

Chin J Plant Ecol ›› 2019, Vol. 43 ›› Issue (4): 374-382.DOI: 10.17521/cjpe.2019.0048
Special Issue: 菌根真菌
• Research Articles • Previous Articles
FAN Zi-Teng,WU Yu-Ling,WANG Xin-Ju,LI Tai-Qiang,GAO Jiang-Yun( )
)
Received:2019-09-05
Revised:2019-04-16
Online:2019-04-20
Published:2019-05-30
Contact:
GAO Jiang-Yun
Supported by:FAN Zi-Teng, WU Yu-Ling, WANG Xin-Ju, LI Tai-Qiang, GAO Jiang-Yun. Effects of symbiotic fungi on seed germination of interspecific hybrid progenies in Orchidaceae[J]. Chin J Plant Ecol, 2019, 43(4): 374-382.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2019.0048

Fig. 1 Seed germination, protocorm and seedling formation of three Dendrobium species under different treatments at different stages. A, Germinating seed and formed protocorm of D. officinale after 30 days incubation with SSCDO-5 strain. B, A seeding of D. devonianum after 58 days incubation with FDd1 strain. C, Seedings of hybrid after 68 days incubation with FDd1 strain. D, Seedings of hybrid after 68 days incubation with SSCDO-5 strain.
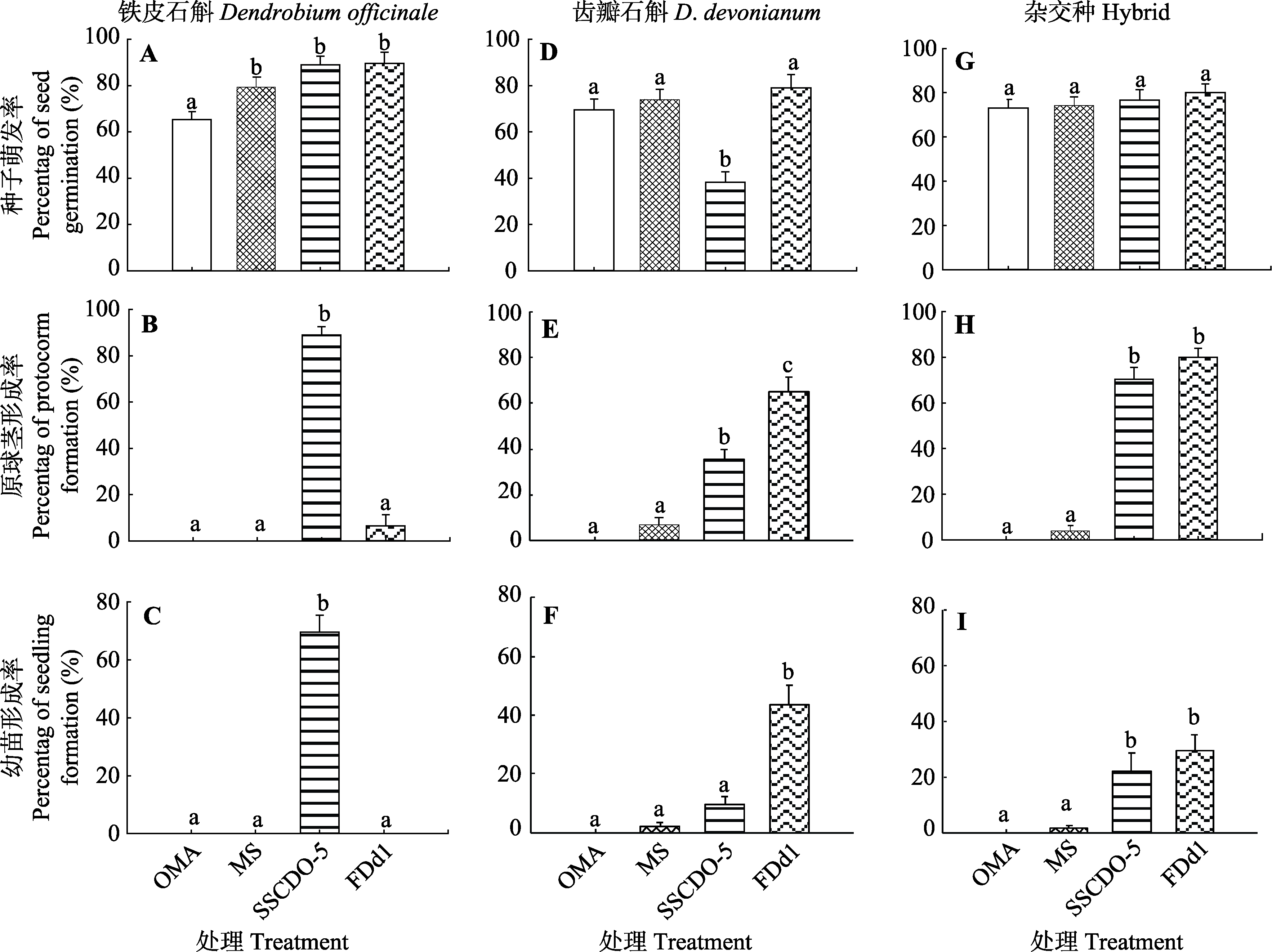
Fig. 2 Percentage of seed germination (A, D, G), protocorm (B, E, H) and seedling (C, F, I) formation of different treatments in three Dendrobium species (mean ± SE). Different lowercase letters above the bars represent significant differences between different treatments at the same stage (α = 0.05). OMA, oatmeal agar medium without fungal inoculation was used as nutrient-poor control treatment; MS, MS medium without fungal inoculation was used as nutrient-rich control treatment; SSCDO-5, treatment of fungal inoculation with SSCDO-5 strain on OMA medium; FDd1, treatment of fungal inoculation with FDd1 strain on OMA medium.
| 多重比较 Multiple comparisons | 铁皮石斛 D. officinale | 齿瓣石斛 D. devonianum | 杂交种 Hybrid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 种子萌发率 Seed germination rate | 原球茎形成率 Protocorms formation rate | 幼苗率 Seedlings formation rate | 种子萌发率 Seed germination rate | 原球茎形成率 Protocorms formation rate | 幼苗率 Seedlings formation rate | 种子萌发率 Seed germination rate | 原球茎形成率 Protocorms formation rate | 幼苗率 Seedlings formation rate | |
| OMA-MS | -0.139 3 ± 0.058 2* | 0.000 0 ± 0.042 7 | 0.000 0 ± 0.053 4 | -0.044 4 ± 0.071 1 | -0.065 5 ± 0.063 9 | -0.021 4 ± 0.051 6 | -0.012 0 ± 0.058 1 | -0.039 3 ± 0.053 0 | -0.017 2 ± 0.064 4 |
| OMA-SSCDO-5 | -0.235 3 ± 0.057 7*** | -0.888 8 ± 0.042 3*** | -0.696 6 ± 0.052 9*** | 0.312 1 ± 0.069 5*** | -0.354 8 ± 0.062 4*** | -0.096 8 ± 0.050 4 | -0.036 9 ± 0.058 1 | -0.704 0 ± 0.053 0*** | -0.221 3 ± 0.064 4** |
| OMA-FDd1 | -0.242 3 ± 0.058 2*** | -0.065 5 ± 0.042 7 | 0.065 5 ± 0.053 4 | -0.094 8 ± 0.070 5 | -0.651 7 ± 0.063 3*** | -0.436 8 ± 0.051 2*** | -0.070 1 ± 0.059 1 | -0.800 4 ± 0.053 9*** | -0.295 3 ± 0.065 5*** |
