

植物生态学报 ›› 2025, Vol. 49 ›› Issue (4): 573-584.DOI: 10.17521/cjpe.2024.0001 cstr: 32100.14.cjpe.2024.0001
所属专题: 植物功能性状
郭李琦1, 闫晓蕾1, 曹磊1, 高景1, 刘瑞强2,*( ), 周旭辉2
), 周旭辉2
收稿日期:2024-01-02
接受日期:2024-05-06
出版日期:2025-04-20
发布日期:2025-04-18
通讯作者:
* (rqliu@nefu.edu.cn)基金资助:
GUO Li-Qi1, YAN Xiao-Lei1, CAO Lei1, GAO Jing1, LIU Rui-Qiang2,*( ), ZHOU Xu-Hui2
), ZHOU Xu-Hui2
Received:2024-01-02
Accepted:2024-05-06
Online:2025-04-20
Published:2025-04-18
Contact:
* (rqliu@nefu.edu.cn)
Supported by:摘要: 根际微生物网络深刻影响土壤碳周转、养分循环与植物生长等诸多生态过程。植物菌根类型与根系性状是影响植物生长与地下养分利用策略的重要因素, 然而不同菌根类型树种根系性状对根际微生物群落组成及网络性质的影响目前尚不清楚。该研究以帽儿山温带次生林为研究对象, 测定了5种丛枝菌根(AM)与7种外生菌根(EcM)树种的根系性状与根际土壤微生物群落组成, 探究不同树种菌根类型根系性状差异及其对根际微生物网络特性的影响。结果显示: (1) AM树种的细根比根长, 根系氮、磷含量均高于EcM树种, 不同菌根类型树种的根组织密度、根直径、根氮磷比无显著差异; (2) AM树种根际罗兹菌门的相对丰度显著高于EcM树种, 拟杆菌门相对丰度显著低于EcM树种, 不同菌根类型树种根际微生物群落多样性无显著差异; (3) EcM树种根际微生物网络更复杂, 细菌负凝聚力(Cohesion)显著强于AM树种; (4) AM树种根际微生物群落及其网络复杂性主要受比根长影响, 而EcM树种根际主要受根系直径、根氮磷比调节。研究结果表明, 树种菌根类型显著影响根系比根长和养分含量等资源获取性状, 并调控根系性状与根际微生物群落的联系, 从而改变微生物网络复杂性。
郭李琦, 闫晓蕾, 曹磊, 高景, 刘瑞强, 周旭辉. 树种菌根类型与根系性状对根际微生物网络复杂性的影响. 植物生态学报, 2025, 49(4): 573-584. DOI: 10.17521/cjpe.2024.0001
GUO Li-Qi, YAN Xiao-Lei, CAO Lei, GAO Jing, LIU Rui-Qiang, ZHOU Xu-Hui. Effects of mycorrhizal types and root traits of tree species on rhizosphere microbial network complexity. Chinese Journal of Plant Ecology, 2025, 49(4): 573-584. DOI: 10.17521/cjpe.2024.0001
| 树种 Tree species | 拉丁名 Latin name | 菌根类型 Mycorrhizal type | 胸径 DBH (cm) |
|---|---|---|---|
| 卫矛 | Euonymus alatus | 丛枝根菌 AM | 2.87 ± 0.12 |
| 青楷槭 | Acer tegmentosum | 丛枝根菌 AM | 5.93 ± 1.05 |
| 色木槭 | Acer mono | 外生根菌 EcM | 7.17 ± 3.67 |
| 春榆 | Ulmus davidiana var. japonica | 外生根菌 EcM | 14.43 ± 3.13 |
| 红松 | Pinus koraiensis | 外生根菌 EcM | 16.02 ± 2.97 |
| 白桦 | Betula platyphylla | 外生根菌 EcM | 21.02 ± 1.25 |
| 落叶松 | Larix gmelinii | 外生根菌 EcM | 24.17 ± 3.10 |
| 黄檗 | Phellodendron amurense | 丛枝根菌 AM | 25.91 ± 1.28 |
| 胡桃楸 | Juglans mandshurica | 丛枝根菌 AM | 27.08 ± 3.17 |
| 山杨 | Populus davidiana | 外生根菌 EcM | 32.01 ± 5.17 |
