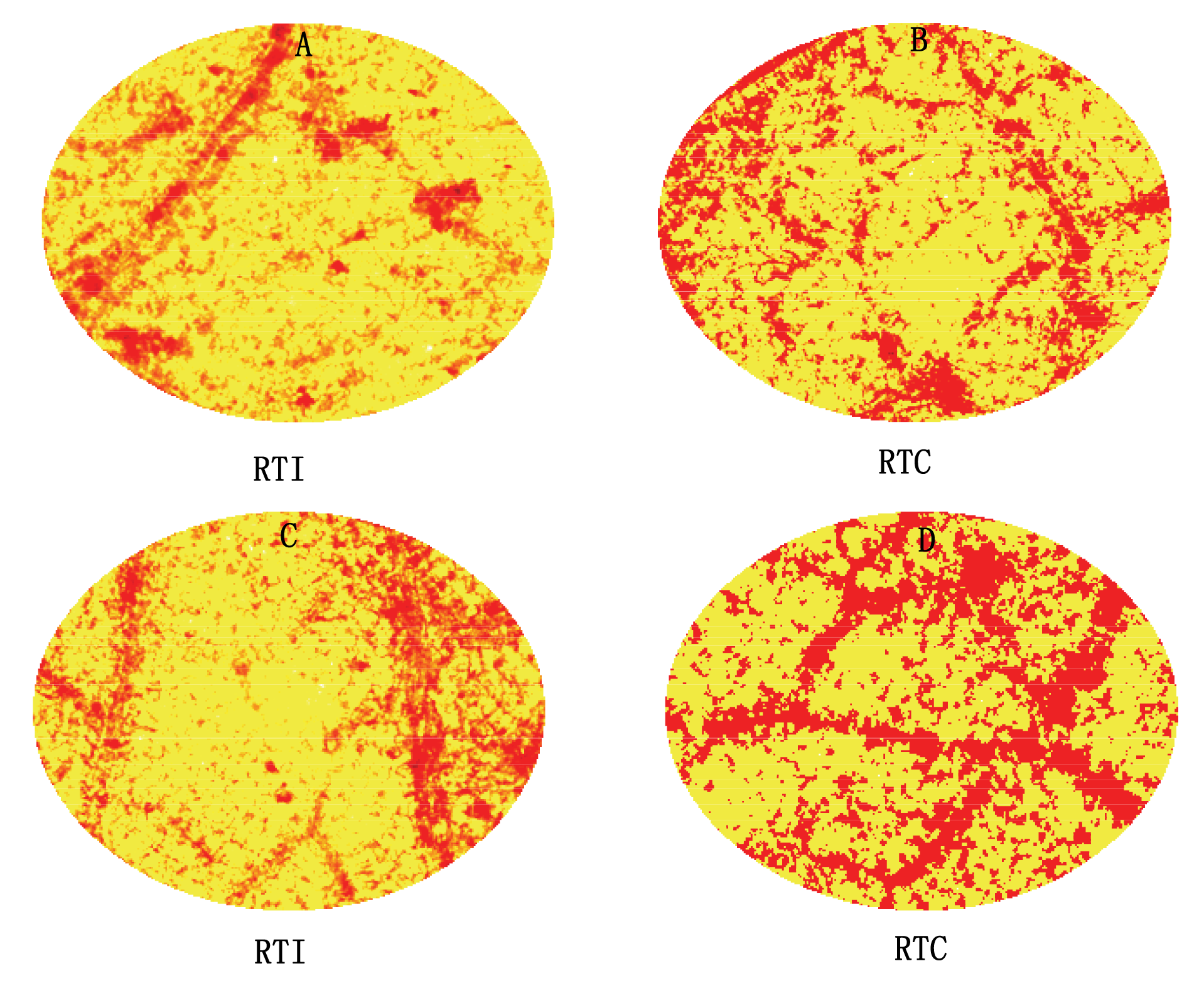
图4 环境高温37 ℃ (A、B)和40 ℃ (C、D)处理8 h条件下光核桃幼苗的根系温度对叶片H2O2 (深色部分) 含量的影响 RTC、RTI: 同表1 See Table 1
Fig. 4 Effects of root temperature of Prunus mira seedlings on H2O2 (dark section) concentration in the P. mira leaves at 37 ℃ (A, B) and 40 ℃ (C, D) for 8 h
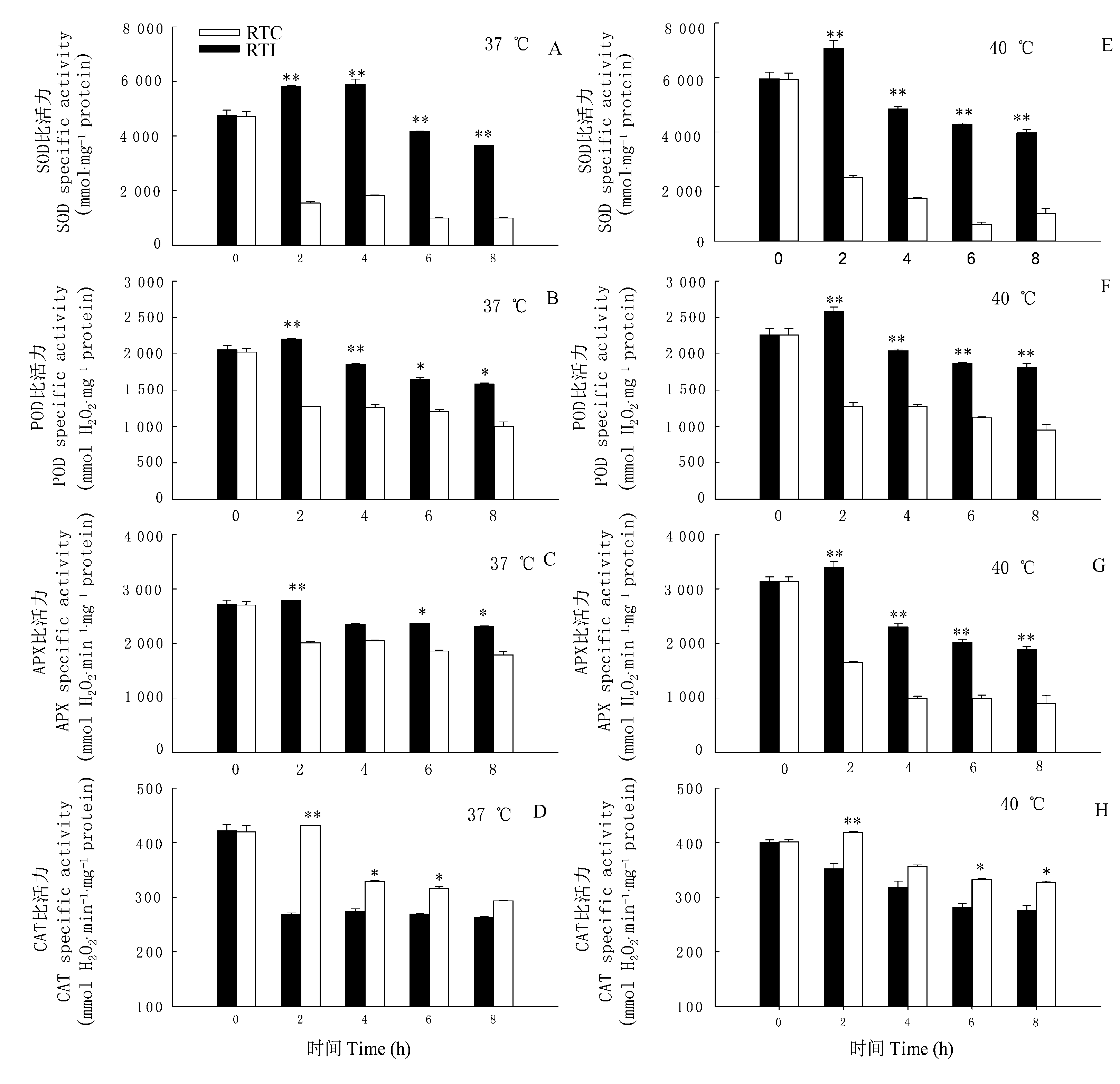
图5 环境高温(37或40 ℃)条件下光核桃幼苗的根系温度对叶片中SOD、POD、APX、CAT的比活力的影响(平均值±标准误差, n=3) 单星号表示Duncan’s检验中差异达到0.05显著水平, 双星号表示达到0.01显著水平 Values within the same columns with single or double asterisk are significantly different at 0.05 or 0.01 probability level using Duncan’s multiple range test RTC、RTI: 同表1 See Table 1
