

植物生态学报 ›› 2023, Vol. 47 ›› Issue (3): 374-388.DOI: 10.17521/cjpe.2022.0032
所属专题: 光合作用
收稿日期:2022-01-19
接受日期:2022-07-06
出版日期:2023-03-20
发布日期:2022-07-15
通讯作者:
刘建新
作者简介:* E-mail: liujx1964@163.com基金资助:
LIU Jian-Xin( ), LIU Rui-Rui, LIU Xiu-Li, JIA Hai-Yan, BU Ting, LI Na
), LIU Rui-Rui, LIU Xiu-Li, JIA Hai-Yan, BU Ting, LI Na
Received:2022-01-19
Accepted:2022-07-06
Online:2023-03-20
Published:2022-07-15
Contact:
LIU Jian-Xin
Supported by:摘要:
为探讨外源H2S对盐碱胁迫下植物光合碳代谢的调控效应, 采用盆栽土培实验, 以裸燕麦(Avena nuda)为材料, 研究喷施50 µmol·L−1 H2S供体硫氢化钠(NaHS)溶液对3.00 g·kg-1盐碱胁迫下叶片光合和荧光参数, 单糖、寡糖和淀粉含量, 卡尔文循环和糖代谢关键酶活性及产量构成因素的影响。结果表明: (1)盐碱胁迫下喷施NaHS显著降低裸燕麦叶片叶绿素含量、胞间CO2浓度、光系统II (PSII)初始荧光、最大荧光、调节性能量耗散量子产量、光化学荧光淬灭和非光化学淬灭, 显著提高Hill反应活力、净光合速率、蒸腾速率、气孔导度、表观CO2利用效率、PSII最大光化学效率和核酮糖1,5-二磷酸羧化酶(Rubisco)、Rubisco活化酶、3-磷酸甘油醛脱氢酶和转酮醇酶活性。说明外源H2S通过减少光能吸收和降低PSII天线色素吸收光能用于光化学电子传递份额、提高原初光能转化效率和促进水的光解、调控卡尔文循环关键酶活性和增强CO2利用效率缓解盐碱胁迫诱导的光抑制和光合速率下降。(2)盐碱胁迫下喷施NaHS后第7天, 裸燕麦叶片α-淀粉酶和蔗糖磷酸合成酶活性显著提高, 淀粉、还原糖含量和中性转化酶活性显著降低; 第14天, 可溶性总糖、还原糖含量和蔗糖合成酶、蔗糖磷酸合成酶活性显著下降, 中性转化酶活性显著提高; 第7天和第14天葡萄糖、果糖、半乳糖含量不同程度提高, 棉子糖含量显著下降, 而总淀粉酶、β-淀粉酶、淀粉磷酸化酶、腺苷二磷酸葡萄糖焦磷酸化酶、酸性转化酶活性和岩藻糖、海藻糖、蔗糖含量无显著变化。这表明外源H2S参与盐碱胁迫下裸燕麦淀粉和蔗糖代谢及多糖和低聚糖之间转化的调控。(3)喷施NaHS对盐碱胁迫下裸燕麦的株高、穗数、穗铃数、千粒质量和生物学产量无显著影响, 而穗粒数和籽粒产量显著提高。综上所述, 外源H2S参与盐碱胁迫下裸燕麦光合碳代谢调控, 它能够增强裸燕麦对盐碱胁迫的耐性。
刘建新, 刘瑞瑞, 刘秀丽, 贾海燕, 卜婷, 李娜. 外源硫化氢对盐碱胁迫下裸燕麦光合碳代谢的调控. 植物生态学报, 2023, 47(3): 374-388. DOI: 10.17521/cjpe.2022.0032
LIU Jian-Xin, LIU Rui-Rui, LIU Xiu-Li, JIA Hai-Yan, BU Ting, LI Na. Regulation of exogenous hydrogen sulfide on photosynthetic carbon metabolism in Avena nude under saline-alkaline stress. Chinese Journal of Plant Ecology, 2023, 47(3): 374-388. DOI: 10.17521/cjpe.2022.0032
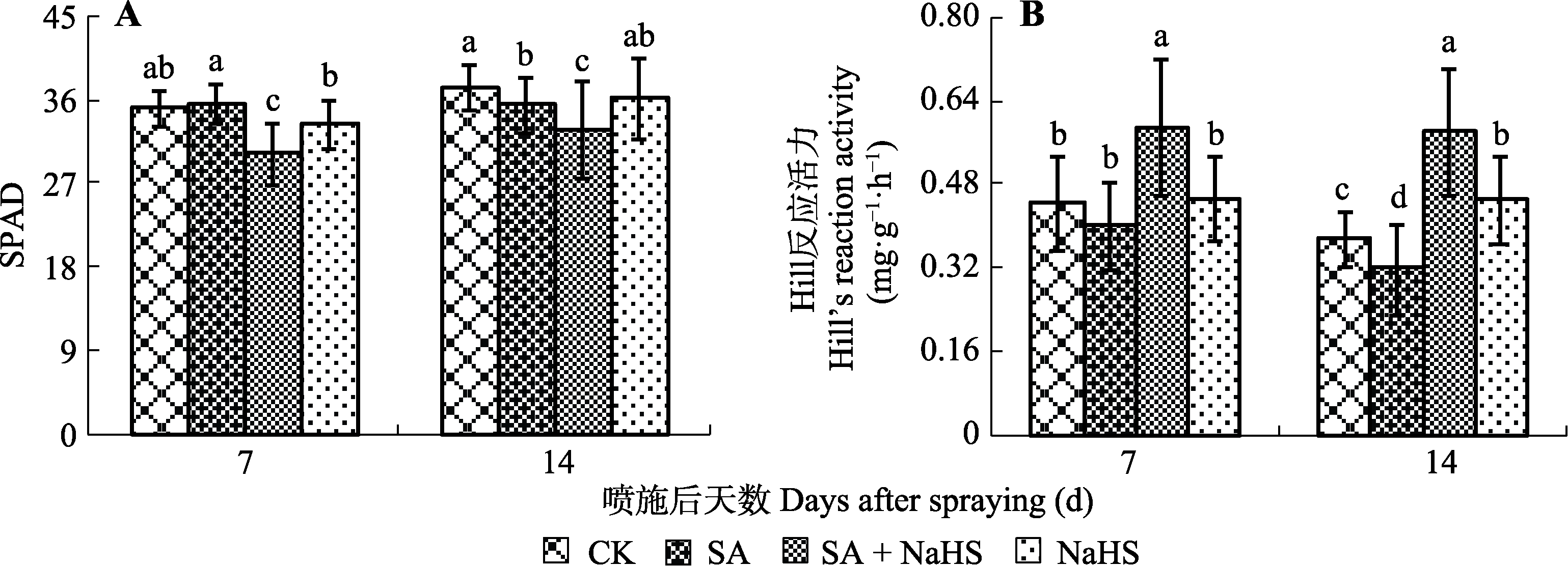
图1 外源H2S对盐碱胁迫下裸燕麦叶片SPAD值(A)和Hill反应活力(B)的影响(平均值±标准差)。CK, 对照; NaHS, 单独喷NaHS; SA, 盐碱胁迫下喷蒸馏水; SA + NaHS, 盐碱胁迫下喷NaHS。不同小写字母表示同一时间不同处理间差异显著(p < 0.05)。
Fig. 1 Effect of exogenous H2S on the SPAD value (A) and Hill’s reaction activity (B) in Avena nude leaves under saline-alkali stress (mean ± SD). CK, control; NaHS, spraying NaHS only; SA, spraying water under salt-alkali stress; SA + NaHS, spraying NaHS under salt-alkali stress. Different lowercase letters indicate significant differences among treatments for the same time (p < 0.05).
