

植物生态学报 ›› 2011, Vol. 35 ›› Issue (2): 176-186.DOI: 10.3724/SP.J.1258.2011.00176
所属专题: 青藏高原植物生态学:生理生态学
收稿日期:2010-07-05
接受日期:2010-11-01
出版日期:2011-07-05
发布日期:2011-01-21
作者简介:师生波, E-mail: sbshi@nwipb.cas.cn
SHI Sheng-Bo1,2,*( ), SHANG Yan-Xia2, ZHU Peng-Jin2, ZHANG De-Gang1
), SHANG Yan-Xia2, ZHU Peng-Jin2, ZHANG De-Gang1
Received:2010-07-05
Accepted:2010-11-01
Online:2011-07-05
Published:2011-01-21
摘要:
采用滤除自然光谱中UV-B辐射成分的方法, 探讨了高山植物美丽风毛菊(Saussurea superba)光合机构对青藏高原强UV-B辐射的响应和适应特性。结果表明, 强太阳光中的UV-B成分能引起净光合速率的降低。连续16天不同天气下的观测表明, 滤除UV-B处理时3 min暗适应的光化学量子效率有升高的趋势; 晴天下稳态光化学效率的分析也显示滤除UV-B处理的实际光化学量子效率和光化学猝灭系数有升高趋势, 意味着自然光中的UV-B成分可限制美丽风毛菊叶片PSII反应中心的激发能捕获效率。PSII有效光化学量子效率的增加和非光化学猝灭系数的降低进一步表明, UV-B辐射能导致有效光化学效率的降低和非光化学能量耗散的增加。由上可知, 自然强UV-B辐射是限制美丽风毛菊叶片光合作用的一个因素。滤除UV-B辐射处理对光合色素含量的影响较小, 无论以叶面积还是叶鲜重为基础的滤除UV-B处理仅有微弱的增加趋势, 说明强UV-B辐射具有加速光合色素的光氧化进程, 促进细胞成熟和叶片衰亡的潜在作用。同样UV-B吸收物质的含量也几乎没有变化, 表明强太阳辐射环境下生活的高山植物美丽风毛菊叶表皮层中已具有较多的紫外线屏蔽物质, 足以抵御目前环境中强太阳UV-B辐射可能引起的伤害, 较少受UV-B辐射波动的影响。
师生波, 尚艳霞, 朱鹏锦, 张德罡. 滤除自然光中UV-B辐射成分对高山植物美丽风毛菊光合生理的影响. 植物生态学报, 2011, 35(2): 176-186. DOI: 10.3724/SP.J.1258.2011.00176
SHI Sheng-Bo, SHANG Yan-Xia, ZHU Peng-Jin, ZHANG De-Gang. Effects of UV-B exclusion on photosynthetic physiology in alpine plant Saussurea superba. Chinese Journal of Plant Ecology, 2011, 35(2): 176-186. DOI: 10.3724/SP.J.1258.2011.00176