| 蒙古栎 | Quercus mongolica | 外生根菌 EcM | 32.23 ± 4.00 |
| 水曲柳 | Fraxinus mandshurica | 丛枝根菌 AM | 36.20 ± 1.38 |
表1 采集树种的胸径状况(平均值±标准误)
Table 1 DBH of collected tree species (mean ± SE)
| 树种 Tree species | 拉丁名 Latin name | 菌根类型 Mycorrhizal type | 胸径 DBH (cm) |
|---|---|---|---|
| 卫矛 | Euonymus alatus | 丛枝根菌 AM | 2.87 ± 0.12 |
| 青楷槭 | Acer tegmentosum | 丛枝根菌 AM | 5.93 ± 1.05 |
| 色木槭 | Acer mono | 外生根菌 EcM | 7.17 ± 3.67 |
| 春榆 | Ulmus davidiana var. japonica | 外生根菌 EcM | 14.43 ± 3.13 |
| 红松 | Pinus koraiensis | 外生根菌 EcM | 16.02 ± 2.97 |
| 白桦 | Betula platyphylla | 外生根菌 EcM | 21.02 ± 1.25 |
| 落叶松 | Larix gmelinii | 外生根菌 EcM | 24.17 ± 3.10 |
| 黄檗 | Phellodendron amurense | 丛枝根菌 AM | 25.91 ± 1.28 |
| 胡桃楸 | Juglans mandshurica | 丛枝根菌 AM | 27.08 ± 3.17 |
| 山杨 | Populus davidiana | 外生根菌 EcM | 32.01 ± 5.17 |
| 蒙古栎 | Quercus mongolica | 外生根菌 EcM | 32.23 ± 4.00 |
| 水曲柳 | Fraxinus mandshurica | 丛枝根菌 AM | 36.20 ± 1.38 |
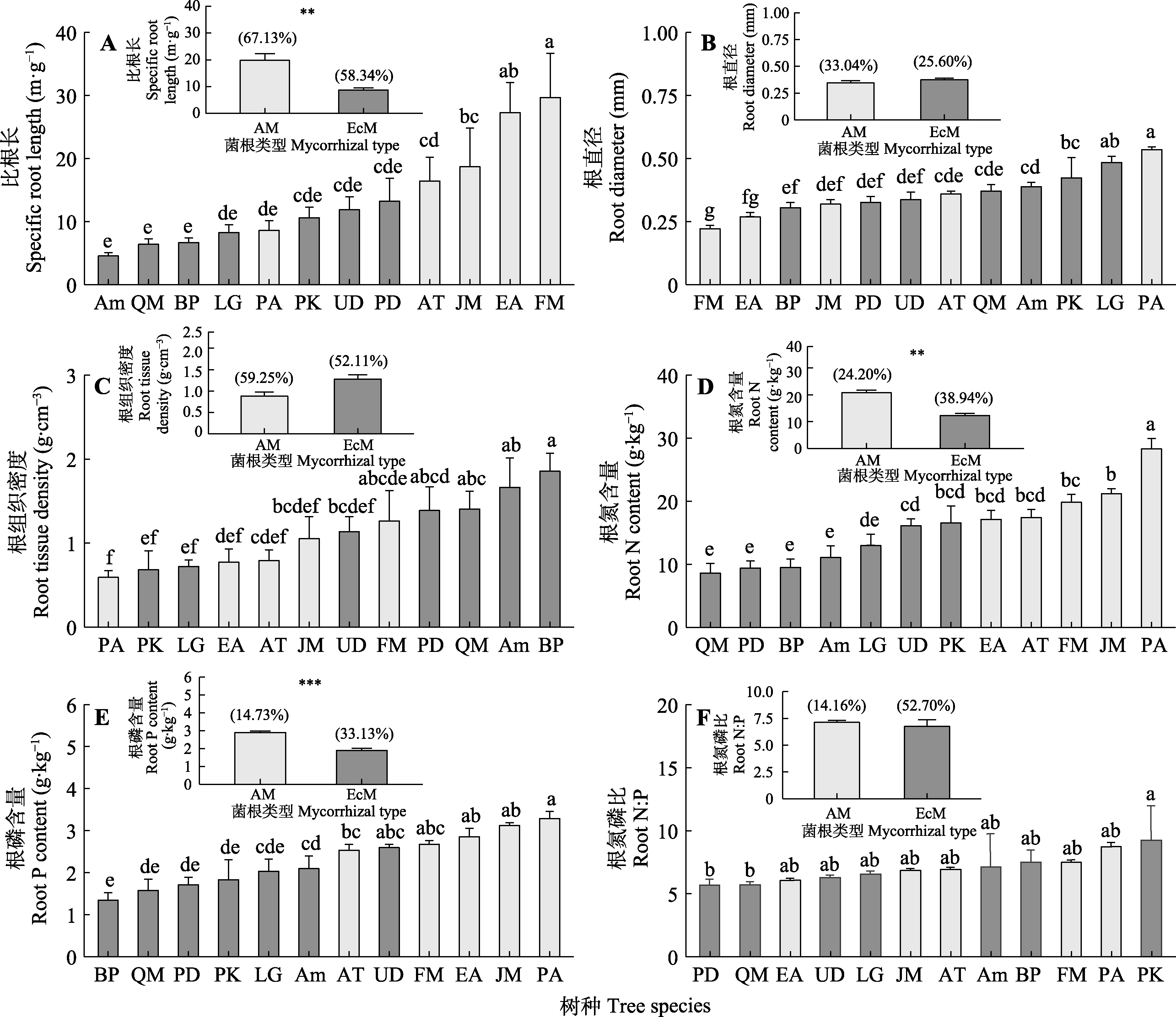
图1 12个树种的根系性状及菌根类型效应(平均值±标准误)。AM, 丛枝菌根; EcM, 外生菌根。Am, 色木槭; AT, 青楷槭; BP, 白桦; EA, 卫矛; FM, 水曲柳; JM, 胡桃楸; LG, 落叶松; PA, 黄檗; PD, 山杨; PK, 红松; QM, 蒙古栎; UD, 春榆。不同小写字母代表不同树种间差异显著(p < 0.05)。括号内的百分数为不同菌根树种的变异系数; *代表混合效应模型中菌根类型效应的显著性水平: **, p < 0.01; ***, p < 0.001。
Fig. 1 Root traits among 12 tree species and the effect of mycorrhizal types on root traits (mean ± SE). AM, arbuscular mycorrhiza; EcM, ectomycorrhiza; N, nitrogen; P, phosphorus. Am, Acer mono; AT, Acer tegmentosum; BP, Betula platyphylla; EA, Euonymus alatus; FM, Fraxinus mandshurica; JM, Juglans mandshurica; LG, Larix gmelinii; PA, Phellodendron amurense; PD, Populus davidiana; PK, Pinus koraiensis; UD, Ulmus davidiana var. japonica; QM, Quercus mongolica. Different lowercase letters indicate significant differences among different tree species (p < 0.05). The percentage in parentheses represent the coefficient of variation on different mycorrhizal tree species; * indicate the significance levels of mycorrhizal type effects in the mixed effects model: **, p < 0.01; ***, p < 0.001.