Fig. 5 Effects of root temperature of Prunus mira seedlings on specific activity of SOD, POD, APX and CAT in P. mira leaves at 37 ℃ or 40 ℃ (mean±SE, n=3)
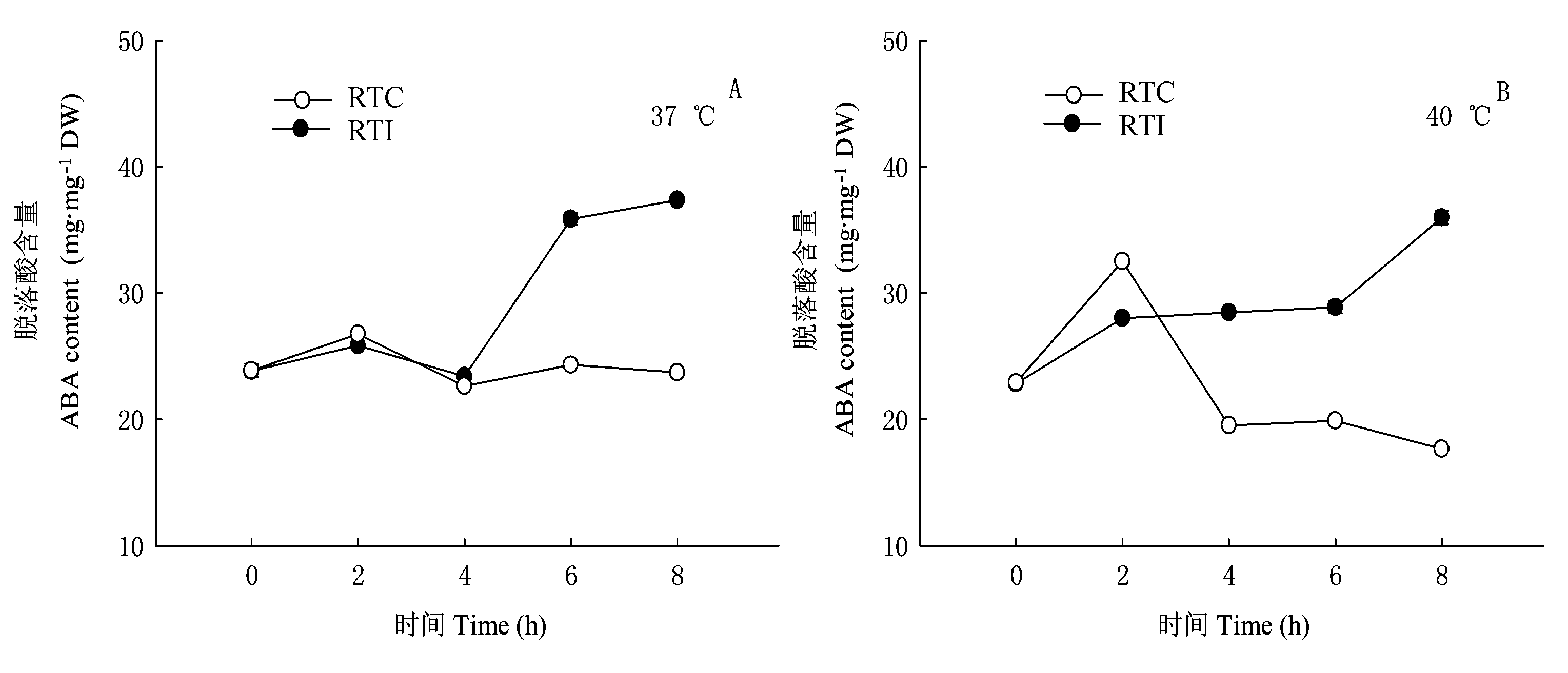
图6 环境高温(37或40 ℃)条件下光核桃幼苗的根系温度对叶片ABA含量的影响(n=3) RTC、RTI: 同表1 See Table 1
Fig. 6 Effects of root temperature of Prunus mira seedlings on ABA content in P. mira leaves at 37 ℃ or 40 ℃ (n=3)
| [1] | Change B, Maehly AC (1955). Assay of catalases and peroxidase. Methods in Enzymology, 2, 764-775. |
| [2] | Dong GZ (董国正) (1991). Investigation of smooth-pit peach in Tibet of China. Forest By-Product and Speciality in China (中国林副特产), (3), 44-45. (in Chinese) |
| [3] | Feng YL (冯玉龙), Zhang YJ (张亚杰), Zhu CQ (朱春全) (2001). The effects of manipulated activated oxygen metabolism on photoinhibition in leaves of poplars suffering root osmotic stress. Acta Phytoecologica Sinica (植物生态学报), 25, 451-459. (in Chinese with English abstract) |
| [4] |
Fodor J, Gullner G, Adam AL, Barna B, Komives T, Kiraly Z (1997). Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco. Plant Physiology, 114, 1443-1451.