| amb UV-B | low UV-B | 差异显著性 Significance | |
|---|---|---|---|
| 光合有效辐射 PAR (μmol photon·m-2·s-1) | 1 767 ± 33 | 1 726 ± 43 | 0.398 |
| 大气相对湿度 Air relative humidity (%) | 65.06 ± 0.23 | 64.88 ± 0.16 | 0.524 |
| 空气温度 Air temperature (℃) | 24.09 ± 0.06 | 24.03 ± 0.02 | 0.340 |
| 紫外线-B UV-B (W·m-2) | 4.05 ± 0.10 | 1.74 ± 0.27 | 0 |
| 紫外线-A UV-A (mW·m -2) | 18.74 ± 0.28 | 15.27 ± 0.25 | 0 |
表1 滤除UV-B辐射试验中主要环境因子的变化(2009年7月18日)(平均值±标准误差, n = 15; p = 0.05)
Table 1 Changes of main environmental factors in UV-B-exclusion experiments in July 18, 2009 (mean ± SE, n = 15; p = 0.05)
| amb UV-B | low UV-B | 差异显著性 Significance | |
|---|---|---|---|
| 光合有效辐射 PAR (μmol photon·m-2·s-1) | 1 767 ± 33 | 1 726 ± 43 | 0.398 |
| 大气相对湿度 Air relative humidity (%) | 65.06 ± 0.23 | 64.88 ± 0.16 | 0.524 |
| 空气温度 Air temperature (℃) | 24.09 ± 0.06 | 24.03 ± 0.02 | 0.340 |
| 紫外线-B UV-B (W·m-2) | 4.05 ± 0.10 | 1.74 ± 0.27 | 0 |
| 紫外线-A UV-A (mW·m -2) | 18.74 ± 0.28 | 15.27 ± 0.25 | 0 |
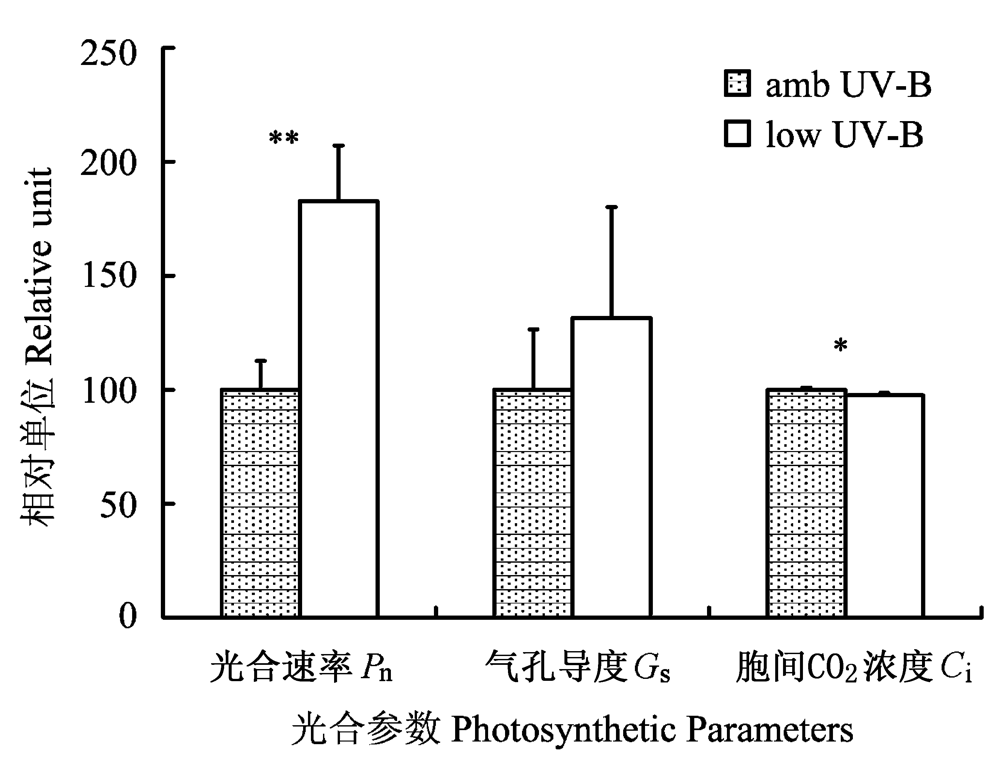
图1 2009年7月滤除自然光中UV-B辐射成分处理对美丽风毛菊叶片光合作用的影响。垂直条表示标准误差。*, p < 0.05; **, p < 0.01。
Fig. 1 Effects of removal of UV-B component from natural sunlight on photosynthesis in Saussurea superba in July 2009. Vertical bar is SE. *, p < 0.05; **, p < 0.01. Ci, intercellular CO2 concentration; Pn, net photosynthetic rate; Gs, stomatal conductance.