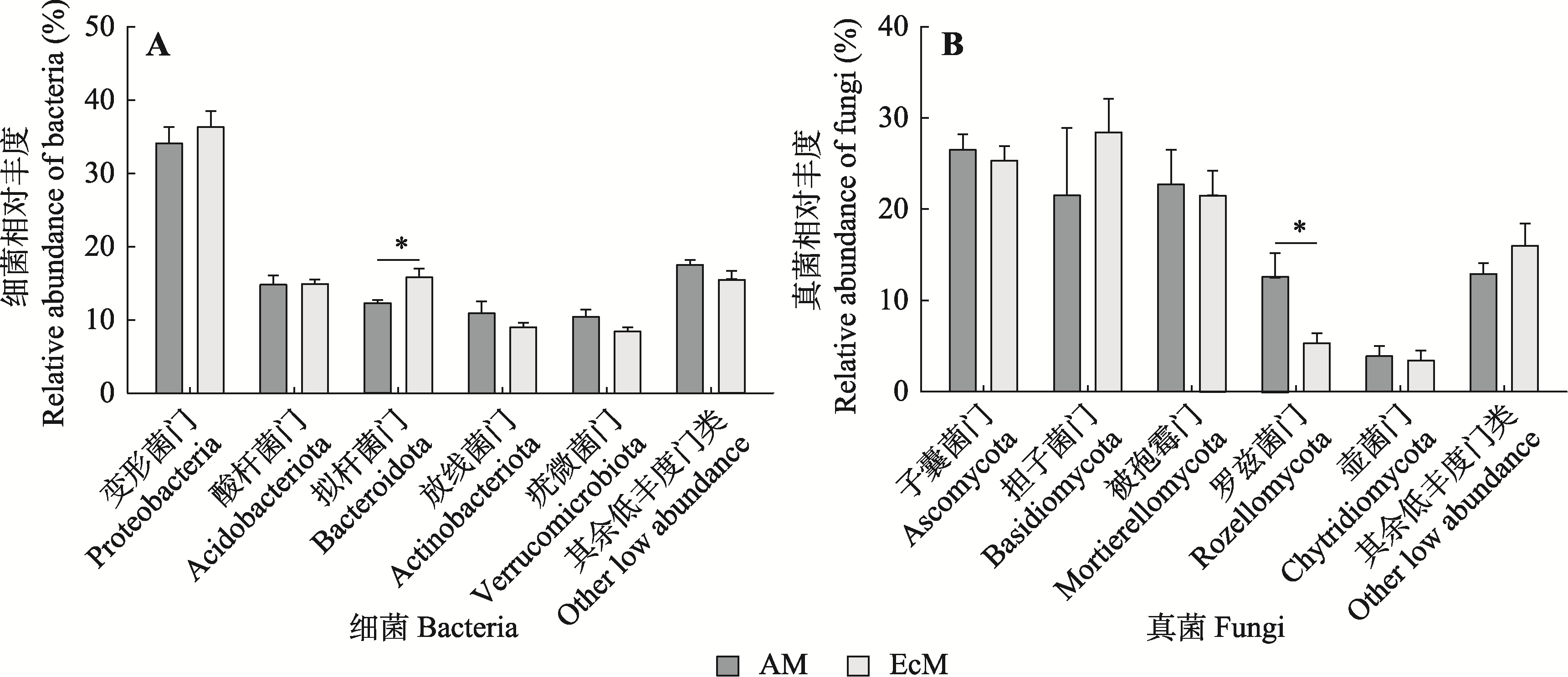
图2 不同菌根类型树种根际微生物群落组成(平均值±标准误)。*代表菌根类型间相对丰度差异显著(p < 0.05)。AM, 丛枝菌根; EcM, 外生菌根。
Fig. 2 Microbial community composition between different mycorrhizal types of tree species (mean ± SE). * indicates a significant difference in relative abundance between mycorrhizal types (p < 0.05). AM, arbuscular mycorrhiza; EcM, ectomycorrhiza.
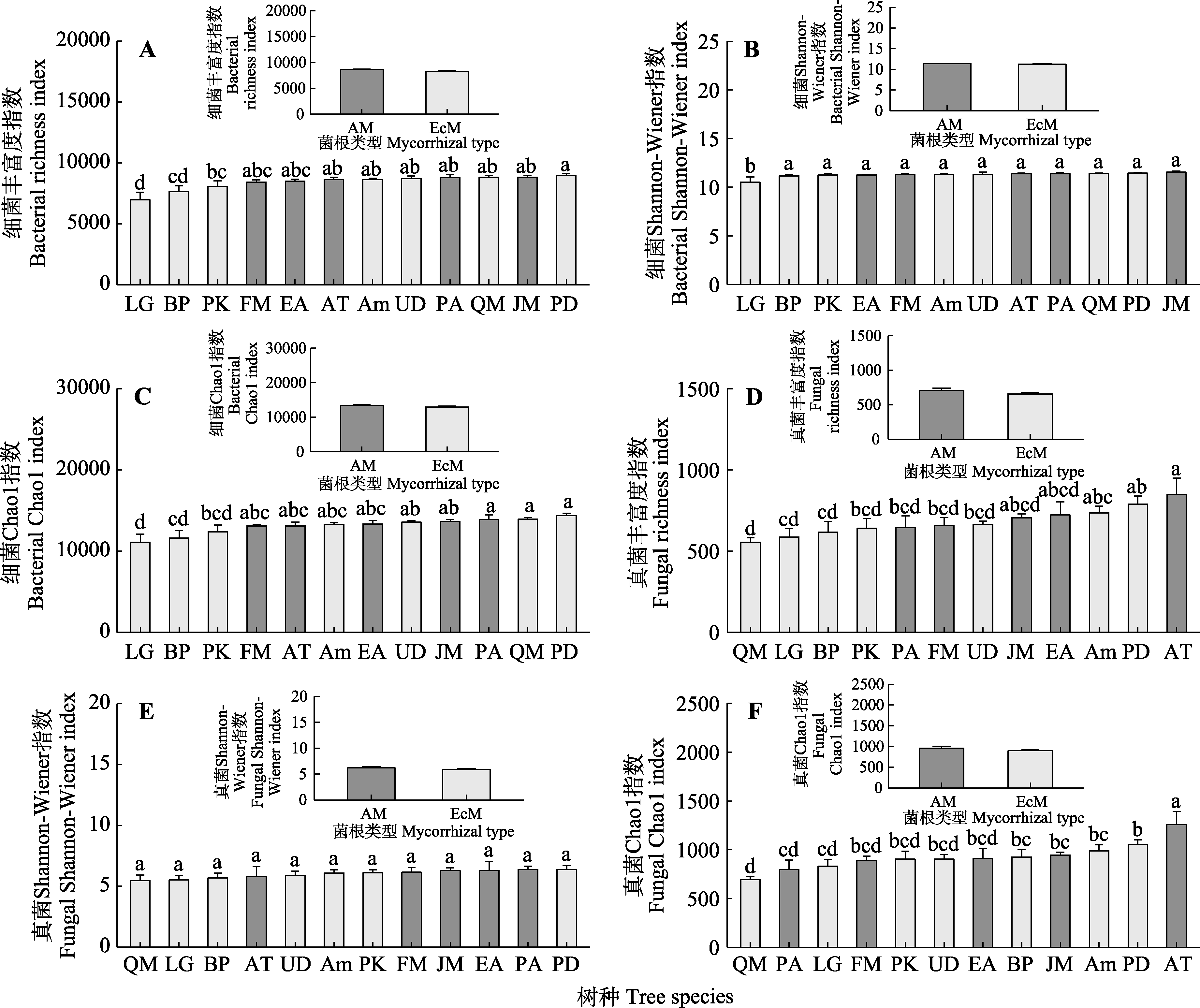
图3 不同菌根类型树种根际微生物群落多样性(平均值±标准误)。AM, 丛枝菌根; EcM, 外生菌根。Am, 色木槭; AT, 青楷槭; BP, 白桦; EA, 卫矛; FM, 水曲柳; JM, 胡桃楸; LG, 落叶松; PA, 黄檗; PD, 山杨; PK, 红松; QM, 蒙古栎; UD, 春榆。不同小写字母代表不同树种间差异显著(p < 0.05)。
Fig. 3 Microbial diversity between different mycorrhizal types of tree species (mean ± SE). AM, arbuscular