| MS-SSCDO-5 | -0.096 0 ± 0.058 2 | -0.888 8 ± 0.042 7*** | -0.696 6 ± 0.053 4*** | 0.356 5 ± 0.068 1*** | -0.289 4 ± 0.061 1*** | -0.075 3 ± 0.049 4 | -0.024 9 ± 0.055 3 | -0.664 8 ± 0.050 4*** | -0.204 0 ± 0.061 3** |
| MS- FDd1 | -0.102 9 ± 0.058 7 | -0.006 6 ± 0.043 1 | 0.065 5 ± 0.053 8 | -0.050 4 ± 0.069 2 | -0.586 2 ± 0.062 1*** | -0.415 4 ± 0.050 2*** | -0.058 1 ± 0.056 3 | -0.761 1 ± 0.0513 4*** | -0.278 0 ± 0.062 4*** |
| SSCDO-5- FDd1 | -0.007 0 ± 0.058 2 | 0.823 3 ± 0.042 7*** | 0.631 0 ± 0.053 4*** | -0.406 9 ± 0.067 5*** | -0.296 9 ± 0.060 6*** | -0.340 0 ± 0.049 0*** | -0.033 2 ± 0.056 3 | -0.096 4 ± 0.051 3 | -0.074 0 ± 0.062 4 |
Table 1 Multiple comparisons on seed germination rate, protocorms formation rate and seedlings formation rate of different treatments for three Dendrobium species (mean ± SE)
| 多重比较 Multiple comparisons | 铁皮石斛 D. officinale | 齿瓣石斛 D. devonianum | 杂交种 Hybrid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 种子萌发率 Seed germination rate | 原球茎形成率 Protocorms formation rate | 幼苗率 Seedlings formation rate | 种子萌发率 Seed germination rate | 原球茎形成率 Protocorms formation rate | 幼苗率 Seedlings formation rate | 种子萌发率 Seed germination rate | 原球茎形成率 Protocorms formation rate | 幼苗率 Seedlings formation rate | |
| OMA-MS | -0.139 3 ± 0.058 2* | 0.000 0 ± 0.042 7 | 0.000 0 ± 0.053 4 | -0.044 4 ± 0.071 1 | -0.065 5 ± 0.063 9 | -0.021 4 ± 0.051 6 | -0.012 0 ± 0.058 1 | -0.039 3 ± 0.053 0 | -0.017 2 ± 0.064 4 |
| OMA-SSCDO-5 | -0.235 3 ± 0.057 7*** | -0.888 8 ± 0.042 3*** | -0.696 6 ± 0.052 9*** | 0.312 1 ± 0.069 5*** | -0.354 8 ± 0.062 4*** | -0.096 8 ± 0.050 4 | -0.036 9 ± 0.058 1 | -0.704 0 ± 0.053 0*** | -0.221 3 ± 0.064 4** |
| OMA-FDd1 | -0.242 3 ± 0.058 2*** | -0.065 5 ± 0.042 7 | 0.065 5 ± 0.053 4 | -0.094 8 ± 0.070 5 | -0.651 7 ± 0.063 3*** | -0.436 8 ± 0.051 2*** | -0.070 1 ± 0.059 1 | -0.800 4 ± 0.053 9*** | -0.295 3 ± 0.065 5*** |
| MS-SSCDO-5 | -0.096 0 ± 0.058 2 | -0.888 8 ± 0.042 7*** | -0.696 6 ± 0.053 4*** | 0.356 5 ± 0.068 1*** | -0.289 4 ± 0.061 1*** | -0.075 3 ± 0.049 4 | -0.024 9 ± 0.055 3 | -0.664 8 ± 0.050 4*** | -0.204 0 ± 0.061 3** |
| MS- FDd1 | -0.102 9 ± 0.058 7 | -0.006 6 ± 0.043 1 | 0.065 5 ± 0.053 8 | -0.050 4 ± 0.069 2 | -0.586 2 ± 0.062 1*** | -0.415 4 ± 0.050 2*** | -0.058 1 ± 0.056 3 | -0.761 1 ± 0.0513 4*** | -0.278 0 ± 0.062 4*** |
| SSCDO-5- FDd1 | -0.007 0 ± 0.058 2 | 0.823 3 ± 0.042 7*** | 0.631 0 ± 0.053 4*** | -0.406 9 ± 0.067 5*** | -0.296 9 ± 0.060 6*** | -0.340 0 ± 0.049 0*** | -0.033 2 ± 0.056 3 | -0.096 4 ± 0.051 3 | -0.074 0 ± 0.062 4 |
| [1] | Aceto S, Caputo P, Cozzolino S, Gaudio L, Moretti A ( 1999). Phylogeny and evolution of Orchis and allied genera based on ITS DNA variation: Morphological gaps and molecular continuity. Molecular Phylogenetics & Evolution, 13, 67-76. |
| [2] | Arditti J ( 1967). Factors affecting the germination of orchid seeds. The Botanical Review, 33, 1-97. |