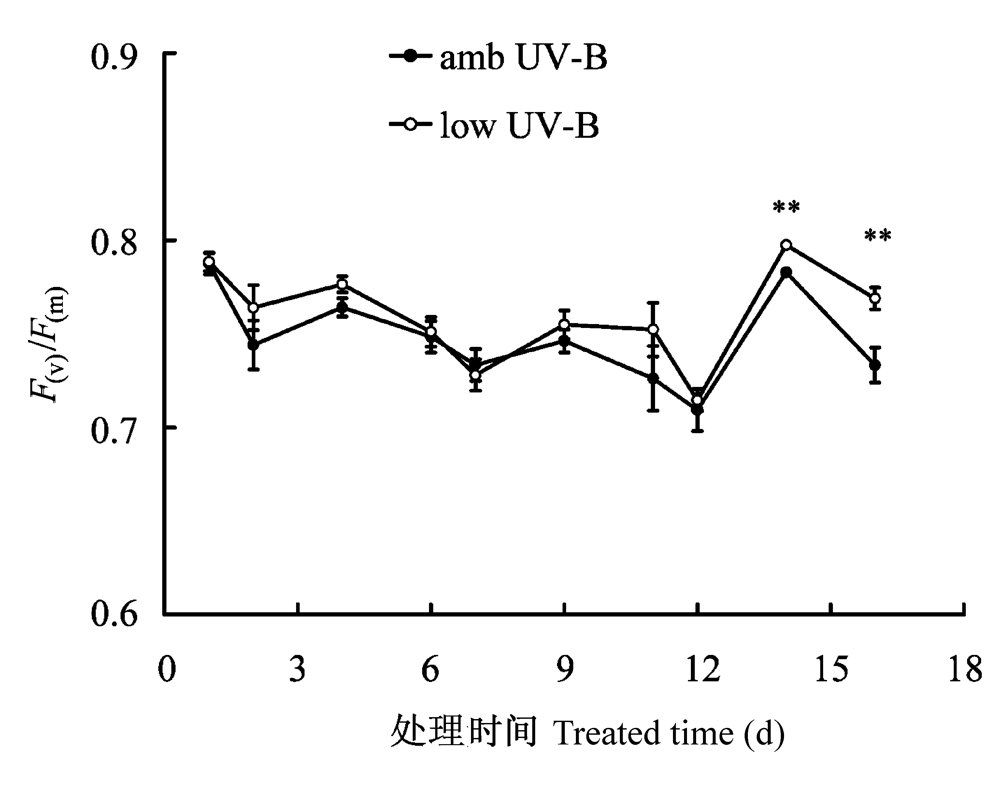
图2 2008年8月滤除自然光中UV-B辐射成分处理对美丽风毛菊叶片暗适应3 min后PSII光化学量子效率(F(v)/F(m))的影响。图中连续16天的测定中, 由于天气等原因每次的样本数目从4到22不等。垂直条表示标准误差。**, p < 0.01。
Fig. 2 Effects of removal of UV-B components from natural sunlight on 3 min dark adapted quantum efficiency of PSII photochemistry (F(v)/F(m)) in Saussurea superba in August 2008. Data numbers were from 4 to 22 during the 16 days of continue measurement due to the influence of weather condition. Vertical bar is SE. **, p < 0.01.
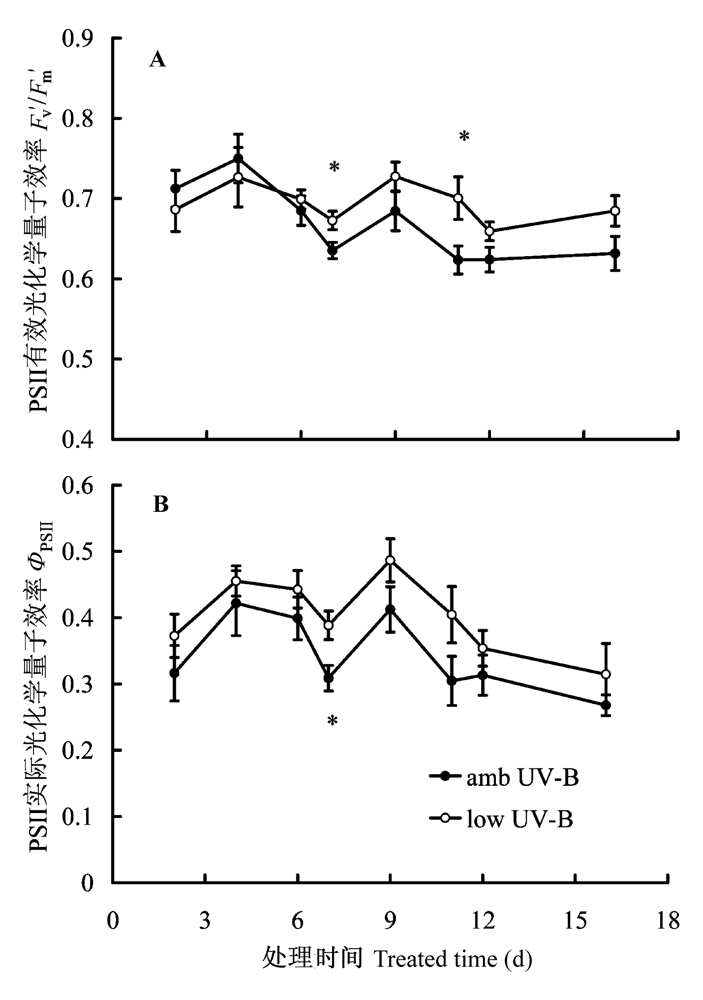
图3 2008年8月滤除自然光中UV-B辐射成分处理对美丽风毛菊叶片稳态PSII光化学效率的影响。垂直条表示标准误差。*, p < 0.05。
Fig. 3 Effects of removal of UV-B components from natural sunlight on light adapted photochemistry efficiency of PSII in Saussurea superba in August 2008. Fv′/Fm′, photochemical efficiency of PSII in the light; ΦPSII, actual photochemical efficiency of PSII. Vertical bar is SE. *, p < 0.05.