mycorrhiza; EcM, ectomycorrhiza. Am, Acer mono; AT, Acer tegmentosum; BP, Betula platyphylla; EA, Euonymus alatus; FM, Fraxinus mandshurica; JM, Juglans mandshurica; LG, Larix gmelinii; PA, Phellodendron amurense; PD, Populus davidiana; PK, Pinus koraiensis; UD, Ulmus davidiana var. japonica; QM, Quercus mongolica. Different lowercase letters indicate significant differences among different tree species (p < 0.05).
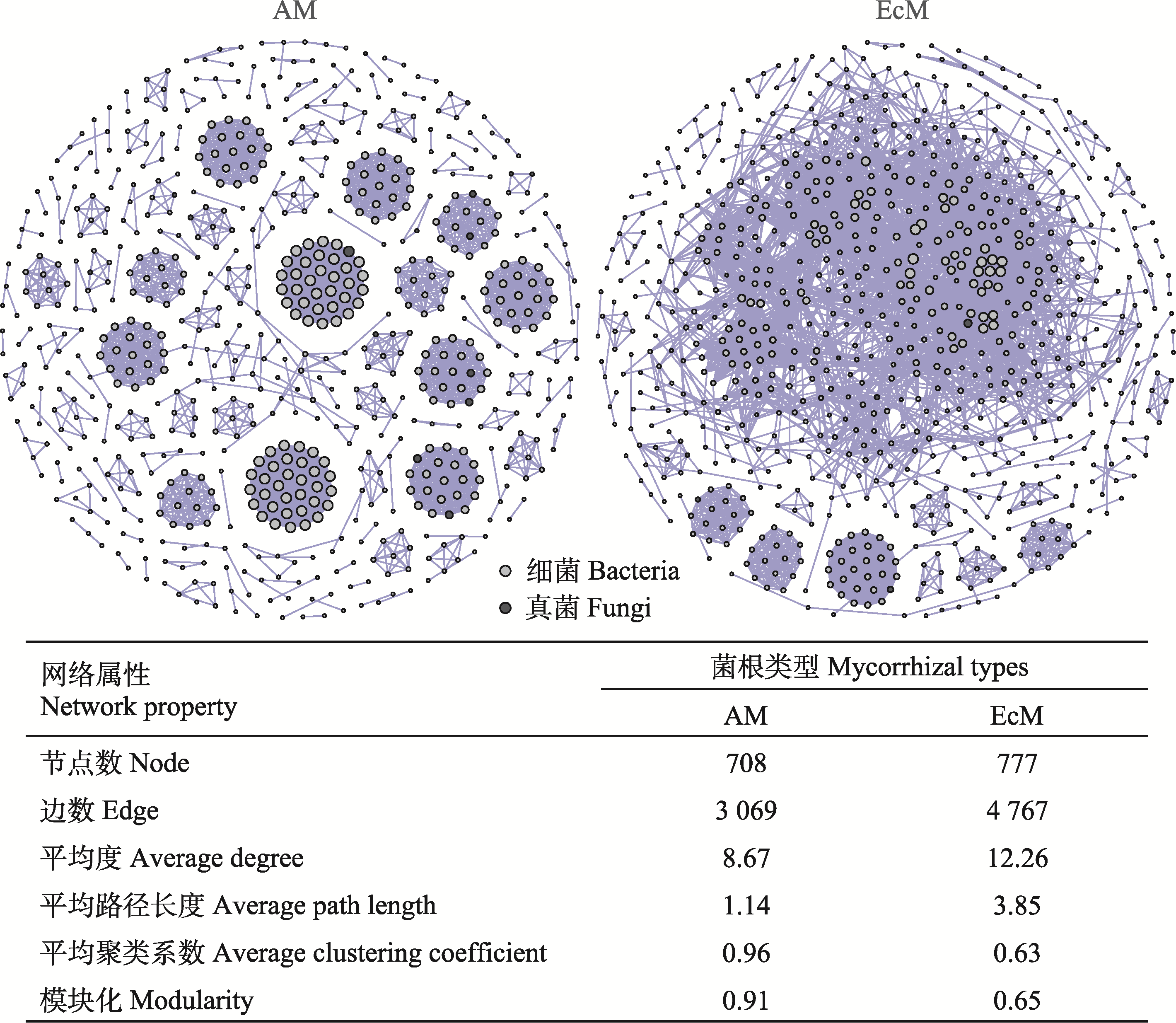
图4 不同菌根类型树种根际微生物网络。点的大小代表微生物类群度的大小。AM, 丛枝菌根; EcM, 外生菌根。
Fig. 4 Microbial network between different mycorrhizal types of tree species. The size of points indicates the degree of microbial taxonomies. AM, arbuscular mycorrhiza; EcM, ectomycorrhiza.