DOI URL PMID |
| [5] |
Giannopolites CN, Ries SK (1977). Superoxide dismutase. I. Occurrence in higher plants. Plant Physiology, 59, 309-314.
DOI URL PMID |
| [6] |
Gong M, Li YJ, Chen SZ (1998a). Abscisic acid induced thermotolerance in maize seedlings is mediated by Ca2+ and associated with antioxidant systems. Journal of Plant Physiology, 153, 488-496.
DOI URL |
| [7] |
Gong M, Vander LA, Knight MR, Trewavas AJ (1998b). Heat shock induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiology, 116, 429-437.
DOI URL |
| [8] | Howarth CJ (2005). Genetic Improvements of Tolerance to High Temperature. The Howarth Press, New York. |
| [9] | Jane LK, Huang BR (2005). Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Journal of Plant Growth Regulation, 47, 17-28. |
| [10] |
Jiang CD, Peng ML, Hui YG, Qi Z, Gao MJ, Ling HL (2005). Enhanced photoprotection at the early stages of leaf expansion in field-grown soybean plants. Plant Science, 168, 911-919.
DOI URL |
| [11] |
Jiang CD, Gao MJ, Xian ZW, Ling HL, Biswas DK, Yong GL (2006). Increased photosynthetic activities and thermostability of photosystem II with leaf development of elm seedlings (Ulmus pumila) probed by the fast fluorescence rise OJIP. Environmental and Experimental Botany, 58, 261-268.
DOI URL |
| [12] | Jia WS (贾文锁), Wang XC (王学臣), Zhang SQ (张蜀秋), Lou CH (娄成后) (1996). The transport of ABA from root to shoot and its distribution in response to water stress in Vicia faba L. Acta Phytophysiologica Sinica (植物生理学报), 22, 363-367. (in Chinese with English abstract) |
| [13] |
Kang G, Wang C, Sun G, Wang Z (2003). Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environment and Experiment Botany, 50, 9-15.
DOI URL |
| [14] |
Knight H (2000). Calcium signalling during abiotic stress in plants. International Review of Cytology, 195, 269-324.
DOI URL PMID |
| [15] | Li ZJ, Oda M, Okada K (1996). Changes in thermotolerance of photosynthetic apparatus in cucumber leaves in response to water stress and exogenous ABA treatments. Journal of the Japanese Society for Horticultural Science, 65, 269-324. |
| [16] |
Liu XZ, Huang BR (2005). Root physiological factors involved in cool-season grass response to high soil temperature. Environment and Experiment Botany, 53, 233-245.
DOI URL |
| [17] | Li HS (李合生) (2000). The Experimental Principle and Technology of Plant Physiology and Biochemistry (植物生理生化实验原理和技术). Higher Education Press, Beijing. (in Chinese) |
| [18] | Luo LL (罗丽兰), Shi L (石雷), Jiang CD (姜闯道), Zhang JZ (张金政) (2008). Photosynthetic characteristics and photoprotective mechanisms under various temperatures in Lilium×formolongo seedlings. Acta Horticulturae Sinica (园艺学报), 35, 131-136. (in Chinese with English abstract) |
| [19] | Maehly AC, Chance B (1954). The Assay of Catalase and Peroxidase. Interscience Publishers, New York. |
| [20] | Nakano Y, Asada K (1987). Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant & Cell Physiology, 28, 131-140. |
| [21] | Schöffl F, Prandl R, Reindl A (1999). Molecular responses to heat stress. In: Shinozaki K, Yamaguchi-Shinozaki K eds. Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants. R. G. Landes Company, Austin, TX, USA, 81-98. |
| [22] |
Ślesak I, Libik M, Karpinska B, Karpinski S, Miszalski Z (2007). The role of hydrogen peroxide in regulation of plant metabolism and cellular signaling in response to environmental stresses. Acta Biochimica Polonica, 54, 39-50.