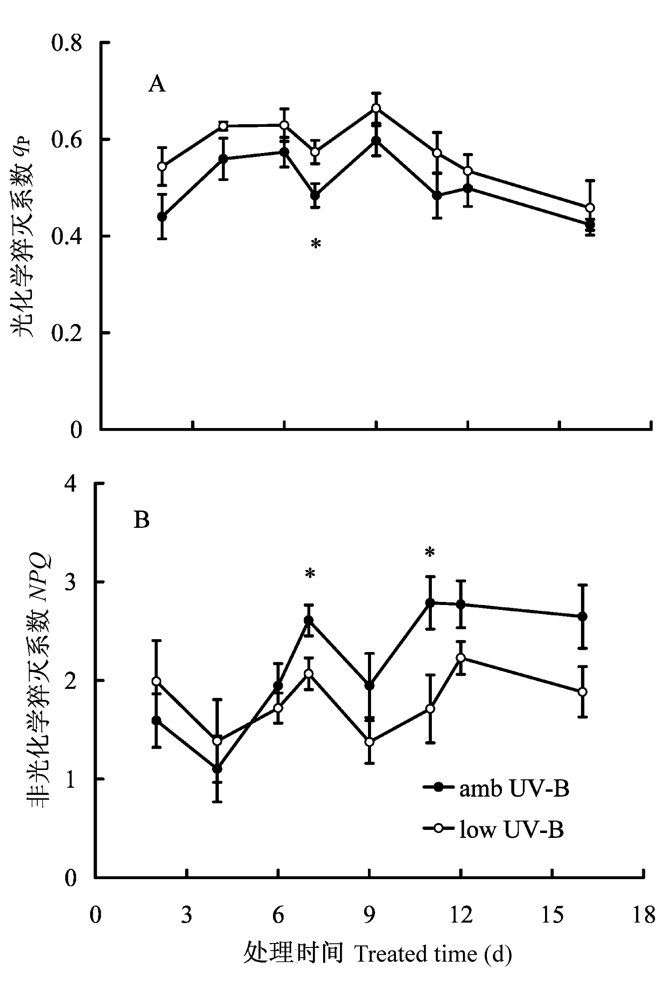
图4 2008年8月滤除自然光中UV-B辐射成分处理对美丽风毛菊叶片光化学和非光化学猝灭系数的影响。垂直条表示标准误差。*, p < 0.05。
Fig. 4 Effects of removal of UV-B components from natural sunlight on photochemical and non-photochemical quenching coefficient in Saussurea superba in August 2008. NPQ, nonphotochemical quenching; qp, the coefficient of photochemical quenching. Vertical bar is SE. *, p < 0.05.