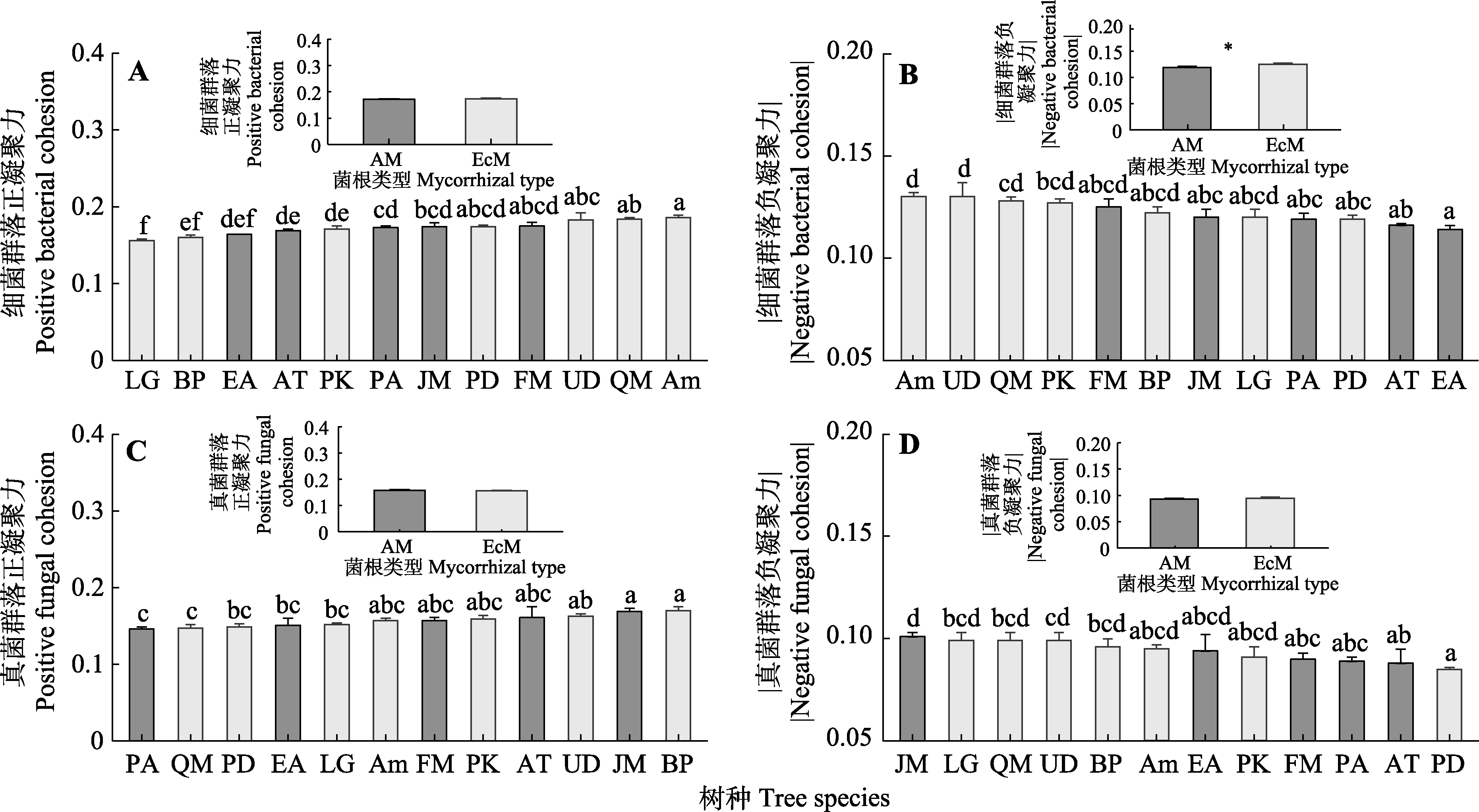
图5 不同菌根类型树种根际微生物群落复杂性(平均值±标准误)。AM, 丛枝菌根; EcM, 外生菌根。Am, 色木槭; AT, 青楷槭; BP, 白桦; EA, 卫矛; FM, 水曲柳; JM, 胡桃楸; LG, 落叶松; PA, 黄檗; PD, 山杨; PK, 红松; QM, 蒙古栎; UD, 春榆。不同小写字母代表不同树种间差异显著(p < 0.05); *代表混合效应模型中菌根类型效应显著(p < 0.05)。
Fig. 5 Microbial community complexity between different mycorrhizal types of tree species (mean ± SE). AM, arbuscular mycorrhiza; EcM, ectomycorrhiza. Am, Acer mono; AT, Acer tegmentosum; BP, Betula platyphylla; EA, Euonymus alatus; FM, Fraxinus mandshurica; JM, Juglans mandshurica; LG, Larix gmelinii; PA, Phellodendron amurense; PD, Populus davidiana; PK, Pinus koraiensis; UD, Ulmus davidiana var. japonica; QM, Quercus mongolica. Different lowercase letters indicate significant differences among different tree species (p < 0.05); * represents a significant mycorrhizal type effect in the mixed effects model (p < 0.05).
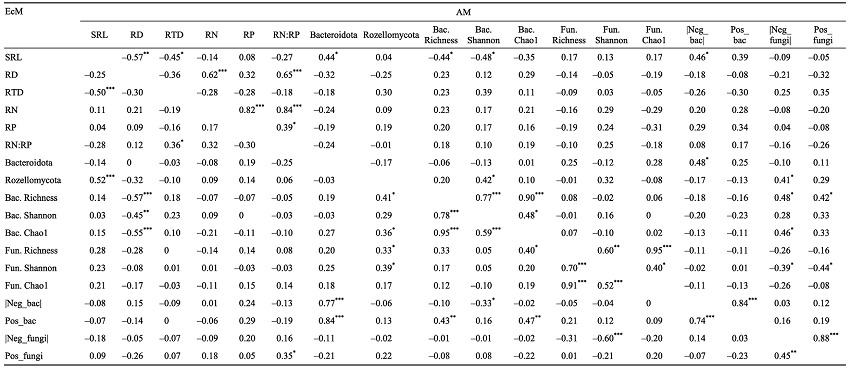 |
表2 不同菌根类型树种根际微生物多样性、群落复杂性与根系性状的联系
Table 2 Relationship among microbial biodiversity, community complexity and root traits between different mycorrhizal types of tree species
 |
| [1] | Averill C, Turner BL, Finzi AC (2014). Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature, 505, 543-545. |
| [2] |
Bahram M, Netherway T, Hildebrand F, Pritsch K, Drenkhan R, Loit K, Anslan S, Bork P, Tedersoo L (2020). Plant nutrient-acquisition strategies drive topsoil microbiome structure and function. New Phytologist, 227, 1189-1199.
DOI PMID |
| [3] |
Brabcová V, Nováková M, Davidová A, Baldrian P (2016). Dead fungal mycelium in forest soil represents a decomposition hotspot and a habitat for a specific microbial community. New Phytologist, 210, 1369-1381.
DOI PMID |
| [4] |
Brundrett MC, Tedersoo L (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytologist, 220, 1108-1115.
DOI PMID |
| [5] |
Brzostek ER, Dragoni D, Brown ZA, Phillips RP (2015). Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytologist, 206, 1274-1282.
DOI PMID |
| [6] |
Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016). Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proceedings of the National Academy of Sciences of the United States of America, 113, 8741-8746.
DOI PMID |
| [7] | Chen YC, Chi JH, Lu XY, Cai YJ, Jiang H, Zhang QF, Zhang KR (2023). Fungal-bacterial composition and network complexity determine soil multifunctionality during ecological restoration. Catena, 230. DOI: 10.1016/j.catena.2023.107251. |
| [8] | Csárdi G, Nepusz T (2006). The igraph software package for complex network research. InterJournal Complex Systems, 1695. http://igraph.sf.net. |
| [9] | Deng MF, Hu SJ, Guo LL, Jiang L, Huang YY, Schmid B, Liu C, Chang PF, Li S, Liu XJ, Ma KP, Liu LL (2023). Tree mycorrhizal association types control biodiversity- productivity relationship in a subtropical forest. Science Advances, 9, eadd4468. DOI: 10.1126/sciadv.add4468. |
| [10] | Eagar AC, Mushinski RM, Horning AL, Smemo KA, Phillips RP, Blackwood CB (2022). Arbuscular mycorrhizal tree communities have greater soil fungal diversity and relative abundances of saprotrophs and pathogens than ectomycorrhizal tree communities. Applied and Environmental Microbiology, 88, e0178221. DOI: 10.1128/AEM.01782-21. |
| [11] | Fang M, Liang MX, Liu XB, Li WB, Huang EH, Yu SX (2020). Abundance of saprotrophic fungi determines decomposition rates of leaf litter from arbuscular mycorrhizal and ectomycorrhizal trees in a subtropical forest. Soil Biology & Biochemistry, 149, 107966. DOI: 10.1016/j.soilbio.2020.107966. |
| [12] | Fernandez CW, Kennedy PG (2016). Revisiting the ‘Gadgil effect’: Do interguild fungal interactions control carbon cycling in forest soils? New Phytologist, 209, 1382-1394. |
| [13] |
Frey SD (2019). Mycorrhizal fungi as mediators of soil organic matter dynamics. Annual Review of Ecology, Evolution, and Systematics, 50, 237-259.
DOI |
| [14] | Gadgil RL, Gadgil PD (1971). Mycorrhiza and litter decomposition. Nature, 233, 133. |
| [15] | Gao J, Zhou MY, Shao JJ, Zhou GY, Liu RQ, Zhou LY, Liu HY, He YH, Chen Y, Zhou XH (2021a). Fine root trait-function relationships affected by mycorrhizal type and climate. Geoderma, 394, 115011. DOI: 10.1016/j.geoderma.2021.115011. |
| [16] | Gao YQ, Yuan Y, Li QK, Kou L, Fu XL, Dai XQ, Wang HM (2021b). Mycorrhizal type governs foliar and root multi-elemental stoichiometries of trees mainly via root traits. Plant and Soil, 460, 229-246. |
| [17] | Gleason FH, Carney LT, Lilje O, Glockling SL (2012). Ecological potentials of species of Rozella (Cryptomycota). Fungal Ecology, 5, 651-656. |
| [18] | Han MG, Sun LJ, Gan DY, Fu LC, Zhu B (2020). Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biology & Biochemistry, 151, 108019. DOI: 10.1016/j.soilbio.2020.108019. |
| [19] | Hernandez DJ, David AS, Menges ES, Searcy CA, Afkhami ME (2021). Environmental stress destabilizes microbial networks. The ISME Journal, 15, 1722-1734. |
| [20] | Herren CM, McMahon KD (2017). Cohesion: a method for quantifying the connectivity of microbial communities. The ISME Journal, 11, 2426-2438. |
| [21] | Huang XF, Chaparro JM, Reardon KF, Zhang R, Shen Q, Vivanco JM (2014). Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany, 92, 267-275. |
| [22] | Jiang Z, Thakur MP, Liu R, Zhou G, Zhou L, Fu Y, Zhang P, He Y, Shao J, Gao J, Li N, Wang X, Jia S, Chen Y, Zhang C, Zhou X (2022). Soil P availability and mycorrhizal type determine root exudation in sub-tropical forests. Soil Biology & Biochemistry, 171, 108722. DOI: 10.1016/j.soilbio.2022.108722. |
| [23] | Jiao S, Qi JJ, Jin CJ, Liu Y, Wang Y, Pan HB, Chen S, Liang CL, Peng ZH, Chen BB, Qian X, Wei GH (2022). Core phylotypes enhance the resistance of soil microbiome to environmental changes to maintain multifunctionality in agricultural ecosystems. Global Change Biology, 28, 6653-6664. |
| [24] | Kong D, Wang J, Wu H, Valverde-Barrantes OJ, Wang R, Zeng H, Kardol P, Zhang H, Feng Y (2019). Nonlinearity of root trait relationships and the root economics spectrum. Nature Communications, 10, 2203. DOI: 10.1038/s41467-019-10245-6. |
| [25] | Langley JA, Hungate BA (2003). Mycorrhizal controls on belowground litter quality. Ecology, 84, 2302-2312. |
| [26] | Li WT, Liu QH, Xie LL, Yin CY (2023). Interspecific plant-plant interactions increase the soil microbial network stability, shift keystone microbial taxa, and enhance their functions in mixed stands. Forest Ecology and Management, 533, 120851. DOI: 10.1016/j.foreco.2023.120851. |
| [27] |
Lin G, McCormack ML, Ma C, Guo D (2017). Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytologist, 213, 1440-1451.
DOI PMID |
| [28] | Liu YP, Sun QB, Li J, Lian B (2018). Bacterial diversity among the fruit bodies of ectomycorrhizal and saprophytic fungi and their corresponding hyphosphere soils. Scientific Reports, 8, 11672. DOI: 10.1038/s41598-018-30120-6. |
| [29] | Midgley MG, Brzostek E, Phillips RP (2015). Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees. Journal of Ecology, 103, 1454-1463. |
| [30] |
Phillips RP, Brzostek E, Midgley MG (2013). The mycorrhizal- associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytologist, 199, 41-51.
DOI PMID |
| [31] | Reich PB (2014). The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. Journal of Ecology, 102, 275-301. |
| [32] | Shi J, Wang Z, Peng YM, Zhang ZY, Fan ZM, Wang J, Wang X (2023). Microbes drive metabolism, community diversity, and interactions in response to microplastic-induced nutrient imbalance. Science of the Total Environment, 877, 162885. DOI: 10.1016/j.scitotenv.2023.162885. |
| [33] | Shi W, Wang ZQ, Liu JL, Gu JC, Guo DL (2008). Fine root morphology of twenty hardwood species in Maoershan natural secondary forest in northeastern China. Journal of Plant Ecology (Chinese Version), 32, 1217-1226. |
|
[师伟, 王政权, 刘金梁, 谷加存, 郭大力 (2008). 帽儿山天然次生林20个阔叶树种细根形态. 植物生态学报, 32, 1217-1226.]
DOI |
|
| [34] | Singavarapu B, Beugnon R, Bruelheide H, Cesarz S, Du J, Eisenhauer N, Guo L, Nawaz A, Wang Y, Xue K, Wubet T (2022). Tree mycorrhizal type and tree diversity shape the forest soil microbiota. Environmental Microbiology, 24, 4236-4255. |
| [35] |
Smith GR, Wan J (2019). Resource-ratio theory predicts mycorrhizal control of litter decomposition. New Phytologist, 223, 1595-1606.
DOI PMID |
| [36] | Steidinger BS, Crowther TW, Liang J, van Nuland ME, Werner GDA, Reich PB, Nabuurs GJ, de-Miguel S, Zhou M, Picard N, Herault B, Zhao X, Zhang C, Routh D, Peay KG, et al. (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature, 569, 404-408. |
| [37] | Sun XF, Liu F, Zhang QZ, Li YC, Zhang LF, Wang J, Zhang HY, Wang CK, Wang XC (2021). Biotic and climatic controls on the interannual variation in canopy litterfall of a deciduous broad-leaved forest. Agricultural and Forest Meteorology, 307, 108483. DOI: 10.1016/j.agrformet.2021.108483. |
| [38] | Tanunchai B, Ji L, Schroeter SA, Wahdan SFM, Thongsuk K, Hilke I, Gleixner G, Buscot F, Schulze ED, Noll M, Purahong W (2023). Tree mycorrhizal type regulates leaf and needle microbial communities, affects microbial assembly and co-occurrence network patterns, and influences litter decomposition rates in temperate forest. Frontiers in Plant Science, 14, 1239600. DOI: 10.3389/fpls.2023.1239600. |
| [39] |
Valverde-Barrantes OJ, Smemo KA, Feinstein LM, Kershner MW, Blackwood CB (2018). Patterns in spatial distribution and root trait syndromes for ecto and arbuscular mycorrhizal temperate trees in a mixed broadleaf forest. Oecologia, 186, 731-741.
DOI PMID |
| [40] |
Wagg C, Schlaeppi K, Banerjee S, Kuramae EE,van der Heijden MGA (2019). Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nature Communications, 10, 4841. DOI: 10.1038/s41467-019-12798-y.