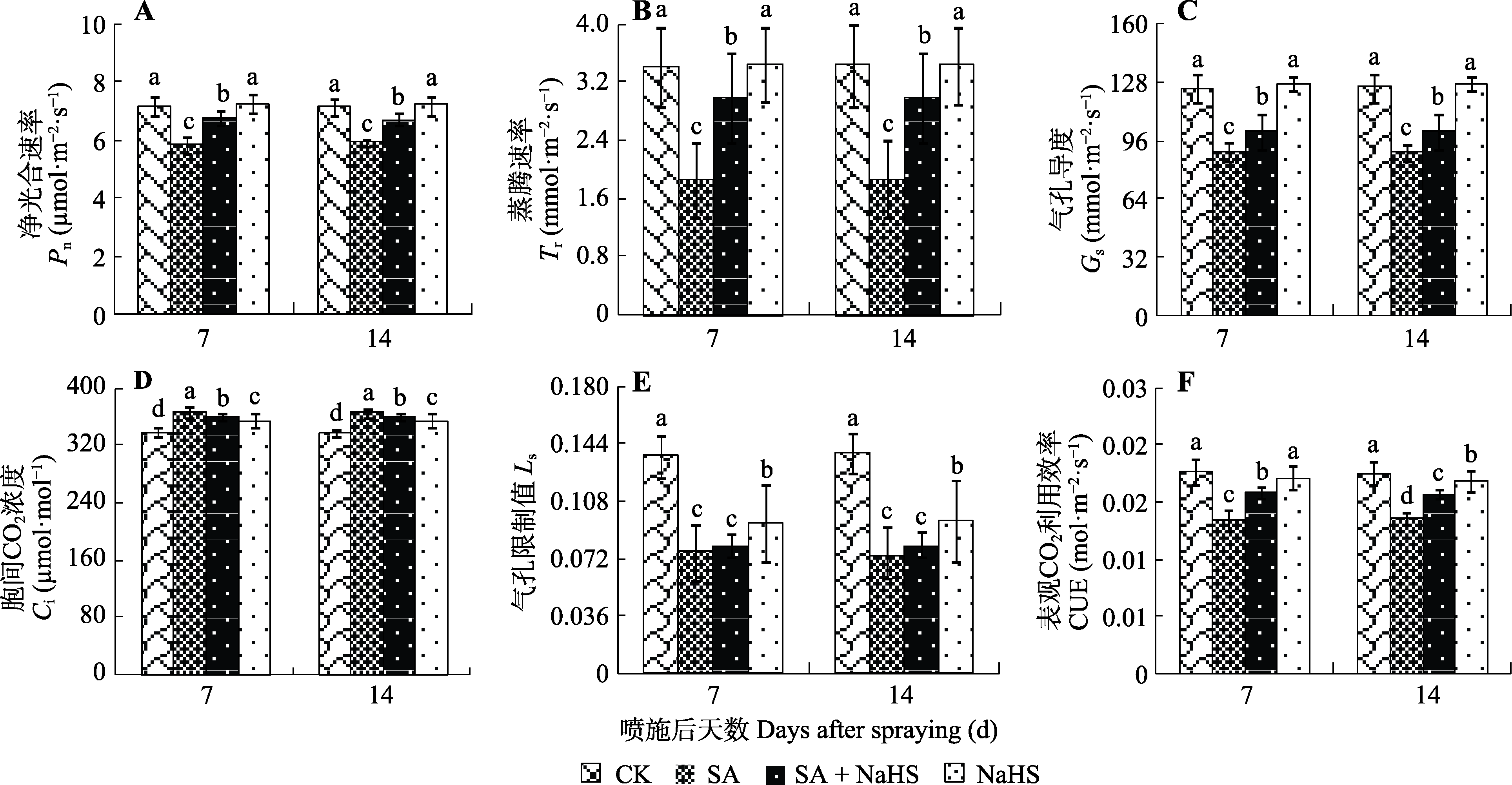
图2 外源H2S对盐碱胁迫下裸燕麦叶片光合气体交换参数的影响(平均值±标准差)。CK, 对照; NaHS, 单独喷NaHS; SA, 盐碱胁迫下喷蒸馏水; SA + NaHS, 盐碱胁迫下喷NaHS。不同小写字母表示同一时间不同处理间差异显著(p < 0.05)。
Fig. 2 Effect of exogenous H2S on photosynthetic gas exchange parameters in Avena nude leaves under saline-alkali stress (mean ± SD). CK, control; NaHS, spraying NaHS only; SA, spraying water under salt-alkali stress; SA + NaHS, spraying NaHS under salt-alkali stress. Ci, intercellular CO2 concentration; CUE, apparent CO2 utility efficiency; Gs, stomatal conductance; Ls, stomatal limitation value; Pn, net photosynthetic rate; Tr, transpiration rate. Different lowercase letters indicate significant differences among treatments at the same time (p < 0.05).