| amb UV-B | low UV-B | 差异显著性 Significant | ||
|---|---|---|---|---|
| 叶绿素a Chl a | mg·g-1 (FW) | 1.068 1 ± 0.029 1 | 1.117 9 ± 0.094 8 | 0.645 |
| μg·cm-2 (leaf area) | 0.034 4 ± 0.000 8 | 0.038 8 ± 0.003 6 | 0.304 | |
| 叶绿素b Chl b | mg·g-1 (FW) | 0.324 7 ± 0.011 6 | 0.337 3 ± 0.024 1 | 0.655 |
| μg·cm-2 (leaf area) | 0.010 5 ± 0.000 4 | 0.011 7 ± 0.000 9 | 0.276 | |
| 总叶绿素 Chl a+b | mg·g-1 (FW) | 1.392 9 ± 0.040 1 | 1.455 2 ± 0.118 7 | 0.637 |
| μg·cm-2 (leaf area) | 0.044 8 ± 0.001 2 | 0.050 5 ± 0.004 5 | 0.297 | |
| 类胡萝卜素 Car | mg·g-1 (FW) | 0.312 4 ± 0.011 9 | 0.335 3 ± 0.039 5 | 0.599 |
| μg·cm-2 (leaf area) | 0.010 1 ± 0.000 5 | 0.011 7 ± 0.001 5 | 0.363 | |
| Car/Chl a+b | 0.224 5 ± 0.008 3 | 0.228 4 ± 0.010 1 | 0.784 | |
| Chl a/b | 3.292 3 ± 0.046 5 | 3.305 1 ± 0.052 8 | 0.861 |
表2 2008年8月滤除自然光中UV-B成分处理对美丽风毛菊叶片光合色素的影响(平均值±标准误差, n = 15; p = 0.05)
Table 2 Effects of removal of UV-B component from natural sunlight on photosynthetic pigment contents in Saussurea superba in August 2008 (mean ± SE, n = 5; p = 0.05)
| amb UV-B | low UV-B | 差异显著性 Significant | ||
|---|---|---|---|---|
| 叶绿素a Chl a | mg·g-1 (FW) | 1.068 1 ± 0.029 1 | 1.117 9 ± 0.094 8 | 0.645 |
| μg·cm-2 (leaf area) | 0.034 4 ± 0.000 8 | 0.038 8 ± 0.003 6 | 0.304 | |
| 叶绿素b Chl b | mg·g-1 (FW) | 0.324 7 ± 0.011 6 | 0.337 3 ± 0.024 1 | 0.655 |
| μg·cm-2 (leaf area) | 0.010 5 ± 0.000 4 | 0.011 7 ± 0.000 9 | 0.276 | |
| 总叶绿素 Chl a+b | mg·g-1 (FW) | 1.392 9 ± 0.040 1 | 1.455 2 ± 0.118 7 | 0.637 |
| μg·cm-2 (leaf area) | 0.044 8 ± 0.001 2 | 0.050 5 ± 0.004 5 | 0.297 | |
| 类胡萝卜素 Car | mg·g-1 (FW) | 0.312 4 ± 0.011 9 | 0.335 3 ± 0.039 5 | 0.599 |
| μg·cm-2 (leaf area) | 0.010 1 ± 0.000 5 | 0.011 7 ± 0.001 5 | 0.363 | |
| Car/Chl a+b | 0.224 5 ± 0.008 3 | 0.228 4 ± 0.010 1 | 0.784 | |
| Chl a/b | 3.292 3 ± 0.046 5 | 3.305 1 ± 0.052 8 | 0.861 |
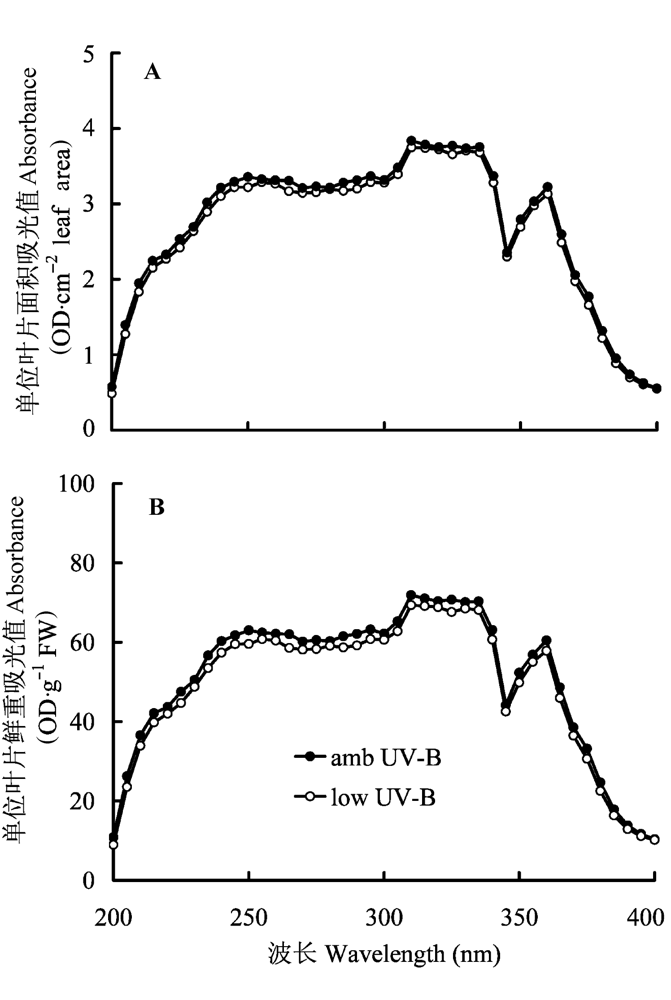
图5 2008年8月滤除自然光中UV-B辐射成分处理对美丽风毛菊叶片中紫外线吸收物质的影响。
Fig. 5 Effects of removal of UV-B components from natural sunlight on UV-B-absorbing compounds in Saussurea superba in August 2008.
| [1] | Baker NR (1996). Photoinhibition of photosynthesis. In: Jennings RC, Zucchelli G, Ghetti F, Colombetti G eds. Light as Energy Source and Information Carrier in Plant Physiology. Plenum Press, New York. 89-96. |
| [2] |
Baker NR (2008). Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology, 59, 89-113.
DOI URL PMID |
| [3] | Beggs CG, Wellmann E (1994). Photocontrol of flavonoid biosynthesis. In: Kendrick RE, Kronenberg GHM eds. Photomorphogenesis in Plants. Volume 2. Kluwer Academic 733-750. |
| [4] |
Bilger W, Björkman O (1990). Role of the xanthophyll cycle photoprotection elucidated by measurements of light- induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research, 25, 173-185.
DOI URL PMID |
| [5] | Björn LO (1999). Ultraviolet-B radiation, the ozone layer and ozonedepletion. In: Rozema J ed. Stratospheric Ozone Depletion: the Effects of Enhanced UV-B Radiation on Terrestrial Ecosystems. Backhuys Publishers, Leiden, The Netherlands. 21-27. |
| [6] | Caldwell MM, Flint SD (1994). Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystem. Climate Change, 28, 375-394. |
| [7] | Costa H, Gallego SM, Tomaro ML (2002). Effects of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Science, 162, 939-945. |
| [8] | Day TA, Neale PJ (2002). Effects of UV-B radiation on terrestrial and aquatic primary producers. Annual Review of Ecological System, 33, 371-396. |
| [9] | Fedina I, Georgieva K, Velitchkova M, Grigorova I (2006). Effect of pretreatment of barley seedlings with different salts on the level of UV-B induced and UV-B absorbing compounds. Environmental and Experimental Botany, 56, 225-230. |
| [10] |
Fiscus EL, Booker FL (1995). Is increased UV-B a threat to crop photosynthesis and productivity? Photosynthesis Research, 43, 81-92.
DOI URL PMID |
| [11] | Fiscus EL, Philbeck R, Britt AB, Booker FL (1999). Growth of Arabidopsis flavonoid mutants under solar radiation and UV filter. Environmental and Experimental Botany, 41, 231-245. |
| [12] | Flint SD, Ryel RJ, Caldwell MM (2003). Ecosystem UV-B experiments in terrestrial communities: a review of recent findings and methodologies. Agricultural and Forest Meteorology, 120, 177-189. |
| [13] |
Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smimoff N, Asami T, Davies WJ, Jones AM, Baker NR, Mullineaux PM (2009). The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. The Plant Cell, 21, 2143-2162.