URL PMID |
| [23] | Song JY (宋金艳), Liu DH (刘东焕), Zhao SW (赵世伟), Zhang ZS (张佐双), Jiang CD (姜闯道), Liu YJ (刘玉军) (2008). Advances in studies on primary site of photosynthetic apparatus injured by high temperature. Chinese Bulletin of Life Sciences (生命科学), (2), 263-265. (in Chinese with English abstract) |
| [24] | Taiz L, Zeiger E (1998). Plant Physiology. Sinauer Associates Press, Sunderland, Mass, USA. |
| [25] |
Wang LJ, Li SH (2006). Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Science, 170, 685-694.
DOI URL |
| [26] | Wei PL, Wei GS, Ma KP (2008). Differential responses of the activities of antioxidant enzymes to thermal stresses between two invasive Eupatorium species in China. Journal of Integrative Plant Biology, 10, 1744-1759. |
| [27] |
Wahid A, Gelani S, Ashraf M, Foolad MR (2007). Heat tolerance in plants: An overview. Environmental and Experimental Botany, 61, 199-233.
DOI URL |
| [28] | Xiu LH, Wei W, Chun QL, Jian HZ, Fan L, Ying Z, Ming YJ (2008). Cross-talks between Ca2+/CaM and H2O2 in abscisic acid-induced antioxidant defense in leaves of maize plants exposed to water stress. Journal of Plant Growth Regulation, 55, 183-198. |
| [29] | Zhang ZS (张宗申), Li RQ (利容千), Wang JB (王建波) (2001). Effects of Ca2+ pretreatment on plasmalemma permeability, GSH and ASA contents, and Calcium distribution in pepper mesophyll cells under heat stress. Acta Phytoecologica Sinica (植物生态学报), 25, 230-234. (in Chinese with English abstract) |
| [1] | 刘建新, 刘瑞瑞, 刘秀丽, 贾海燕, 卜婷, 李娜. 外源硫化氢对盐碱胁迫下裸燕麦光合碳代谢的调控[J]. 植物生态学报, 2023, 47(3): 374-388. |
| [2] | 杜英东, 袁相洋, 冯兆忠. 不同形态氮对杨树光合特性及生长的影响[J]. 植物生态学报, 2023, 47(3): 348-360. |
| [3] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [4] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [5] | 余玉蓉, 吴浩, 高娅菲, 赵媛博, 李小玲, 卜贵军, 薛丹, 刘正祥, 武海雯, 吴林. 模拟氮沉降对鄂西南湿地泥炭藓生理及形态特征的影响[J]. 植物生态学报, 2023, 47(11): 1493-1506. |
| [6] | 叶洁泓, 于成龙, 卓少菲, 陈新兰, 杨科明, 文印, 刘慧. 木兰科植物叶片光合系统耐热性与叶片形态及温度生态位的关系[J]. 植物生态学报, 2023, 47(10): 1432-1440. |
| [7] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [8] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| [9] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [10] | 周稳, 迟永刚, 周蕾. 基于日光诱导叶绿素荧光的北半球森林物候研究[J]. 植物生态学报, 2021, 45(4): 345-354. |
| [11] | 丁键浠, 周蕾, 王永琳, 庄杰, 陈集景, 周稳, 赵宁, 宋珺, 迟永刚. 叶绿素荧光主动与被动联合观测应用前景[J]. 植物生态学报, 2021, 45(2): 105-118. |
| [12] | 郭庆华, 胡天宇, 马勤, 徐可心, 杨秋丽, 孙千惠, 李玉美, 苏艳军. 新一代遥感技术助力生态系统生态学研究[J]. 植物生态学报, 2020, 44(4): 418-435. |
| [13] | 刘校铭, 杨晓芳, 王璇, 张守仁. 暖温带落叶阔叶林辽东栎和五角枫生长和光合生理生态特征对模拟氮沉降的响应[J]. 植物生态学报, 2019, 43(3): 197-207. |
| [14] | 汪俊宇, 王小东, 马元丹, 傅卢成, 周欢欢, 王彬, 张汝民, 高岩. ‘波叶金桂’对干旱和高温胁迫的生理生态响应[J]. 植物生态学报, 2018, 42(6): 681-691. |
| [15] | 许红梅, 李进, 张元明. 水分条件对人工培养齿肋赤藓光化学效率及生理特性的影响[J]. 植物生态学报, 2017, 41(8): 882-893. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19