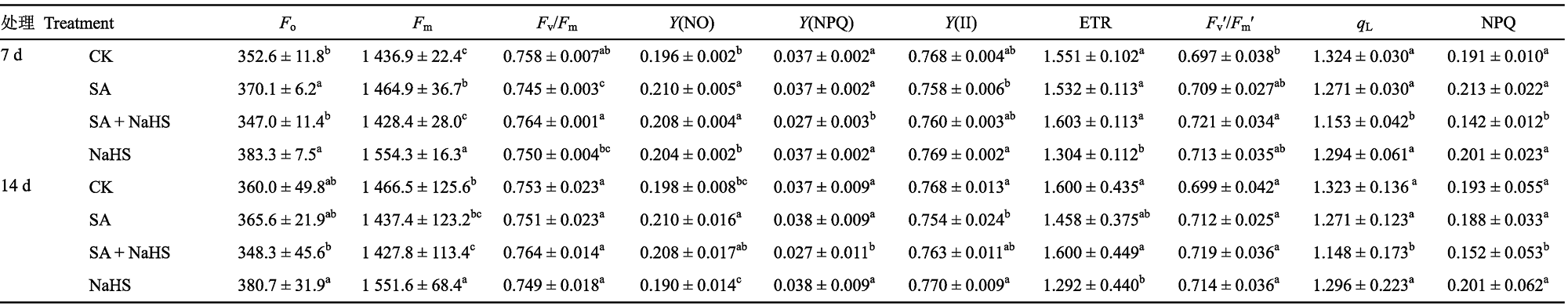 |
表1 外源H2S对盐碱胁迫下裸燕麦叶片叶绿素荧光参数的影响(平均值±标准差)
Table 1 Effect of exogenous H2S on the chlorophyll fluorescence parameters in leaves of Avena nude under saline-lkali stress (mean± SD)
 |
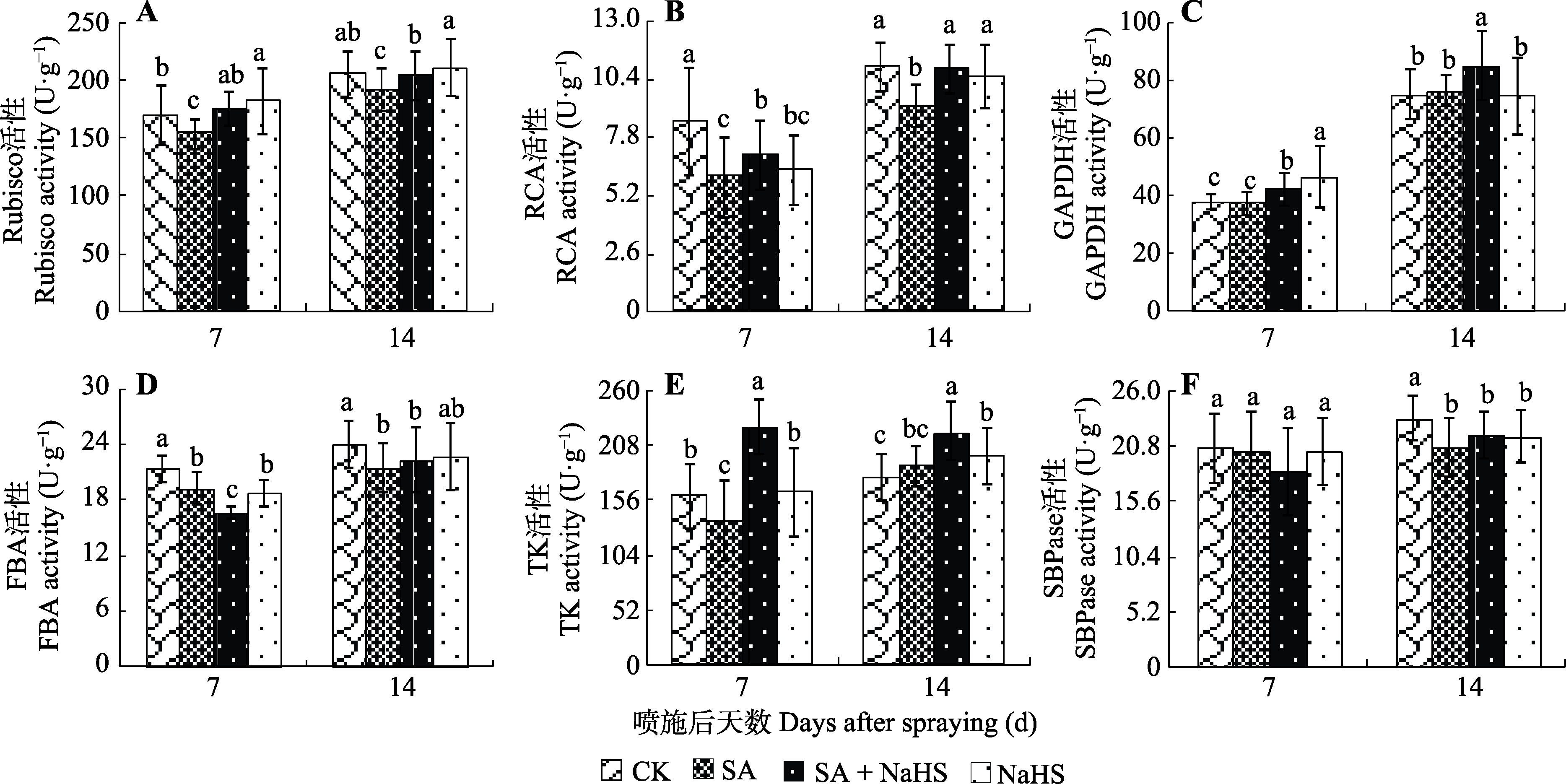
图3 外源H2S对盐碱胁迫下裸燕麦叶片卡尔文循环关键酶活性的影响(平均值±标准差)。CK, 对照; NaHS, 单独喷NaHS; SA, 盐碱胁迫下喷蒸馏水; SA + NaHS, 盐碱胁迫下喷NaHS。FBA, 果糖-1,6-二磷酸醛缩酶; GAPDH, 3-磷酸甘油醛脱氢酶; RCA, Rubisco活化酶; Rubisco, 核酮糖-1,5-二磷酸羧化酶; SBPase, 景天庚酮糖-1,7-二磷酸酶; TK, 转酮醇酶。不同小写字母表示同一时间不同处理间差异显著(p < 0.05)。
Fig. 3 Effect of exogenous H2S on the activities of key enzymes in Calvin cycle in Avena nude leaves under saline-alkali stress (mean ± SD). CK, control; NaHS, spraying NaHS only; SA, spraying water under salt-alkali stress; SA + NaHS, spraying NaHS under salt-alkali stress. FBA, fructose-1,6-bisphosphate aldolase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RCA, Rubisco activase; Rubisco, ribulose-1,5-bisphophate carboxylase; SBPase, sedoheptulose-1,7-bisphosphatase; TK, transketolase. Different lowercase letters indicate significant differences among treatments at the same time (p < 0.05).
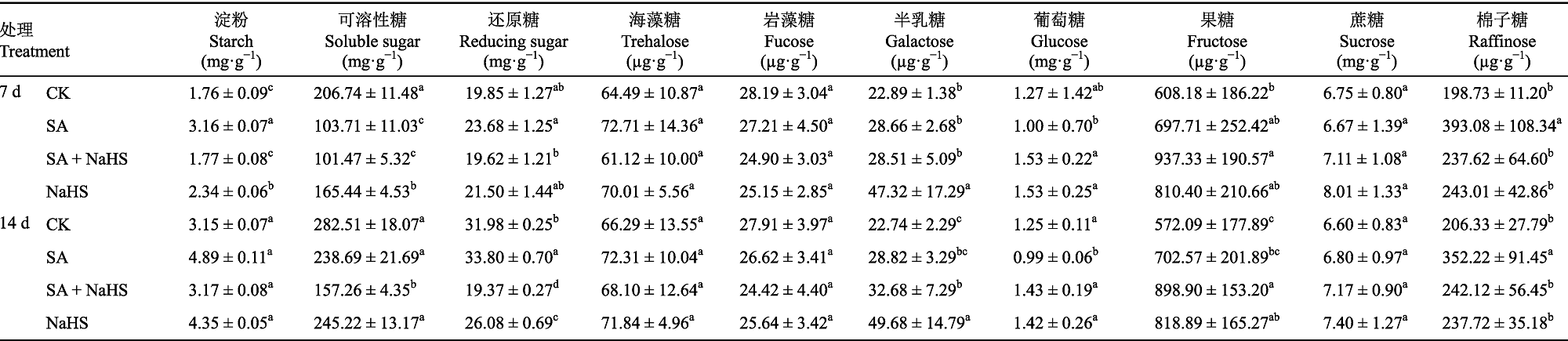 |
表2 外源H2S对盐碱胁迫下裸燕麦叶片不同类型糖含量的影响(平均值±标准差)
Table 2 Effect of exogenous H2S on the contents of different sugars in leaves of Avena nude under saline-alkali stress (mean ±SD)
 |
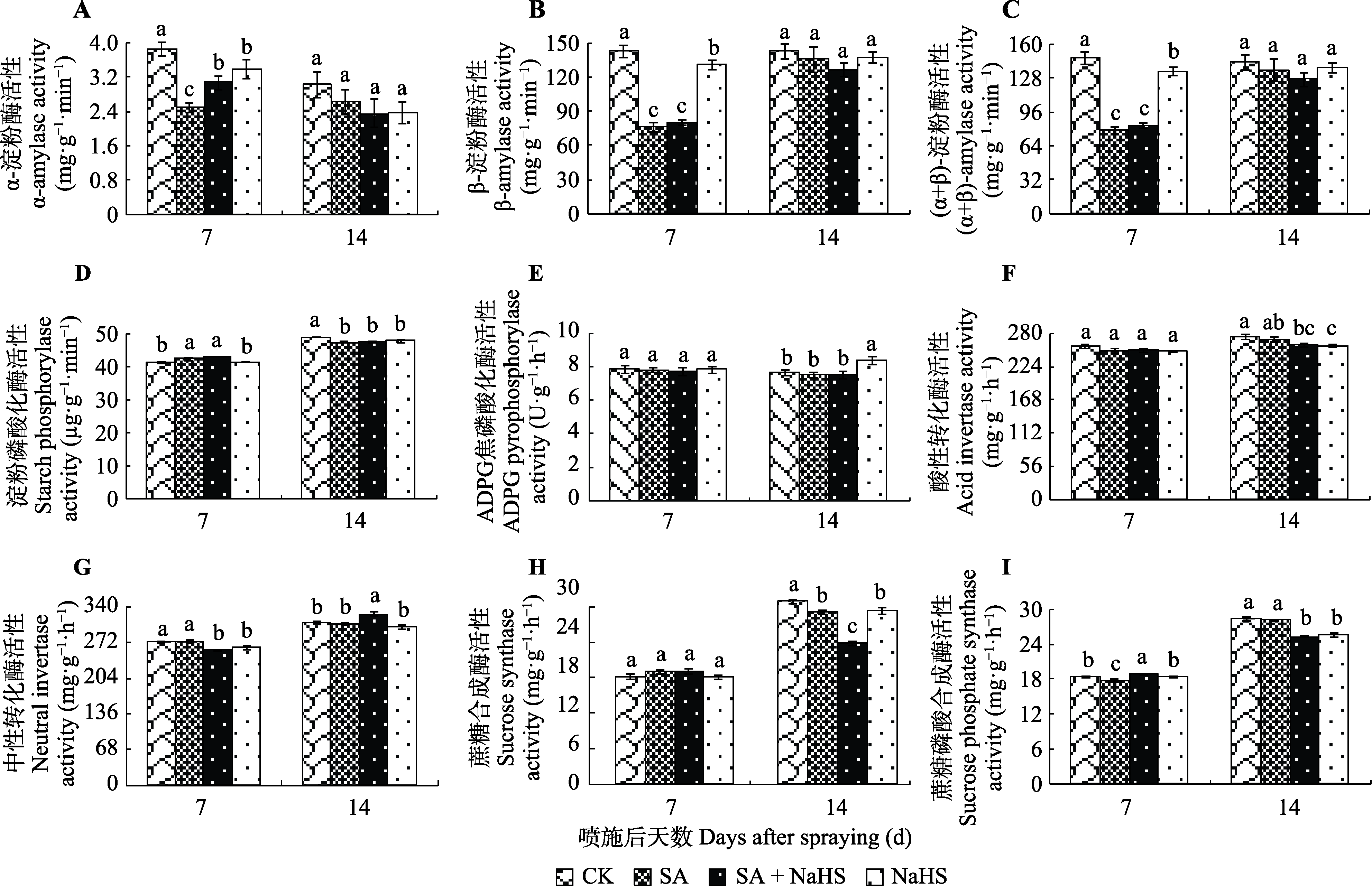
图4 外源H2S对盐碱胁迫下裸燕麦叶片淀粉和蔗糖代谢酶活性的影响(平均值±标准差)。ADPG, 腺苷二磷酸葡萄糖。CK, 对照; NaHS, 单独喷NaHS; SA, 盐碱胁迫下喷蒸馏水; SA + NaHS, 盐碱胁迫下喷NaHS。不同小写字母表示同一时间不同处理间差异显著(p < 0.05)。
Fig. 4 Effect of exogenous H2S on the metabolic enzyme activities of starch and sucrose in Avena nude leaves under saline-alkali stress (mean ± SD). ADPG, adenosine diphosphate glucose. CK, control; NaHS, spraying NaHS only; SA, spraying water under salt-alkali stress; SA + NaHS, spraying NaHS under salt-alkali stress. Different lowercase letters indicate significant differences among treatments at the same time (p < 0.05).