| [3] | Arditti J, Ghani AKA ( 2000). Numerical and physical properties of orchid seeds and their biological implications. New Phytologist, 145, 367-421. |
| [4] | Bidartondo MI, Read DJ (2008). Fungal specificity bottlenecks during orchid germination and development. Molecular Ecology, 17, 3707-3716. |
| [5] | Brundrett MC, Scade A, Batty AL, Dixon KW, Sivasithamparam K ( 2003). Development of in situ and ex situ seed baiting techniques to detect mycorrhizal fungi from terrestrial orchid habitats. Mycological Research, 107, 1210-1220. |
| [6] | Chase MW, Cameron KM, Freudenstein JV, Pridgeon AM, Salazar G, Berg C, Schuiteman A ( 2015). An updated classification of Orchidaceae. Botanical Journal of the Linnean Society, 177, 151-174. |
| [7] | Coyne JA, Orr HA ( 2004). Speciation. Sinauer Associates, Sunderland, USA. |
| [8] | Cozzolino S, Widmer A ( 2005). Orchid diversity: An evolutionary consequence of deception? Trends in Ecology & Evolution, 20, 487-494. |
| [9] | Dafni A, Ivri Y (1979). Pollination ecology of, and hybridization between,Orchis coriophora L. and O. collina Sol. ex Russ.(Orchidaceae) in Israel. New Phytologist, 83, 181-187. |
| [10] | Dearnaley JDW, Martos F, Selosse MA (2012). Orchid mycorrhizas: Molecular ecology, physiology, evolution and conservation aspects. In: Hock B ed. Fungal Associations. 2nd edn. Springer, Berlin,Germany. 207-230. |
| [11] | Dearnaley JWD, Perotto S, Selosse MA (2016). Structure and development of orchid mycorrhizas. In: Martin F ed. Molecular Mycorrhizal Symbiosis. Springer, Berlin. 63-86. |
| [12] | Gao JY, Liu Q, Yu DL (2014). Orchids of Xishuangbanna: Diversity and Conservation. China Forestry Publishing House, Beijing. 15-31. |
| [ 高江云, 刘强, 余东莉 (2014). 西双版纳的兰科植物: 多样性和保护. 中国林业出版社, 北京. 15-31.] | |
| [13] | Hegarty MJ, Hiscock SJ ( 2005). Hybrid speciation in plants: New insights from molecular studies. New Phytologist, 165, 411-423. |
| [14] | Hollick PS, Taylor RJ, McComb JA, Dixon KW ( 2005). If orchid mycorrhizal fungi are so specific, how do natural hybrids cope? Selbyana, 26, 159-170. |
| [15] | Huang H, Zi XM, Lin H, Gao JY ( 2018). Host-specificity of symbiotic mycorrhizal fungi for enhancing seed germination, protocorm formation and seedling development of over-collected medicinal orchid,Dendrobium devonianum. Journal of Microbiology, 56, 42-48. |
| [16] | Kauth P, Dutra D, Johnson T (2008). Techniques and applications of in vitro orchid seed germination. In: Teixeira da Silva JA ed. Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues. Global Science Books, Iselworth,UK. 375-391. |
| [17] | Lee YI ( 2007). The asymbiotic seed germination of six Paphiopedilum species in relation to the time of seed collection and seed pretreatment. Acta Horticulturae, 755, 381-386. |
| [18] | Liu HX, Luo YB, Liu H ( 2010). Studies of mycorrhizal fungi of Chinese orchids and their role in orchid conservation in China―A review. The Botanic Review, 76, 241-262. |