DOI URL PMID |
| [14] | Gassi-Lit M, Whitecross MJ, Nayudu M, Tanner GJ (1997). UV-B irradiation induces differential leaf damage, ultra-structural changes and accumulation of specific phenolic compounds in rice cultivars. Australian Journal of Plant Physiology, 24, 261-274. |
| [15] | Genty B, Briantais JM, Baker NR (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta, 990, 87-92. |
| [16] | Jansen MAK, Gaba V, Greenberg BM (1998). Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends in Plant Science, 3, 131-135. |
| [17] | Lau TSL, Eno E, Goldstein G, Smith C, Christopher DA (2006). Ambient levels of UV-B in Hawaii combined with nutrient deficiency decrease photosynthesis in near- isogenic maize lines varying in leaf flavonoids: Flavonoids decrease photoinhibition in plants exposed to UV-B. Photosynthetica, 44, 394-403. |
| [18] | Lizana XC, Hess S, Calderini DF (2009). Crop phenology modifies wheat responses to increased UV-B radiation. Agricultural and Forest Meteorology, 149, 1964-1974. |
| [19] | Madronich S, McKenzie RL, Caldwell MM, Björn LO (1995). Changes in ultraviolet radiation reaching the earth’s surface. Ambio, 24, 143-152. |
| [20] | Middleton EM, Teramura AH (1993). The role of flavonol glycoside and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiology, 103, 475-480. |
| [21] | Oxborough K, Baker NR (1997). Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components-calculation of qP and Fv′/Fm′ without measuring F0′. Photosynthesis Research, 54, 135-142. |
| [22] | Paul ND, Gwynn-Jones D (2003). Ecological roles of solar UV radiation: towards an integrated approach. Trends in Ecology and Evolution, 18, 48-55. |
| [23] | Petropoulou Y, Georgiou O, Psaras GK, Manetas Y (2001). The growth, flower properties and demography of Anthemis arvensis exposed to enhanced UV-B radiation. Plant Ecology, 154, 59-64. |
| [24] | Pinto ME, Casati P, Hus TP, Ku MSB, Edwards GE (1999). Effects of UV-B radiation on growth, photosynthesis, UV-B-absorbing compounds and NADP-malic enzyme in bean ( Phaseolus vulgaris L.) grown under different nitrogen condition. Journal of Photochemistry and Photobiology B: Biology, 48, 200-209. |
| [25] | Quick WP, Stitt M (1989). An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochimica et Biophysica Acta, 977, 287-296. |
| [26] | Rozema J, Björn LO, Bornman JF, Gaberščik A, Häder DP, Trošt T, Germ M, Klisch M, Gröniger A, Sinha RP, Lebert M, He YY, Buffoni-Hall R, de Bakker NVJ, van de Staaij J, Meijkamp BB (2002). The role of UV-B radiation in aquatic and terrestrial ecosystem—an experimental and functional analysis of evolution of UV-absorbing compounds. Journal of Photochemistry and Photobiology B: Biology, 66, 2-12. |
| [27] |
Shi SB, Zhu WY, Li HM, Zhou DW, Han F, Zhao XQ, Tang YH (2004). Photosynthesis of Saussurea superba and Gentiana straminea is not reduced after long-term enhancement of UV-B radiation. Environmental and Experimental Botany, 51, 75-83.
DOI URL |
| [28] |
Sicora C, Máté Z, Vass I (2003). The interaction of visible and UV-B light during photodamage and repair of photosystem II. Photosynthesis Research, 75, 127-137.
DOI URL |
| [29] | Sicora C, Szilárd A, Sass L, Turcsányi E, Máté Z, Vass I (2006). UV-B and UV-A radiation effects on photosynthesis at the molecular level. In: Ghetti F, Checcucci G, Bornman JF eds. Environmental UV Radiation: Impact on Ecosystem and Human Health and Predictive Model. Springer, The Netherlands. 121-135. |
| [30] |
Šprtová M, Špunda V, Kalina J, Marek MV (2003). Photosynthetic UV-B response of beach ( Fagus sylvatica L.) saplings. Photosynthetic, 41, 533-543.