PMID |
| [41] | Wan XH, Yu ZP, Wang MJ, Zhang Y, Huang ZQ (2022). Litter and root traits control soil microbial composition and enzyme activities in 28 common subtropical tree species. Journal of Ecology, 110, 3012-3022. |
| [42] | Wang C, Pan X, Yu W, Ye X, Erdenebileg E, Wang C, Ma L, Wang R, Huang Z, Indree T, Liu G (2023). Aridity and decreasing soil heterogeneity reduce microbial network complexity and stability in the semi-arid grasslands. Ecological Indicators, 151, 110342. DOI: 10.1016/j.ecolind.2023.110342. |
| [43] | Weil SS, Martinez-Almoyna C, Piton G, Renaud J, Boulangeat L, Foulquier A, Saillard A, Choler P, Poulenard J, Consortium O, Münkemüller T, Thuiller W (2021). Strong links between plant traits and microbial activities but different abiotic drivers in mountain grasslands. Journal of Biogeography, 48, 2755-2770. |
| [44] | Wen Z, White PJ, Shen J, Lambers H (2022). Linking root exudation to belowground economic traits for resource acquisition. New Phytologist, 233, 1620-1635. |
| [45] | Yin L, Dijkstra FA, Phillips RP, Zhu B, Wang P, Cheng W (2021). Arbuscular mycorrhizal trees cause a higher carbon to nitrogen ratio of soil organic matter decomposition via rhizosphere priming than ectomycorrhizal trees. Soil Biology & Biochemistry, 157, 108246. DOI: 10.1016/j.soilbio.2021.108246. |
| [46] | Yuan M, Guo X, Wu L, Zhang Y, Xiao N, Ning D, Shi Z, Zhou X, Wu L, Yang Y, Tiedje JM, Zhou J (2021). Climate warming enhances microbial network complexity and stability. Nature Climate Change, 11, 343-348. |
| [47] | Zhang BG, Zhang J, Liu Y, Shi P, Wei GH (2018). Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biology & Biochemistry, 118, 178-186. |
| [48] | Zhang L, Zhou J, George TS, Limpens E, Feng G (2022). Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends in Plant Science, 27, 402-411. |
| [49] | Zhang SQ, Chen C, Xu S, Yin SX (2003). Improvement in sulfuric-acid-hydrogen-peroxide assimilating method for determination of NPK in plant. Soil, 35, 174-175. |
| [张山泉, 陈川, 徐沭, 殷士学 (2003). 硫酸-过氧化氢消化法测定植株氮磷钾方法的改进. 土壤, 35, 174-175.] | |
| [50] | Zhang Y, Yu Z, Luan JW, Wang Y, Ye XD, Liu SR (2023). Spatiotemporal variations of vegetation greenness in the forest belt of Northeast China during 1982-2020. Acta Ecologica Sinica, 43, 6670-6681. |
| [张宇, 余振, 栾军伟, 王一, 叶晓丹, 刘世荣 (2023). 1982-2020年东北森林带植被绿度时空变化特征. 生态学报, 43, 6670-6681.] | |
| [51] | Zhao YX, Lou YC, Qin WZ, Cai JJ, Zhang P, Hu BL (2022). Interval aeration improves degradation and humification by enhancing microbial interactions in the composting process. Bioresource Technology, 358, 127296. DOI: 10.1016/j.biortech.2022.127296. |
| [1] | 杨密, 鲁梦珍, 冯志洋, 袁旭东, 赵小祥, 田秋香, 刘峰. 亚热带森林土壤磷有效性与外生菌根优势度的关联分析[J]. , 2026, 50(菌根生态学): 0-. |
| [2] | 沈会涛, 俞筱押, 秦彦杰, 武爱彬. 太行山东麓核桃林碳氮磷化学计量及碳储量随林龄变化特征[J]. 植物生态学报, 2025, 49(地上地下生态过程关联): 1-. |
| [3] | 王娟, 张登山, 肖元明, 裴全帮, 王博, 樊博, 周国英. 长期围封后高寒草原植物根系分泌物特征与环境因子关系[J]. 植物生态学报, 2025, 49(4): 596-609. |
| [4] | 姚博, 陈云, 曹雯婕, 龚相文, 罗永清, 郑成卓, 王旭洋, 王正文, 李玉强. 呼伦贝尔退化沙地植被-土壤碳氮磷互馈关系及微生物驱动机制[J]. 植物生态学报, 2025, 49(1): 59-73. |
| [5] | 王燕, 张全智, 王传宽, 郭万桂, 蔺佳玮. 恢复方式对东北东部森林土壤碳氮磷计量特征的影响[J]. 植物生态学报, 2024, 48(7): 943-954. |
| [6] | 郑莉莉, 余林兰, 戴萍, 薛跃规, 龙萍. 广西大石围天坑群植物叶片养分特征及其适应性[J]. 植物生态学报, 2024, 48(7): 872-887. |
| [7] | 王艺彤, 叶尔江·拜克吐尔汉, 廖丹, 王娟. 雌雄异株植物髭脉槭不同生长阶段叶片元素计量特征与性二态间的相互关系[J]. 植物生态学报, 2024, 48(6): 760-769. |
| [8] | 张文瑾, 佘维维, 秦树高, 乔艳桂, 张宇清. 氮和水分添加对黑沙蒿群落优势植物叶片氮磷化学计量特征的影响[J]. 植物生态学报, 2024, 48(5): 590-600. |
| [9] | 臧妙涵, 王传宽, 梁逸娴, 刘逸潇, 上官虹玉, 全先奎. 基于纬度移栽的落叶松叶、枝、根生态化学计量特征对气候变暖的响应[J]. 植物生态学报, 2024, 48(4): 469-482. |
| [10] | 吴君梅, 曾泉鑫, 梅孔灿, 林惠瑛, 谢欢, 刘苑苑, 徐建国, 陈岳民. 土壤磷有效性调控亚热带森林土壤酶活性和酶化学计量对凋落叶输入的响应[J]. 植物生态学报, 2024, 48(2): 242-253. |
| [11] | 李林, 孙毅, 杨晓琼, 方海东, 闫帮国. 七彩花生根瘤内生菌对氮添加的响应及其与植物化学计量特征的关系[J]. 植物生态学报, 2024, 48(10): 1374-1384. |
| [12] | 刘倩愿, 俞振东, 张微微. 河北坝上乔灌木植物根系无机和有机氮吸收速率及其偏好[J]. 植物生态学报, 2024, 48(10): 1361-1373. |
| [13] | 韩路, 冯宇, 李沅楷, 王雨晴, 王海珍. 地下水埋深对灰胡杨叶片与土壤养分生态化学计量特征及其内稳态的影响[J]. 植物生态学报, 2024, 48(1): 92-102. |
| [14] | 李红琴, 张法伟, 仪律北. 高寒草甸表层土壤和优势植物叶片的化学计量特征对降水改变和氮添加的响应[J]. 植物生态学报, 2023, 47(7): 922-931. |
| [15] | 李兆光, 文高, 和桂青, 徐天才, 和琼姬, 侯志江, 李燕, 薛润光. 滇西北藜麦氮磷钾生态化学计量特征的物候期动态[J]. 植物生态学报, 2023, 47(5): 724-732. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19