| [19] | Long B, Niemiera AX, Cheng ZY, Long CL ( 2010). In vitro propagation of four threatened Paphiopedilum species (Orchidaceae). Plant Cell Tissue and Organ Culture, 101, 151-162. |
| [20] | Mallet J ( 2005). Hybridization as an invasion of the genome. Trends in Ecology & Evolution, 20, 229-237. |
| [21] | Martos F, Francios M, Pailler T, Kottke I, Cédric G, Marc- André Selosse ( 2012). The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Molecular Ecology, 21, 5098-5109. |
| [22] | Masuhara G, Katsuya K (1994). In situ and in vitro specificity between Rhizoctonia spp. and Spiranthes sinensis(Persoon) Ames. var. amoena 127, 711-718. |
| [23] | McCormick MK, Jacquemyn H ( 2014). What constrains the distribution of orchid populations? New Phytologist, 202, 392-400. |
| [24] | McKendrick SL, Leake JR, Read DJ ( 2000). Symbiotic germination and development of myco-heterotrophic plants in nature: Transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytologist, 145, 539-548. |
| [25] | Meng YY, Shao SC, Liu SJ, Gao JY ( 2019). Do the fungi associated with roots of adult plants support seed germination? A case study on Dendrobium exile(Orchidaceae). Global Ecology and Conservation, 17, e00582. DOI: 10.1016/ j.gecco.2019.e00582. |
| [26] | Nontachaiyapoom S, Sasirat S, Manoch L ( 2010). Isolation and identification of Rhizoctonia-like fungi from roots of three orchid genera, Paphiopedilum, Dendrobium, and Cymbidium, collected in Chiang Rai and Chiang Mai provinces of Thailand. Mycorrhiza, 20, 459-471. |
| [27] | Ramsay MM, Stewart J ( 1998). Re-establishment of the lady’s slipper orchid (Cypripedium Calceolus L.) in Britain. Botanical Journal of the Linnean Society, 126, 173-181. |
| [28] | Rasmussen HN, Dixon KW, Jersakova J, Tesitelova T ( 2015). Germination and seedling establishment in orchids: A complex of requirements. Annals of Botany, 116, 391-402. |
| [29] | Roberts DL (2003). Pollination biology: The role of sexual reproduction in orchid conservation. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ eds. Orchid Conservation. Natural History Publications, Kota Kinabalu, Sabah. 113-136. |
| [30] | Schatz B ( 2006). Fine scale distribution of pollinator explains the occurrence of the natural orchid hybrid × Orchis bergonii. Ecoscience, 13, 111-118. |
| [31] | Schatz B, Geoffroy A, Dainat B, Bessiere JM, Buatois B, Hossaert-Mckey M, Selosse MA ( 2010). A case study of modified interactions with symbionts in a hybrid mediterranean orchid. American Journal of Botany, 97, 1278-1288. |
| [32] | Sheng CL, Li YY, Gao JY ( 2012). Ex situ symbiotic seed germination, isolation and identification of effective symbiotic fungus in Cymbidium mannii(Orchidaceae). Chinese Journal of Plant Ecology, 36, 859-869. |
| [ 盛春玲, 李勇毅, 高江云 ( 2012). 硬叶兰种子的迁地共生萌发及有效共生真菌的分离和鉴定. 植物生态学报, 36, 859-869.] | |