DOI URL |
| [31] | Strid A, Porra RJ (1992). Alterations in pigments content in leaves of Pisum sativum after expose to supplementary UV-B. Plant Cell Physiology, 33, 1015-1023. |
| [32] | van Rensen JJ, Vredenberg WJ, Rodrigues GC (2007). Time sequence damage to the acceptor and donor sides of photosystem II by UV-B radiation as evaluated by chlorophyll a fluorescence. Photosynthesis Research, 94, 219-297. |
| [33] | Wang GH, Hao ZJ, Anken RH, Lu JY, Liu YD (2010). Effects of UV-B radiation on photosynthesis activity of Wolffia arrhiza as probed by chlorophyll fluorescence transients. Advances in Space Research, 45, 839-845 |
| [34] | Xu DQ (许大全) (2002). Photosynthetic Efficiency (光合作用效率). Shanghai Scientific and Technical Publishers, Shanghai. (in Chinese) |
| [35] | Zhang SR (张守仁) (1999). A discussion on chlorophyll fluorescence kinetics parameters and their significance. Chinese Bulletin of Botany (植物学通报), 16, 444-448. (in Chinese with English abstract) |
| [36] | Zhu GL (朱广廉), Zhong HW (钟诲文), Zhang AQ (张爱琴) (1990). The Plant Physiological Experiment (植物生理学实验). Beijing University Press, Beijing. 51-54. (in Chinese) |
| [37] | Ziska LH, Termura AH, Sullivan JH (1992). Physiological sensitivity of plants along an elevational gradient to UV-B radiation. American Journal of Botany, 79, 863-871. |
| [1] | 赵艳超, 陈立同. 土壤养分对青藏高原高寒草地生物量响应增温的调节作用[J]. 植物生态学报, 2023, 47(8): 1071-1081. |
| [2] | 任培鑫, 李鹏, 彭长辉, 周晓路, 杨铭霞. 洞庭湖流域植被光合物候的时空变化及其对气候变化的响应[J]. 植物生态学报, 2023, 47(3): 319-330. |
| [3] | 师生波, 周党卫, 李天才, 德科加, 杲秀珍, 马家麟, 孙涛, 王方琳. 青藏高原高山嵩草光合功能对模拟夜间低温的响应[J]. 植物生态学报, 2023, 47(3): 361-373. |
| [4] | 师生波, 师瑞, 周党卫, 张雯. 低温对高山嵩草叶片光化学和非光化学能量耗散特征的影响[J]. 植物生态学报, 2023, 47(10): 1441-1452. |
| [5] | 林马震, 黄勇, 李洋, 孙建. 高寒草地植物生存策略地理分布特征及其影响因素[J]. 植物生态学报, 2023, 47(1): 41-50. |
| [6] | 朱玉英, 张华敏, 丁明军, 余紫萍. 青藏高原植被绿度变化及其对干湿变化的响应[J]. 植物生态学报, 2023, 47(1): 51-64. |
| [7] | 魏瑶, 马志远, 周佳颖, 张振华. 模拟增温改变青藏高原植物繁殖物候及植株高度[J]. 植物生态学报, 2022, 46(9): 995-1004. |
| [8] | 金伊丽, 王皓言, 魏临风, 侯颖, 胡景, 吴铠, 夏昊钧, 夏洁, 周伯睿, 李凯, 倪健. 青藏高原植物群落样方数据集[J]. 植物生态学报, 2022, 46(7): 846-854. |
| [9] | 卢晶, 马宗祺, 高鹏斐, 樊宝丽, 孙坤. 祁连山区演替先锋物种西藏沙棘的种群结构及动态对海拔梯度的响应[J]. 植物生态学报, 2022, 46(5): 569-579. |
| [10] | 张玉林, 尹本丰, 陶冶, 李永刚, 周晓兵, 张元明. 早春首次降雨时间及降雨量对古尔班通古特沙漠两种短命植物形态特征与叶绿素荧光的影响[J]. 植物生态学报, 2022, 46(4): 428-439. |
| [11] | 胡潇飞, 魏临风, 程琦, 吴星麒, 倪健. 青藏高原地区气候图解数据集[J]. 植物生态学报, 2022, 46(4): 484-492. |
| [12] | 吴赞, 彭云峰, 杨贵彪, 李秦鲁, 刘洋, 马黎华, 杨元合, 蒋先军. 青藏高原高寒草地退化对土壤及微生物化学计量特征的影响[J]. 植物生态学报, 2022, 46(4): 461-472. |
| [13] | 郑周涛, 张扬建. 1982-2018年青藏高原水分利用效率变化及归因分析[J]. 植物生态学报, 2022, 46(12): 1486-1496. |
| [14] | 吴霖升, 张永光, 章钊颖, 张小康, 吴云飞. 日光诱导叶绿素荧光遥感及其在陆地生态系统监测中的应用[J]. 植物生态学报, 2022, 46(10): 1167-1199. |
| [15] | 薛金儒, 吕肖良. 黄土高原生态工程实施下基于日光诱导叶绿素荧光的植被恢复生产力效益评价[J]. 植物生态学报, 2022, 46(10): 1289-1304. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19