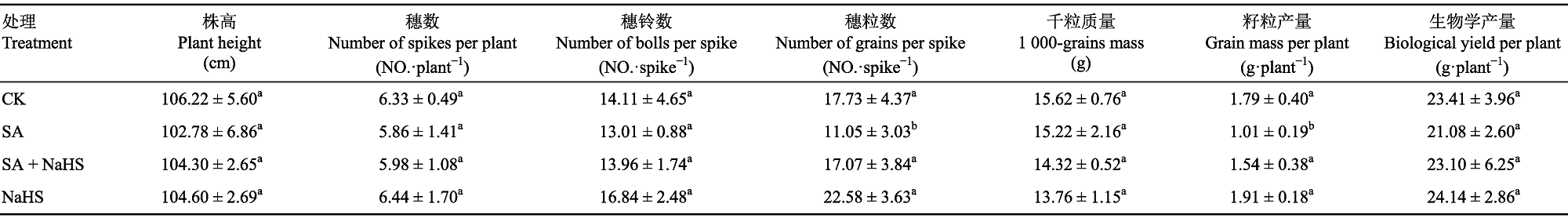 |
表3 外源H2S对盐碱胁迫下裸燕麦生长和产量性状的影响(平均值±标准差)
Table 3 Effect of exogenous H2S on the growth and yield traits of Avena nudes under saline-alkali stress (mean±SD)
 |
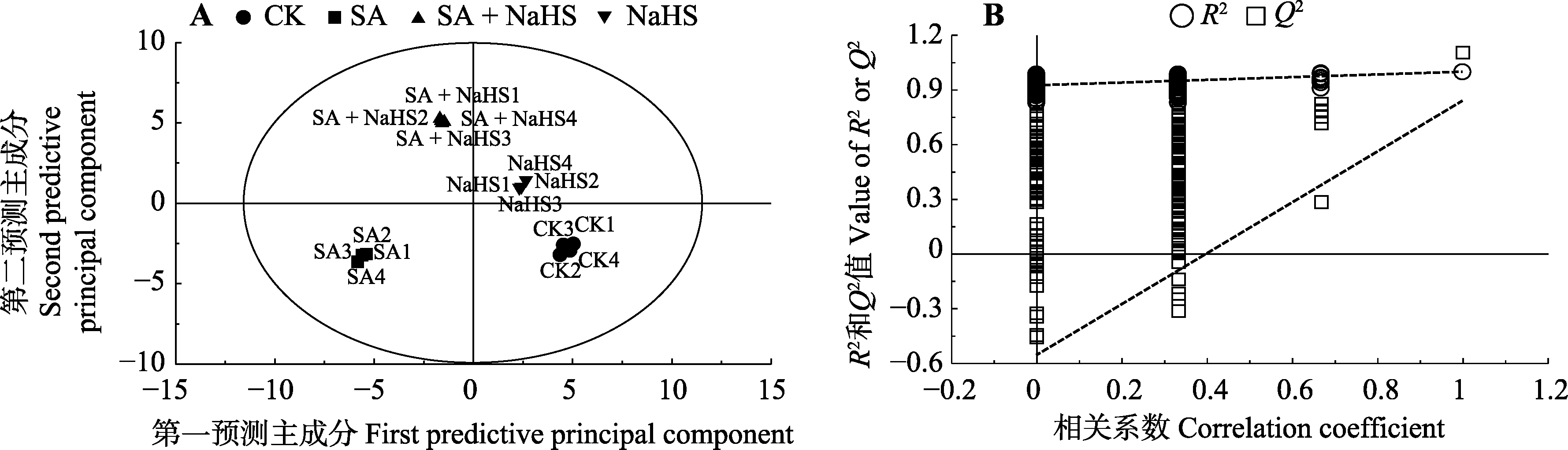
图5 不同处理下裸燕麦叶片光合碳代谢隐结构正交投影判别分析(OPLS-DA)得分图及200次模型的置换检验。CK, 对照; NaHS, 单独喷NaHS; SA, 盐碱胁迫下喷蒸馏水; SA + NaHS, 盐碱胁迫下喷NaHS。
Fig. 5 Orthogonal projection to latent structure-discriminant analysis (OPLS-DA) score plot of photosynthetic carbon metabolism in leaves of Avena nude under different treatments and 200 permutation tests of the model. CK, control; NaHS, spraying NaHS only; SA, spraying water under salt-alkali stress; SA + NaHS, spraying NaHS under salt-alkali stress.
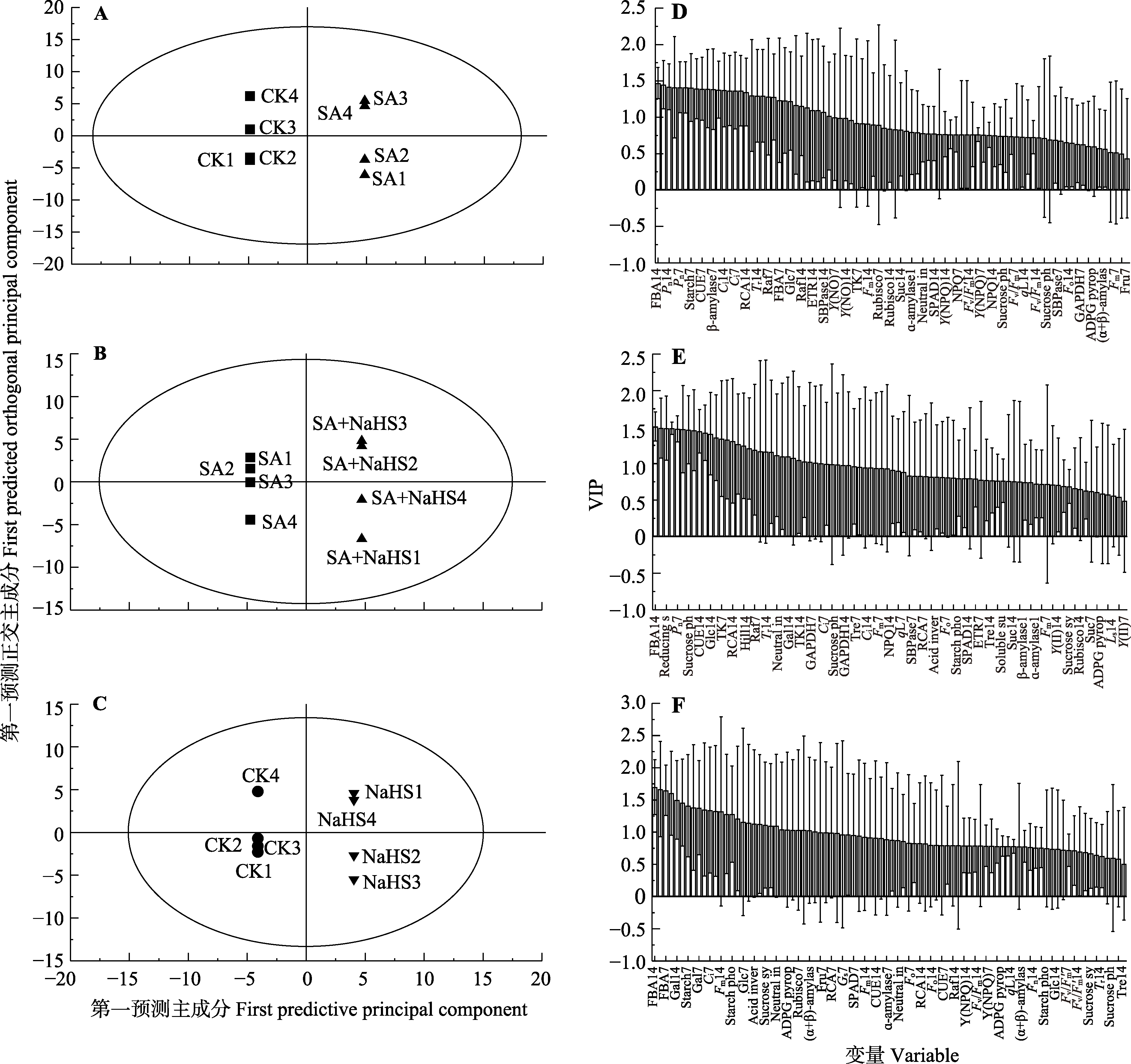
图6 不同处理下裸燕麦光合碳代谢隐结构正交投影判别分析(OPLS-DA)得分图(A、B、C)和变量投射重要度(VIP)图(平均值±标准差) (D、E、F)。CK, 对照; NaHS, 单独喷NaHS; SA, 盐碱胁迫下喷蒸馏水; SA + NaHS, 盐碱胁迫下喷NaHS。 Acid inver, 酸性转化酶; ADPG pyrop, 腺苷二磷酸葡萄糖焦磷酸化酶; α-amylase, α-淀粉酶; β-amylase, β-淀粉酶; α+β-amylas, (α+β)-淀粉酶; Ci, 胞间CO2浓度; CUE, 表观CO2利用效率; ETR, 电子传递速率; FBA, 果糖-1,6-二磷酸醛缩酶; Fm, 最大荧光; Fo, 初始荧光; Fru, 果糖; Fuc, 岩藻糖; Fv/Fm, 最大光化学效率; Fv′/Fm′, 有效光量子产量; Gal, 半乳糖; GAPDH, 3-磷酸甘油醛脱氢酶; Glc, 葡萄糖; Gs, 气孔导度; Hill, Hill反应活力; Ls, 气孔限制值; Neutral in, 中性转化酶; NPQ, 非光化学淬灭系数; Pn, 净光合速率; qL,光化学淬灭系数; Raf, 棉子糖; RCA, Rubisco活化酶; Reducing s, 还原性糖; Rubisco, 核酮糖-1,5-二磷酸羧化酶; SBPase, 景天庚酮糖-1,7-二磷酸酶; Soluble su, 可溶性糖; SPAD, 叶绿素相对含量; Starch, 淀粉; Starch pho, 淀粉磷酸化酶; Suc, 蔗糖; Sucrose sy, 蔗糖合成酶; Sucroseph, 蔗糖磷酸合成酶; TK, 转酮醇酶; Tr, 蒸腾速率; Tre, 海藻糖; Y(NO), 非调节性能量耗散量子产量; Y(NPQ), 调节性能量耗散量子产量; Y(II), 实际光化学量子产量。7和14分别表示第7天和第14天。
Fig. 6 Orthogonal projection to latent structure-discriminant analysis (OPLS-DA) score plot (A, B, C) and varibale importance for the projection (VIP)-plot (mean ± SD) (D, E, F) of photosynthetic carbon metabolism in leaves of Avena nude under different treatment. CK, control; NaHS, spraying NaHS only; SA, spraying water under salt-alkali stress; SA + NaHS, spraying NaHS under salt-alkali stress. Acid inver, acid invertase; ADPG pyrop, adenosine diphosphate glucose pyrophosphorylase; α+β-amylas, (α+β)-amylase; Ci, intercellular CO2 concentration; CUE, apparent CO2 utility efficiency; ETR, electron transport rate; FBA, fructose-1,6-bisphosphate aldolase; Fm, maximum fluorescence; Fo, initial fluorescence; Fru, fructose; Fuc, fucose; Fv/Fm, maximum photochemical efficiency; Fv′/Fm′, effective light quantum yield; Gal, galactose; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Glc, glucose; Gs, stomatal conductance; Hill, Hill’s reaction activity; Ls, stomatal limitation value; Neutral in, neutral invertase; NPQ, non-photochemical quenching coefficient; Pn, net photosynthetic rate; qL, photochemical quenching coefficient; Raf, raffinose; RCA, Rubisco activase; Reducing s, reducing sugar; Rubisco, ribulose-1,5-bisphophate carboxylase; SBPase, sedoheptulose-1,7-bisphosphatase; Soluble su, soluble sugar; SPAD, chlorophyll relative content; Starch pho, starch phosphorylase; Suc, sucrose; Sucrose sy, sucrose synthase; Sucrose ph, sucrose phosphate synthase; TK, transketolase; Tr, transpiration rate; Tre, trehalose; Y(NO), non-adjusting energy dissipation quantum yield; Y(NPQ), adjusting energy dissipation quantum yield; Y(II), actual photochemical quantum yield. 7 and 14 represent the 7th and 14th day, respectively.
| [1] |
Andersson I (1996). Large structures at high resolution: the 1.6 Å crystal structure of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase complexed with 2-carboxyarabinitol bisphosphate. Journal of Molecular Biology, 259, 160-174.
PMID |
| [2] | Che T, Zhang LP, Jin ZP, Pei YX (2019). Hydrogen sulfide regulates the photosynthetic physiology of Brassica pekinensis (Lour.) Rupr. under cadmium stress. Journal of Agro-Environment Science, 38, 1008-1016. |
| [车涛, 张丽萍, 金竹萍, 裴雁曦 (2019). 硫化氢对镉胁迫下大白菜幼苗光合生理的影响. 农业环境科学学报, 38, 1008-1016.] | |
| [3] |
Chen J, Wang WH, Wu FH, He EM, Liu X, Shangguan ZP, Zheng HL (2015). Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Scientific Reports, 5, 12516. DOI: 10.1038/srep12516.
DOI |
| [4] |
Chen J, Wu FH, Wang WH, Zheng CJ, Lin GH, Dong XJ, He JX, Pei ZM, Zheng HL (2011). Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. Journal of Experimental Botany, 62, 4481-4493.
DOI URL |
| [5] |
Demmig-Adams B, AdamsIII WW, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996). Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiologia Plantarum, 98, 253-264.
DOI URL |
| [6] |
Farquhar GD, Sharkey TD (1982). Stomatal conductance and photosynthesis. Annual Review of Plant Physiology, 33, 317-345.
DOI URL |
| [7] | Gao JF (2006). Plant Physiology Experiment Guide. Higher Education Press, Beijing. 107-108. |
| [高俊凤 (2006). 植物生理学实验指导. 高等教育出版社, 北京. 107-108.] | |
| [8] |
Gao WY, Feng Z, Bai QQ, He JJ, Wang YJ (2019). Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. International Journal of Molecular Sciences, 20, 1176-1193.
DOI URL |
| [9] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [10] |
Huang H, Guo SS, Chen LC, Xiao B (2017). Effects of exogenous hydrogen sulfide on the antioxidant characteristics of tea plant (Camellia sinensis) under salt stress. Plant Physiology Journal, 53, 497-504.
DOI URL |
| [黄菡, 郭莎莎, 陈良超, 肖斌 (2017). 外源硫化氢对盐胁迫下茶树抗氧化特性的影响. 植物生理学报, 53, 497-504.] | |
| [11] |
Jia XM, Wang H, Svetla S, Zhu YF, Hu Y, Cheng L, Zhao T, Wang YX (2019). Comparative physiological responses and adaptive strategies of apple Malus halliana to salt, alkali and saline-alkali stress. Scientia Horticulturae, 245, 154-162.
DOI URL |
| [12] |
Jin ZP, Pei YX (2015). Physiological implications of hydrogen sulfide in plants: pleasant exploration behind its unpleasant odour. Oxidative Medicine and Cellular Longevity, 2015, 397502. DOI: 10.1155/2015/397502.
DOI |
| [13] |
Khan MN, Mukherjee S, Al-Huqail AA, Basahi RA, Ali HM, Al-Munqedhi BMA, Siddiqui MH, Kalaji HM (2021). Exogenous potassium (K+) positively regulates Na+/H+ antiport system, carbohydrate metabolism, and ascorbate-glutathione cycle in H2S-dependent manner in NaCl-stressed tomato seedling roots. Plants, 10, 948. DOI: 10.3390/PLANTS10050948.
DOI |
| [14] |
Lai DW, Mao Y, Zhou H, Li F, Wu MZ, Zhang J, He ZY, Cui WT, Xie YJ (2014). Endogenous hydrogen sulfide enhances salt tolerance by coupling the reestablishment of redox homeostasis and preventing salt-induced K+ loss in seedlings of Medicago sativa. Plant Science, 225, 117-129.
DOI URL |
| [15] | Li D, Shen HT, Wang YF, Wang LJ, Zhao SM, Liu L (2019). Effect of exogenous hydrogen sulfide on photosynthetic fluorescence parameters and antioxidant system of flue-cured tobacco seedlings under drought stress. Acta Botanica Boreali-Occidentalia Sinica, 39, 1609-1617. |
| [李冬, 申洪涛, 王艳芳, 王丽君, 赵世民, 刘领 (2019). 干旱胁迫下外源硫化氢对烤烟幼苗光合荧光参数及抗氧化系统的影响. 西北植物学报, 39, 1609-1617.] | |
| [16] | Li XF, Zhang ZL (2016). Guidance of Plant Physiology Experiments. Higher Education Press, Beijing. 59-60, 91-104. |
| [李小方, 张志良 (2016). 植物生理学实验指导. 高等教育出版社, 北京. 59-60, 91-104.] | |
| [17] | Liu JX, Liu RR, Jia HY, Bu T, Li N (2021a). Effects of exogenous hydrogen sulfide on growth and physiological characteristics of naked oat seedlings under saline-alkali mixed stress. Journal of Triticeae Crops, 41, 245-253. |
| [刘建新, 刘瑞瑞, 贾海燕, 卜婷, 李娜 (2021a). 外源H2S对盐碱胁迫下裸燕麦幼苗生长和生理特性的影响. 麦类作物学报, 41, 245-253.] | |
| [18] | Liu JX, Liu RR, Jia HY, Liu XL, Bu T, Li N (2021b). Regulation effects of hydrogen sulfide on ascorbate-glutathione cycle in naked oat leavesunder saline-alkali stress. Chinese Journal of Applied Ecology, 32, 3988-3996. |
| [刘建新, 刘瑞瑞, 贾海燕, 刘秀丽, 卜婷, 李娜 (2021b). 硫化氢对盐碱胁迫下裸燕麦叶片抗坏血酸-谷胱甘肽循环的调控效应. 应用生态学报, 32, 3988-3996.] | |
| [19] | Liu JX, Liu RR, Liu XL, Jia HY, Bu T, Li N (2021c). Effects of spraying NaHSat different growth stages on osmotic adjustment substance and antioxidant activity in leaves of naked oat under saline-alkali stress. Chinese Journal of Ecology, 40, 3620-3632. |
| [刘建新, 刘瑞瑞, 刘秀丽, 贾海燕, 卜婷, 李娜 (2021c). 不同时期喷施NaHS对盐碱胁迫下裸燕麦叶片渗透调节物质和抗氧化活性的影响. 生态学杂志, 40, 3620-3632.] | |
| [20] | Liu JX, Wang JC, Wang RJ, Liu XL (2017). Effect of complex saline-alkali stress on the mineral ions absorption and photosynthetic characteristics of oat seedlings. Agricultural Research in the Arid Areas, 35(1), 178-184. |
| [刘建新, 王金成, 王瑞娟, 刘秀丽 (2017). 混合盐碱胁迫对燕麦幼苗矿质离子吸收和光合特性的影响. 干旱地区农业研究, 35(1), 178-184.] | |
| [21] | Mei YD, Jin XX, Huang LQ (2018). S-sulfhydration: a new post-translational modification of proteins. Chinese Journal of Biochemistry and Molecular Biology, 34, 911-920. |
| [梅玉东, 金欣欣, 黄丽琴 (2018). 硫巯基化:一种新的蛋白质翻译后修饰. 中国生物化学与分子生物学报, 34, 911-920.] | |
| [22] |
Mostofa MG, Saegusa D, Fujita M, Tran LSP (2015). Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Frontiers in Plant Science, 6, 1055. DOI: 10.3389/fpls.2015.01055.
DOI |
| [23] |
Ozfidan-Konakci C, Yildiztugay E, Elbasan F, Kucukoduk M, Turkan I (2020). Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. Journal of Hazardous Materials, 388, 122061. DOI: 10.1016/j.jhazmat.2020.122061.
DOI |
| [24] | Pan DY, Fu X, Zhang XW, Liu FJ, Bi HG, Ai XZ (2020). Hydrogen sulfide acted as a downstream signal was involved in the regulation of salicylic acid on photosynthesis of cucumber seedlings under low temperature and low light intensity. Chinese Journal of Applied Ecology, 31, 3023-3032. |
|
[潘东云, 付鑫, 张晓伟, 刘丰娇, 毕焕改, 艾希珍 (2020). H2S作为下游信号参与SA对低温弱光下黄瓜幼苗光合作用的调控. 应用生态学报, 31, 3023-3032.]
DOI |
|
| [25] |
Raines CA (2003). The Calvin cycle revisited. Photosynthesis Research, 75, 1-10.
DOI PMID |
| [26] |
Shan C, Liu H, Zhao L, Wang X (2014). Effects of exogenous hydrogen sulfide on the redox states of ascorbate and glutathione in maize leaves under salt stress. Biologia Plantarum, 58, 169-173.
DOI URL |
| [27] |
Siddiqui MH, Khan MN, Mukherjee S, Alamri S, Basahi RA, Al-Amri AA, Alsubaie QD, Al-Munqedhi BMA, Ali HM, Almohisen IAA (2021). Hydrogen sulfide (H2S) and potassium (K+) synergistically induce drought stress tolerance through regulation of H+-ATPase activity, sugar metabolism, and antioxidative defense in tomato seedlings. Plant Cell Reports, 40, 1543-1564.
DOI PMID |
| [28] |
Steffens B, Sauter M (2009). Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. The Plant Cell, 21, 184-196.
DOI URL |
| [29] | Sun LM, Jin ZP, Qian JH, Pei YX (2017). H2S enhanced photosynthesis in response to drought stress. Chinese Journal of Cell Biology, 39, 1583-1591. |
| [孙丽敏, 金竹萍, 钱嘉航, 裴雁曦 (2017). 干旱胁迫下H2S信号增强植物叶片的光合作用. 中国细胞生物学学报, 39, 1583-1591.] | |
| [30] | Wang HJ, Zhang LP, Liu ZQ, Liu DM, Jin ZP, Pei YX (2015). Influence of H2S on growth and photosynthesis of Brassica rapa var. pekinensis under chilling stress. Acta Botanica Boreali-Occidentalia Sinica, 35, 780-786. |
| [王鸿蕉, 张丽萍, 刘志强, 刘旦梅, 金竹萍, 裴雁曦 (2015). 外源硫化氢对冷胁迫下白菜幼苗生长和光合作用的影响. 西北植物学报, 35, 780-786.] | |
| [31] | Wu GX, Li SL, Li Y, Li YM, Bi HG, Ai XZ (2020). Effects of hydrogen sulfide, nitric oxide and their interaction on photosynthesis of cucumber seedlings under chilling stress. Plant Physiology Journal, 56, 2221-2232. |
| [吴帼秀, 李胜利, 李阳, 李严曼, 毕焕改, 艾希珍 (2020). H2S和NO及其互作对低温胁迫下黄瓜幼苗光合作用的影响. 植物生理学报, 56, 2221-2232.] | |
| [32] | Wu XH, Feng JM (2018). SNP on the seed germination and photosynthetic carbon metabolism of pumpkin seedlings under NaCl stress. Seed, 37, 100-103. |
| [吴旭红, 冯晶旻 (2018). SNP对盐胁迫下南瓜种子萌发和幼苗光合碳代谢的影响. 种子, 37, 100-103.] | |
| [33] | Yadav SP, Bharadwaj R, Nayak H, Mahto R, Prasad SK (2019). Impact of salt stress on growth, productivity and physicochemical properties of plants: a review. International Journal of Chemical Studies, 7, 1793-1798. |
| [34] | Yan YQ, Wang WJ, Zhu H, Shi XC, Liu XL, Zu YG (2009). Effects of salt-alkali stress on osmoregulation substance and active oxygen metabolism of Qingshan poplar (Populus pseudo-cathayana × P. deltoides). Chinese Journal of Applied Ecology, 20, 2085-2091. |
| [闫永庆, 王文杰, 朱虹, 石溪婵, 刘兴亮, 祖元刚 (2009). 混合盐碱胁迫对青山杨渗透调节物质及活性氧代谢的影响. 应用生态学报, 20, 2085-2091.] | |
| [35] |
Zhao ZJ, Zhang HL, Wang MJ, Zhang XM, Li LX (2020). Salt stress-related regulation mechanism of intracellular pH and ion homeostasis in plants. Plant Physiology Journal, 56, 337-344.
DOI URL |
| [赵振杰, 张海龙, 王明晶, 张小萌, 李立新 (2020). 植物耐盐性相关细胞内pH和离子稳态的调控机制. 植物生理学报, 56, 337-344.] | |
| [36] | Zheng DS, Zhang ZW (2011). Discussion on the origin and taxonomy of naked oat (Avena nuda L.). Journal of Plant Genetic Resources, 12, 667-670. |
| [郑殿升, 张宗文 (2011). 大粒裸燕麦(莜麦) (Avena nuda L.) 起源及分类问题的探讨. 植物遗传资源学报, 12, 667-670.] | |
| [37] | Zheng ZY, Lin HR, Cui HM (2017). Effect of exogenous hydrogen sulfide on photosynthesis parameters and chlorophyll fluorescence characteristics of processing tomato (Lycopersicon esculentum Mill ssp. subspontaneum Brezh) seedlings under NaCl stress. Journal of Nuclear Agricultural Sciences, 31, 1426-1435. |
|
[郑州元, 林海荣, 崔辉梅 (2017). 外源硫化氢对盐胁迫下加工番茄幼苗光合参数及叶绿素荧光特性的影响. 核农学报, 31, 1426-1435.]
DOI |
|
| [38] | Zhou CF, Wu CT, Li DD, Zhang XW, Bi HG, Ai XZ (2018). Hydrogen sulfide promotes chilling tolerance of cucumber seedlings by alleviating low-temperature photoinhibition. Plant Physiology Journal, 54, 411-420. |
| [周超凡, 吴春涛, 李丹丹, 张晓伟, 毕焕改, 艾希珍 (2018). 外源H2S通过减轻低温光抑制增强黄瓜幼苗耐冷性. 植物生理学报, 54, 411-420.] | |
| [39] | Zhou CF, Wu GX, Li T, Bi HG, Li QM, Ai XZ (2016). Effect of exogenous hydrogen sulfide on photosynthesis and antioxidant system of cucumber leaves under low temperature in solar-greenhouse. Acta Horticulturae Sinica, 43, 462-472. |
|
[周超凡, 吴帼秀, 李婷, 毕焕改, 李清明, 艾希珍 (2016). 外源H2S对低温下日光温室黄瓜光合作用及抗氧化系统的影响. 园艺学报, 43, 462-472.]
DOI |
| [1] | 杜英东, 袁相洋, 冯兆忠. 不同形态氮对杨树光合特性及生长的影响[J]. 植物生态学报, 2023, 47(3): 348-360. |
| [2] | 周洁, 杨晓东, 王雅芸, 隆彦昕, 王妍, 李浡睿, 孙启兴, 孙楠. 梭梭和骆驼刺对干旱的适应策略差异[J]. 植物生态学报, 2022, 46(9): 1064-1076. |
| [3] | 林雍, 陈智, 杨萌, 陈世苹, 高艳红, 刘冉, 郝彦宾, 辛晓平, 周莉, 于贵瑞. 中国干旱半干旱区生态系统光合参数的时空变异及其影响因素[J]. 植物生态学报, 2022, 46(12): 1461-1472. |
| [4] | 李义博, 宋贺, 周莉, 许振柱, 周广胜. C4植物玉米的光合-光响应曲线模拟研究[J]. 植物生态学报, 2017, 41(12): 1289-1300. |
| [5] | 冯汉青, 焦青松, 田武英, 孙坤, 贾凌云. 不同光强下细胞外三磷酸腺苷对菜豆叶片光化学反应特性的影响[J]. 植物生态学报, 2014, 38(10): 1117-1123. |
| [6] | 王荣荣, 夏江宝, 杨吉华, 赵艳云, 刘京涛, 孙景宽. 贝壳砂生境干旱胁迫下杠柳叶片光合光响应模型比较[J]. 植物生态学报, 2013, 37(2): 111-121. |
| [7] | 王慧, 周广胜, 蒋延玲, 石耀辉, 许振柱. 降水与CO2浓度协同作用对短花针茅光合特性的 影响[J]. 植物生态学报, 2012, 36(7): 597-606. |
| [8] | 李磊, 李向义, 林丽莎, 王迎菊, 薛伟. 两种生境条件下6种牧草叶绿素含量及荧光参数的比较[J]. 植物生态学报, 2011, 35(6): 672-680. |
| [9] | 耿云霞, 李依玲, 朱莎, 朱精精, 姜俊丞, 牛洪昊, 介冬梅. 盐碱胁迫下羊草植硅体的形态变化[J]. 植物生态学报, 2011, 35(11): 1148-1155. |
| [10] | 张想英, 樊大勇, 谢宗强, 熊高明, 李兆佳. 克隆整合有助于狗牙根抵御水淹[J]. 植物生态学报, 2010, 34(9): 1075-1083. |
| [11] | 闫永庆, 刘兴亮, 王崑, 樊金萍, 石溪婵. 白刺对不同浓度混合盐碱胁迫的生理响应[J]. 植物生态学报, 2010, 34(10): 1213-1219. |
| [12] | 赵平, 孙谷畴, 曾小平. 适度高温下亚热带阔叶树种叶片的光合速率和吸收光能的分配[J]. 植物生态学报, 2008, 32(2): 413-423. |
| [13] | 姚芳芳, 王效科, 陈展, 冯兆忠, 郑启伟, 段晓男, 欧阳志云, 冯宗炜. 农田冬小麦生长和产量对臭氧动态暴露的响应[J]. 植物生态学报, 2008, 32(1): 212-219. |
| [14] | 彭长连, 温学, 林植芳, 周厚诚, 陈少薇, 林桂珠. 龙须菜对海水氮磷富营养化的响应[J]. 植物生态学报, 2007, 31(3): 505-512. |
| [15] | 刘建辉, 孙建云, 戴廷波, 姜东, 荆奇, 曹卫星. 不同小麦进化材料生育后期光合特性和产量[J]. 植物生态学报, 2007, 31(1): 138-144. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19