| [33] | Stewart SL, Kane ME ( 2007). Symbiotic seed germination and evidence for in vitro mycobiont specificity in Spiranthes brevilabris(Orchidaceae) and its implications for species-level conservation. In Vitro Cellular & Developmental Biology-Plant, 43, 178-186. |
| [34] | Stimart DP, Ascher PD ( 1981). In vitro germination of Paphiopedilum seed on a completely defined medium. Scientia Horticulturae, 14, 165-170. |
| [35] | Swarts ND, Dixon KW ( 2009). Terrestrial orchid conservation in the age of extinction. Annals of Botany, 104, 543-556. |
| [36] | Vujanovic V, St-Arnaud M, Barabe D, Thibeault G ( 2000). Viability testing of orchid seed and the promotion of colouration and germination. Annals of Botany, 86, 79-86. |
| [37] | Whitehead MR, Peakall R ( 2014). Pollinator specificity drives strong pre-pollination reproductive isolation in sympatric sexually deceptive orchids. Evolution, 68, 1561-1575. |
| [38] | Yamazaki J, Miyoshi K ( 2006). In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata(Orchidaceae). Annals of Botany, 98, 1197-1206. |
| [39] | Zelmer CD, Cuthbertson L, Currah RS ( 1996). Fungi associated with terrestrial orchid mycorrhizas, seeds and protocorms. Mycoscience, 37, 439-448. |
| [40] | Zettler LW, Piskin KA, Stewart SL, Hartsock JJ, Bowles ML, Bell TJ ( 2005). Protocorm mycobionts of the federally threatened eastern prairie fringed orchid,Platanthera leucophaea(Nutt.) Lindley, and a technique to prompt leaf elongation in seedlings. Studies in Mycology, 53, 163-171. |
| [41] | Zhou X, Gao JY ( 2016). Highly compatible Epa-01 strain promotes seed germination and protocorm development of Papilionanthe teres(Orchidaceae). Plant Cell Tissue and Organ Culture, 125, 479-493. |
| [42] | Zi XM, Sheng CL, Goodale UM, Shao SC, Gao JY ( 2014). In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum(Orchidaceae). Mycorrhiza, 24, 487-499. |
| [43] | Zotz G ( 2013). The systematic distribution of vascular epiphytes—A critical update. Botanical Journal of the Linnean Society, 171, 453-481. |
| [1] | Xu Zi-Yi Guang-Ze JIN. Variation and trade-offs in fine root functional traits of seedlings of different mycorrhizal types in mixed broadleaved-Korean pine forests [J]. Chin J Plant Ecol, 2024, 48(5): 612-622. |
| [2] | Ke-Yu CHEN Sen Xing Yu Tang Sun JiaHui Shijie Ren Bao-Ming JI. Arbuscular mycorrhizal fungal community characteristics and driving factors in different grassland types [J]. Chin J Plant Ecol, 2024, 48(5): 660-674. |
| [3] | Die Hu Xinqi Jiang DAI Zhicong Daiyi Chen Yu Zhang Shan-Shan Qi. Arbuscular mycorrhizal fungi enhance the herbicide tolerance of an invasive weed Sphagneticola trilobata [J]. Chin J Plant Ecol, 2024, 48(5): 651-659. |
| [4] | CHEN Bao-Dong, FU Wei, WU Song-Lin, ZHU Yong-Guan. Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling [J]. Chin J Plant Ecol, 2024, 48(1): 1-20. |
| [5] | REN Yue, GAO Guang-Lei, DING Guo-Dong, ZHANG Ying, ZHAO Pei-Shan, LIU Ye. Species composition and driving factors of the ectomycorrhizal fungal community associated with Pinus sylvestris var. mongolica at different growth periods [J]. Chin J Plant Ecol, 2023, 47(9): 1298-1309. |
| [6] | HU Tong-Xin, LI Bei, LI Guang-Xin, REN Yue-Xiao, DING Hai-Lei, SUN Long. Effects of fire originated black carbon on species composition of ectomycorrhizal fungi in a Larix gmelinii forest in growing season [J]. Chin J Plant Ecol, 2023, 47(6): 792-803. |
| [7] | YANG Jia-Rong, DAI Dong, CHEN Jun-Fang, WU Xian, LIU Xiao-Lin, LIU Yu. Insight into recent studies on the diversity of arbuscular mycorrhizal fungi in shaping plant community assembly and maintaining rare species [J]. Chin J Plant Ecol, 2023, 47(6): 745-755. |
| [8] | HE Fei, LI Chuan, Faisal SHAH, LU Xie-Min, WANG Ying, WANG Meng, RUAN Jia, WEI Meng-Lin, MA Xing-Guang, WANG Zhuo, JIANG Hao. Carbon transport and phosphorus uptake in an intercropping system of Robinia pseudoacacia and Amorphophallus konjac mediated by arbuscular mycorrhizal hyphal networks [J]. Chin J Plant Ecol, 2023, 47(6): 782-791. |
| [9] | ZHAO Rong-Jiang, CHEN Tao, DONG Li-Jia, GUO Hui, MA Hai-Kun, SONG Xu, WANG Ming-Gang, XUE Wei, YANG Qiang. Progress of plant-soil feedback in ecology studies [J]. Chin J Plant Ecol, 2023, 47(10): 1333-1355. |
| [10] | ZHANG Hui, ZENG Wen-Jing, GONG Xin-Tao, MA Ze-Qing. Relationships between root hairs and mycorrhizal fungi across typical subtropical tree species [J]. Chin J Plant Ecol, 2023, 47(1): 88-100. |
| [11] | XIE Wei, HAO Zhi-Peng, ZHANG Xin, CHEN Bao-Dong. Research progress and prospect of signal transfer among plants mediated by arbuscular mycorrhizal networks [J]. Chin J Plant Ecol, 2022, 46(5): 493-515. |
| [12] | SHAN Ting-Ting, CHEN Tong-Yao, CHEN Xiao-Mei, GUO Shun-Xing, WANG Ai-Rong. Advance on the association between mycorrhizal fungi and Orchidaceae in nitrogen nutrition [J]. Chin J Plant Ecol, 2022, 46(5): 516-528. |
| [13] | MA Ju-Feng, XIN Min, XU Chen-Chao, ZHU Wan-Ying, MAO Chuan-Zao, CHEN Xin, CHENG Lei. Effects of arbuscular mycorrhizal fungi and nitrogen addition on nitrogen uptake of rice genotypes with different root morphologies [J]. Chin J Plant Ecol, 2021, 45(7): 728-737. |
| [14] | PANG Fang, XIA Wei-Kang, HE Min, QI Shan-Shan, DAI Zhi-Cong, DU Dao-Lin. Nitrogen-fixing bacteria alleviates competition between arbuscular mycorrhizal fungi and Solidago canadensis for nutrients under nitrogen limitation [J]. Chin J Plant Ecol, 2020, 44(7): 782-790. |
| [15] | TANG Jin-Qi, GUO Xiao-Cheng, LU Xin-Yu, LIU Ming-Chao, ZHANG Hai-Yan, FENG Yu-Long, KONG De-Liang. A review on the effects of invasive plants on mycorrhizal fungi of native plants and their underlying mechanisms [J]. Chin J Plant Ecol, 2020, 44(11): 1095-1112. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn