

植物生态学报 ›› 2023, Vol. 47 ›› Issue (10): 1407-1421.DOI: 10.17521/cjpe.2022.0276
所属专题: 植物功能性状
收稿日期:2022-07-04
接受日期:2023-03-13
出版日期:2023-10-20
发布日期:2023-11-23
通讯作者:
* (基金资助:
CHEN Tu-Qiang, XU Gui-Qing( ), LIU Shen-Si, LI Yan
), LIU Shen-Si, LI Yan
Received:2022-07-04
Accepted:2023-03-13
Online:2023-10-20
Published:2023-11-23
Contact:
* (Supported by:摘要:
梭梭(Haloxylon ammodendron)是古尔班通古特沙漠的主要建群种, 在生物多样性保护和防止旱地退化等生态系统服务方面有重要作用。气候变化引起的频发干旱对梭梭生存有显著的影响, 明晰干旱胁迫下梭梭的抗旱策略, 对于荒漠生态系统的可持续发展至关重要。水力性状和碳收益作为抗旱机制中的重要部分, 目前对干旱胁迫下梭梭生存的水力性状阈值尚不明确。该研究以成年梭梭为对象, 分别设置对照组和干旱处理组, 对梭梭上、中、下3个高度的同化枝水分状况、枝条木质部导度损失率、气体交换特征、非结构性碳水化合物含量和形态特征等进行了测定, 利用单因素方差分析检验不同处理及枝条高度间的各项性状差异, 结合线性回归了解梭梭气孔敏感性, 通过主成分分析解析梭梭的抗旱策略。研究表明: (1)梭梭的黎明和正午同化枝水势、同化枝含水量和枝条含水量均因干旱胁迫而下降, 但并未随高度增加而降低; P50和P88 (最大导水度损失50%和88%的木质部水势)未因干旱胁迫和枝条高度的增加显著变化, 两个处理下3个枝条高度的P50平均值为-4.12 MPa, P88为-7.10 MPa, 而水力安全边界在干旱胁迫下显著降低; (2)梭梭的气孔行为对水分亏缺敏感性低, 干旱胁迫和枝条高度增加总体上未对其净光合速率和气孔导度产生显著影响; (3)同化枝和枝条非结构性碳水化合物含量未因干旱胁迫和枝条高度的增加而降低, 反而略有升高, 干旱胁迫下同化枝和枝条非结构性碳水化合物含量相较对照组分别升高22.11%和13.10%; (4)梭梭在干旱胁迫下的胡伯尔值相较对照组升高73.78%; 比叶面积相较对照组降低14.60%, 但两者均与对照组无显著差异。总之, 梭梭的水力性状受干旱胁迫影响显著, 但不受枝条高度的影响, 并不存在随枝条高度增加的水力限制; 干旱胁迫下, 梭梭树冠外缘枝条同时发生水力失效的风险较大, 水力安全边界(正午同化枝水势与P88的差值)只有对照组的40.85%; 但由于梭梭气孔对水分亏缺的低敏感性, 这使得其光合固碳并未受到影响, 反而同化枝和枝条的非结构性碳水化合物含量会有所升高。
陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态. 植物生态学报, 2023, 47(10): 1407-1421. DOI: 10.17521/cjpe.2022.0276
CHEN Tu-Qiang, XU Gui-Qing, LIU Shen-Si, LI Yan. Hydraulic traits adjustments and nonstructural carbohydrate dynamics of Haloxylon ammodendron under drought stress. Chinese Journal of Plant Ecology, 2023, 47(10): 1407-1421. DOI: 10.17521/cjpe.2022.0276
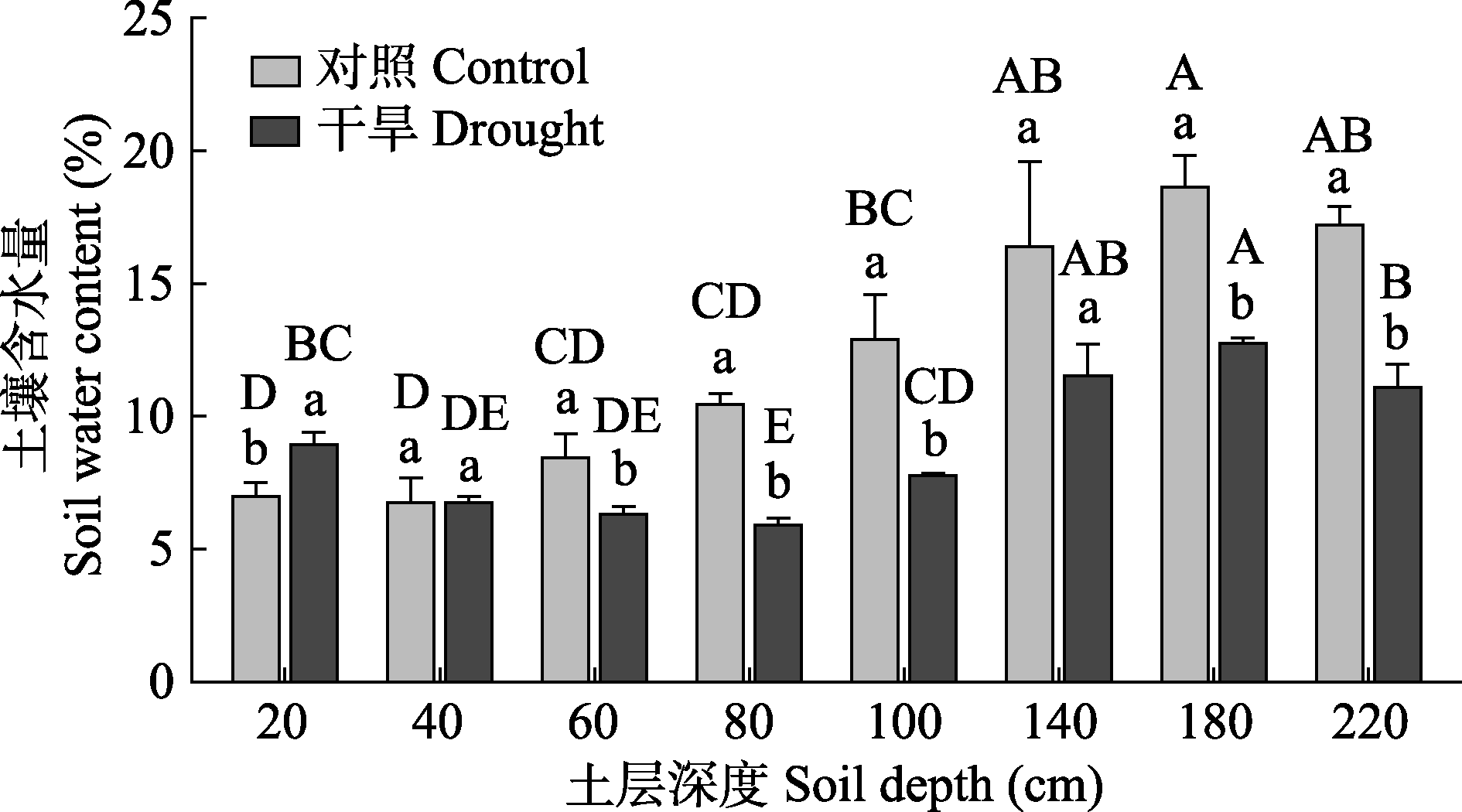
图1 梭梭样地不同土层深度的土壤含水量(平均值±标准误)。不同大写字母表示同一处理不同土层深度存在显著差异(p < 0.05), 不同小写字母表示同一土层深度不同处理间差异显著(p < 0.05)。
Fig. 1 Soil water content at different depths in Haloxylon ammodendron sampling plot (mean ± SE). Different uppercase letters indicate significant differences among soil depths of the same treatment (p < 0.05), and different lowercase letters indicate significant differences between treatments of the same soil depth (p < 0.05).
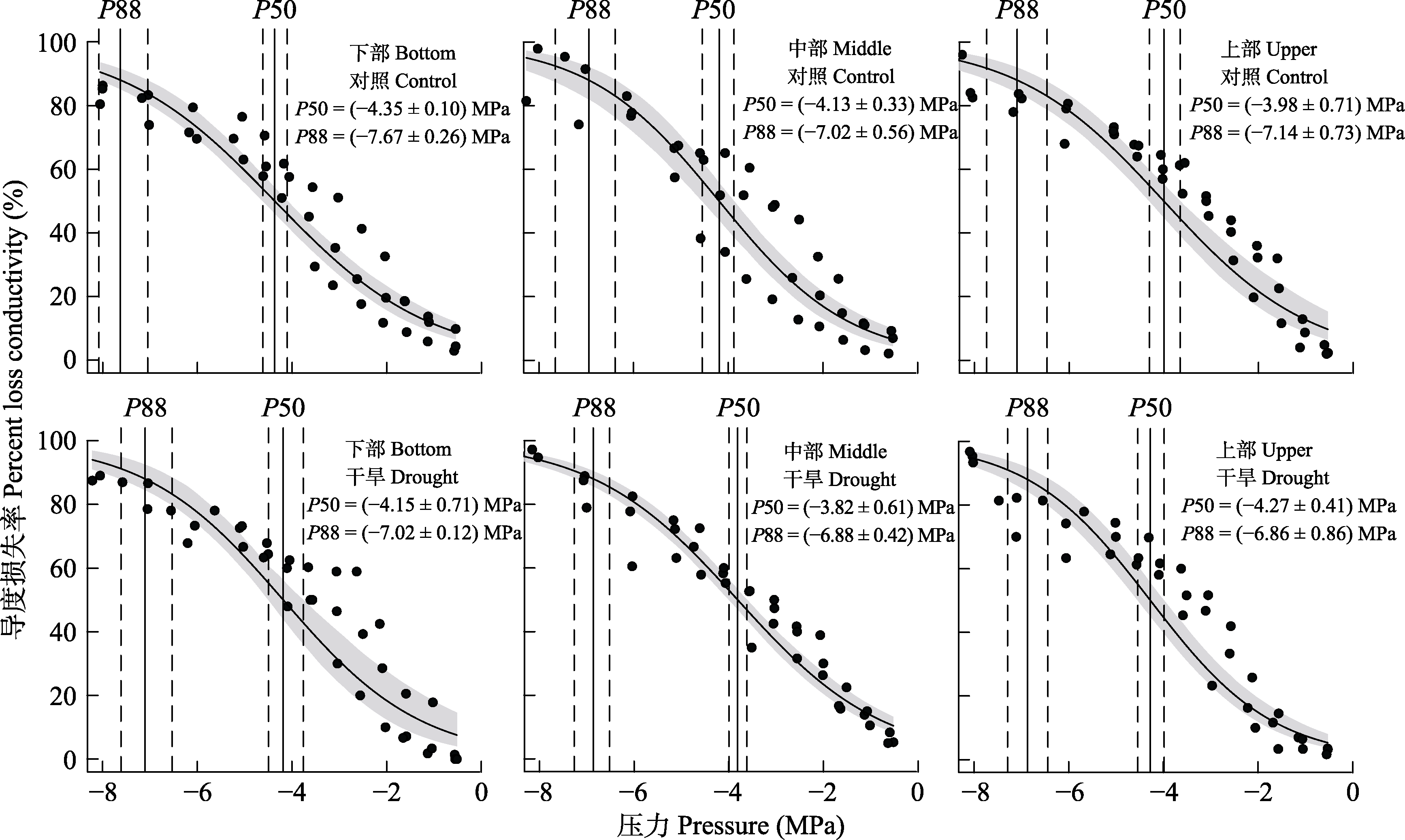
图2 梭梭木质部导度损失率曲线。垂直的实线分别表示最大导水度损失50%和88%时木质部受到的压力(即木质部的水势) (P50、P88); 虚线和阴影为95%的置信区间。
Fig. 2 Percent loss of xylem conductivity for Haloxylon ammodendron. The vertical solid lines indicate the pressure on the xylem (i.e. the water potential of the xylem) for 50% and 88% loss of maximum hydraulic conductivity (P50, P88), respectively; the dashed lines and shaded areas are 95% confidence intervals.
| P50 (MPa) | P88 (MPa) | |||||
|---|---|---|---|---|---|---|
| 下部 Bottom | 中部 Middle | 上部 Upper | 下部 Bottom | 中部 Middle | 上部 Upper | |
| 对照组 Control | -4.35 ± 0.10Aa | -4.13 ± 0.33Aa | -3.98 ± 0.17Aa | -7.67 ± 0.26Aa | -7.02 ± 0.56Aa | -7.10 ± 0.07Aa |
| 干旱组 Drought | -4.15 ± 0.17Aa | -3.82 ± 0.16Aa | -4.27 ± 0.14Aa | -7.03 ± 0.21Aa | -6.88 ± 0.24Aa | -6.86 ± 0.09Aa |
表1 对照组和干旱处理组梭梭上、中和下部枝条最大导水度损失50%和88%的木质部水势(P50和P88) (平均值±标准误)
Table 1 Xylem water potential for 50% and 88% loss of maximum hydraulic conductivity (P50, P88) in upper, middle and lower branches of control and drought-treated Haloxylon ammodendron (mean ± SE)
| P50 (MPa) | P88 (MPa) | |||||
|---|---|---|---|---|---|---|
| 下部 Bottom | 中部 Middle | 上部 Upper | 下部 Bottom | 中部 Middle | 上部 Upper | |
| 对照组 Control | -4.35 ± 0.10Aa | -4.13 ± 0.33Aa | -3.98 ± 0.17Aa | -7.67 ± 0.26Aa | -7.02 ± 0.56Aa | -7.10 ± 0.07Aa |
| 干旱组 Drought | -4.15 ± 0.17Aa | -3.82 ± 0.16Aa | -4.27 ± 0.14Aa | -7.03 ± 0.21Aa | -6.88 ± 0.24Aa | -6.86 ± 0.09Aa |
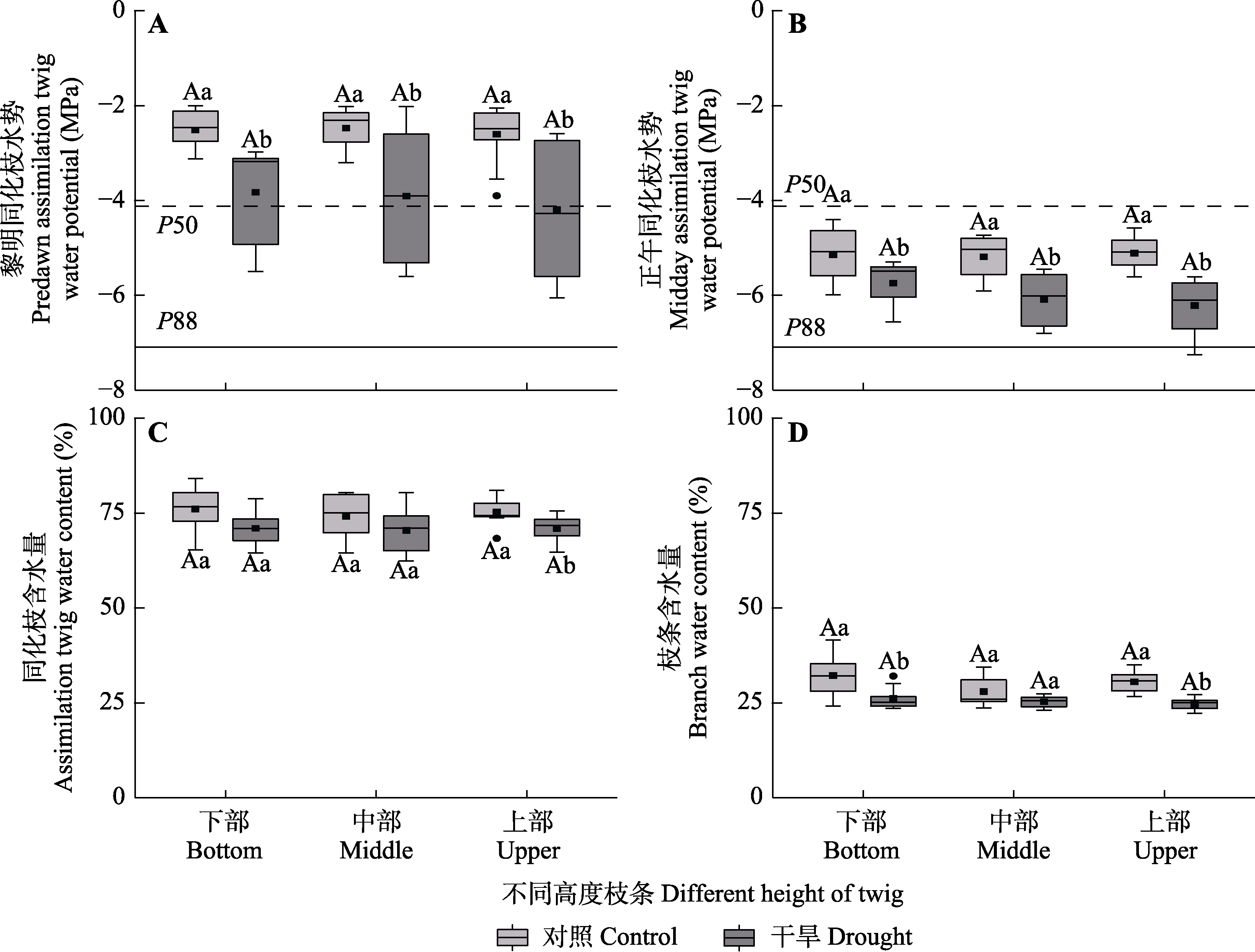
图3 对照组和干旱处理组梭梭不同高度枝条的黎明同化枝水势、正午同化枝水势、同化枝含水量和枝条含水量(平均值±标准误)。P50和P88分别为最大导水度损失50%和88%的木质部水势。不同大写字母表示同一处理不同高度水平间存在显著差异(p < 0.05), 不同小写字母表示同一高度水平不同处理间差异显著(p < 0.05)。
Fig. 3 Predawn assimilation twig water potential, midday assimilation twig water potential, twig water content and branch water content among different heights of Haloxylon ammodendron in control and drought treatment groups (mean ± SE). P50 and P88 are the xylem water potentials for 50% and 88% loss of maximum hydraulic conductivity, respectively. Different uppercase letters indicate significant differences among different heights of the same treatment (p < 0.05) and different lowercase letters indicate significant differences between different treatments of the same height (p < 0.05).
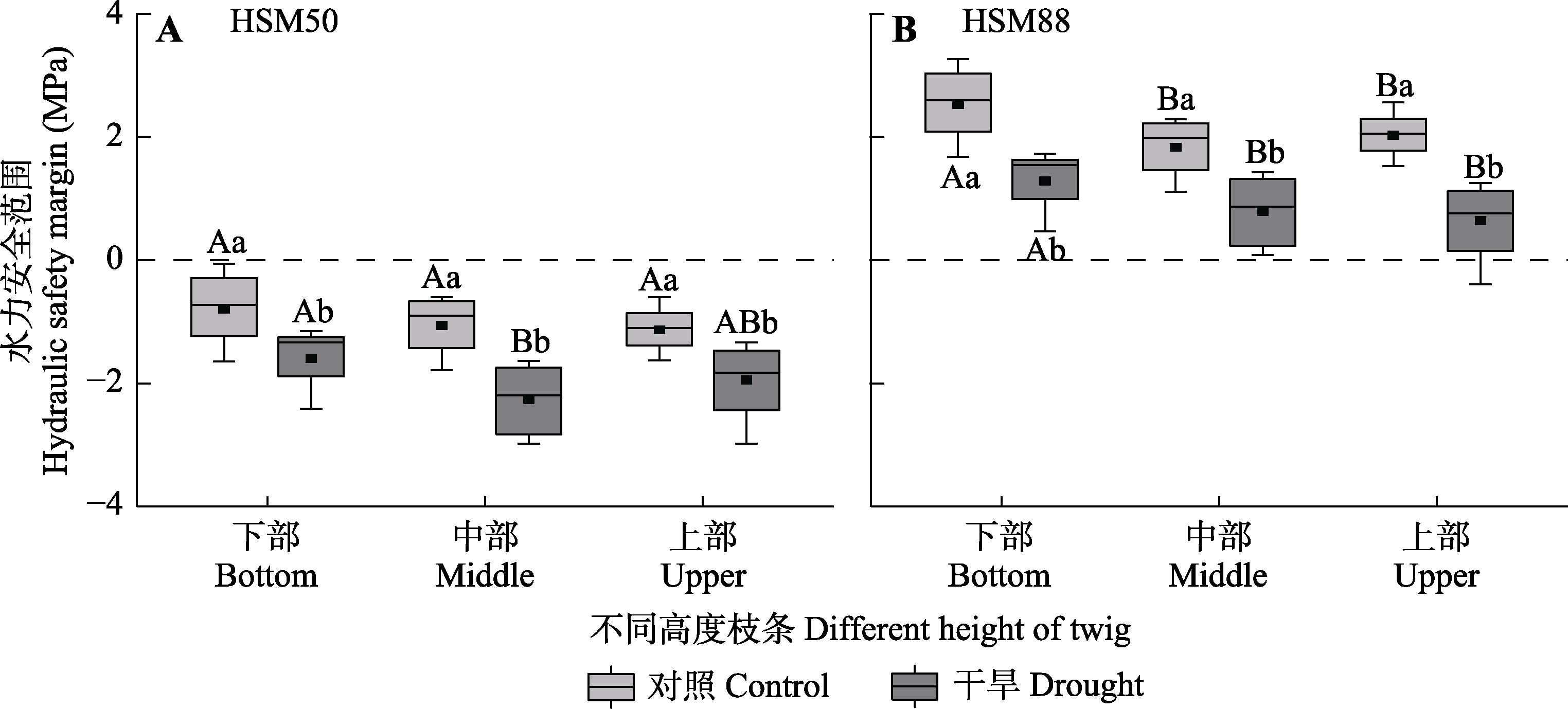
图4 对照组和干旱处理组梭梭不同高度枝条的水力安全边界(平均值±标准误)。HSM50和HSM88分别为正午同化枝水势与最大导水度损失50%和88%的木质部水势之差。不同大写字母表示同一处理不同高度水平间存在显著差异(p < 0.05), 不同小写字母表示同一高度水平不同处理间差异显著(p < 0.05)。
Fig. 4 Hydraulic safety margin among different heights of Haloxylon ammodendron in control and drought treatment groups (mean ± SE). HSM50 and HSM88 are the difference between the midday assimilation twig water potential and the xylem water potential for 50% and 88% loss of maximum hydraulic conductivity, respectively. Different uppercase letters indicate significant differences among different heights of the same treatment (p < 0.05) and different lowercase letters indicate significant differences between different treatments of the same height (p < 0.05).
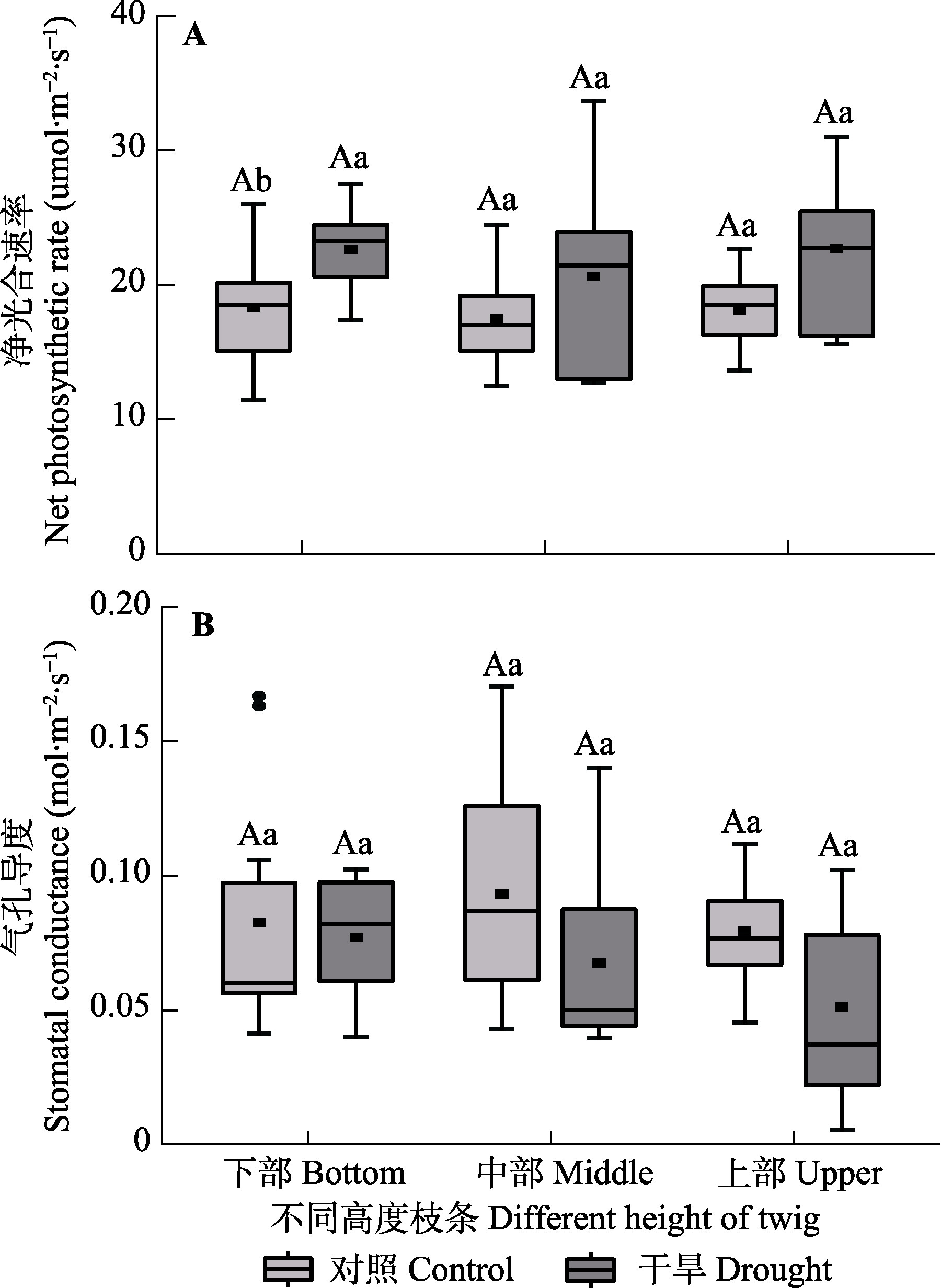
图5 对照组和干旱处理组梭梭不同高度枝条的净光合速率和气孔导度(平均值±标准误)。不同大写字母表示同一处理不同高度水平间存在显著差异(p < 0.05), 不同小写字母表示同一高度水平不同处理间差异显著(p < 0.05)。
Fig. 5 Net photosynthetic rate and stomatal conductance among different heights of Haloxylon ammodendron in control and drought treatment groups (mean ± SE). Different uppercase letters indicate significant differences among different heights of the same treatment (p < 0.05) and different lowercase letters indicate significant differences between different treatments of the same height (p < 0.05).
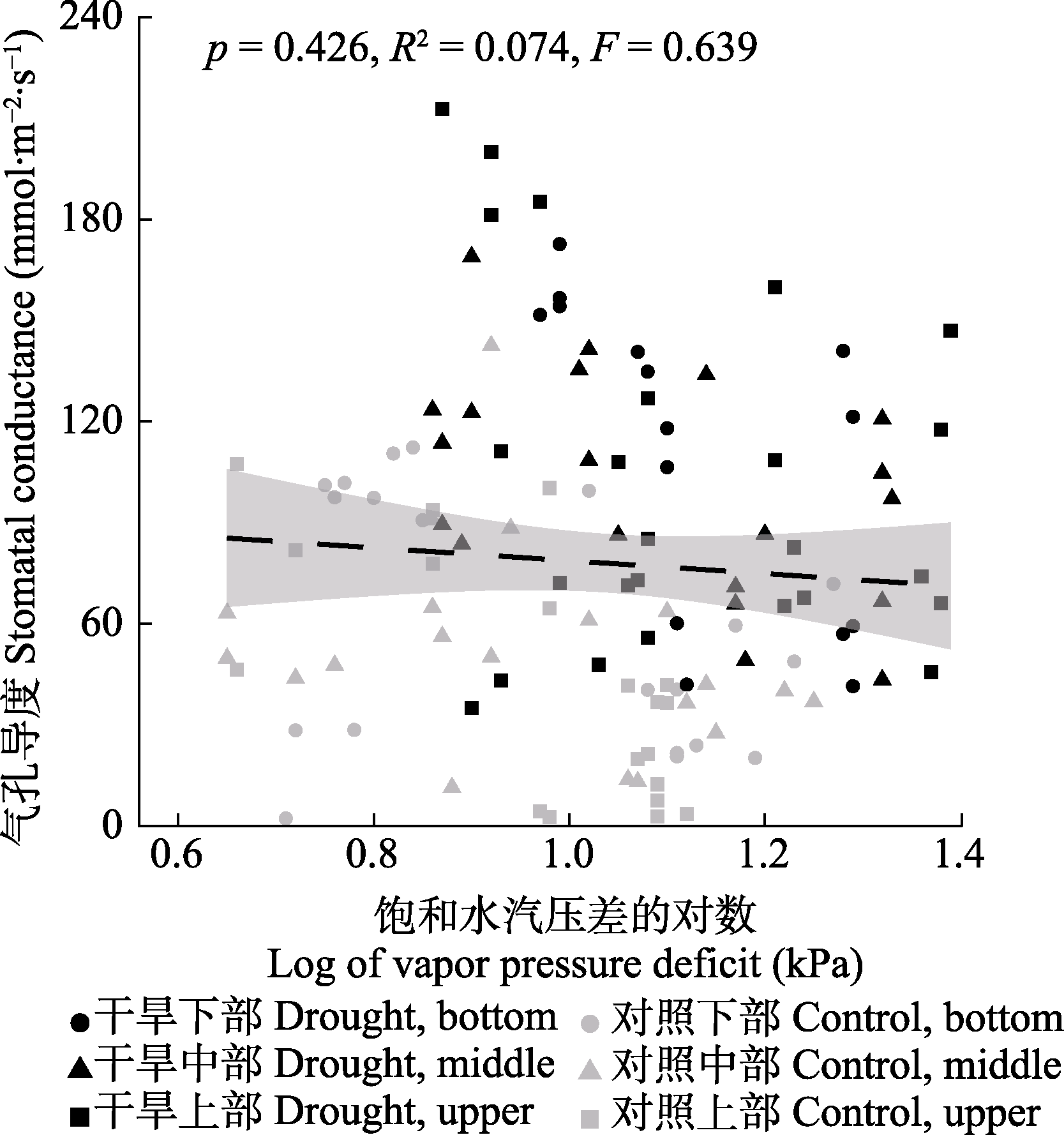
图6 梭梭气孔导度对饱和水汽压差的响应。阴影为95%的置信区间。
Fig. 6 Response of Haloxylon ammodendron stomatal conductance to vapor pressure deficit. The shaded area are 95% confidence intervals.
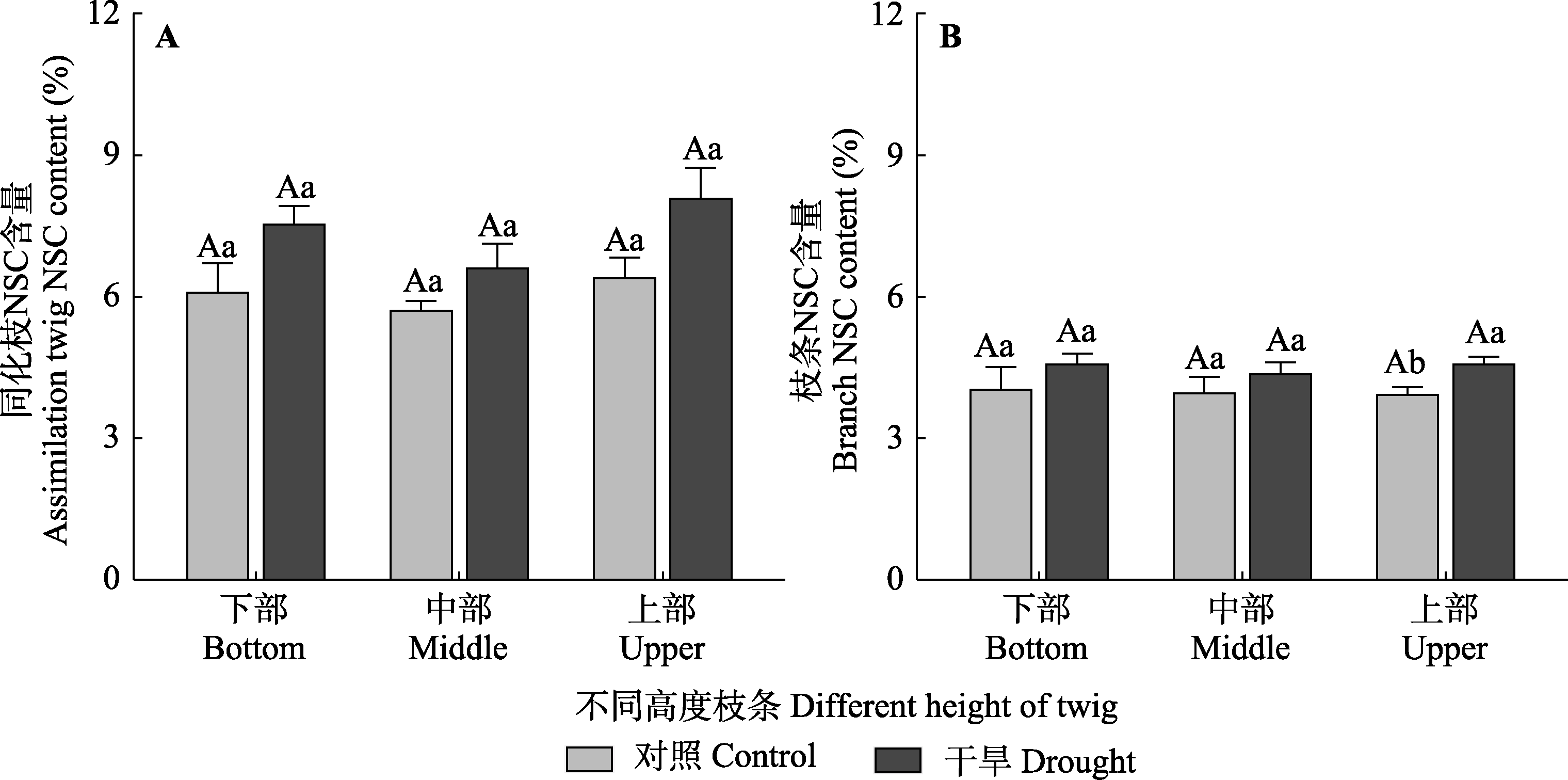
图7 对照组和干旱处理组梭梭不同高度枝条的同化枝和枝条的非结构性碳水化合物(NSC)含量(平均值±标准误)。不同大写字母表示同一处理不同高度水平间存在显著差异(p < 0.05), 不同小写字母表示同一高度水平不同处理间差异显著(p < 0.05)。
Fig. 7 Nonstructural carbohydrate (NSC) content of assimilation twigs and branches among different heights of Haloxylon ammodendron in control and drought treatment groups (mean ± SE). Different uppercase letters indicate significant differences among different heights of the same treatment (p < 0.05) and different lowercase letters indicate significant differences between different treatments of the same height (p < 0.05).
| 处理 Treatment | 高度 Height | 同化枝 Assiassimilation twig | 枝条 Branch | ||
|---|---|---|---|---|---|
| 可溶性糖 Soluble sugar (%) | 淀粉 Starch (%) | 可溶性糖 Soluble sugar (%) | 淀粉 Starch (%) | ||
| 对照 Control | 下部 Bottom | 2.48 ± 0.45Ab | 3.61 ± 0.62Aa | 2.62 ± 0.70Aa | 1.41 ± 0.17Aa |
| 中部 Middle | 2.57 ± 0.14Ab | 3.13 ± 0.30Aa | 2.21 ± 0.39Ab | 1.75 ± 0.28Aa | |
| 上部 Upper | 2.83 ± 0.24Ab | 3.57 ± 0.59Aa | 2.22 ± 0.20Ab | 1.71 ± 0.29Aa | |
| 干旱 Drought | 下部 Bottom | 3.81 ± 0.37Aa | 3.72 ± 0.56Aa | 3.01 ± 0.27Aa | 1.56 ± 0.07Aa |
| 中部 Middle | 3.48 ± 0.58Aa | 3.12 ± 0.59Aa | 3.01 ± 0.14Aa | 1.34 ± 0.33Aa | |
| 上部 Upper | 4.43 ± 0.49Aa | 3.65 ± 0.71Aa | 3.03 ± 0.19Aa | 1.53 ± 0.13Aa | |
表2 对照组和干旱处理组梭梭不同高度枝条的同化枝和枝条的可溶性糖和淀粉含量(平均值±标准误)
Table 2 Soluble sugar and starch contents of assimilation twigs and branches among different heights of Haloxylon ammodendron in control and drought treatment groups (mean ± SE)
| 处理 Treatment | 高度 Height | 同化枝 Assiassimilation twig | 枝条 Branch | ||
|---|---|---|---|---|---|
| 可溶性糖 Soluble sugar (%) | 淀粉 Starch (%) | 可溶性糖 Soluble sugar (%) | 淀粉 Starch (%) | ||
| 对照 Control | 下部 Bottom | 2.48 ± 0.45Ab | 3.61 ± 0.62Aa | 2.62 ± 0.70Aa | 1.41 ± 0.17Aa |
| 中部 Middle | 2.57 ± 0.14Ab | 3.13 ± 0.30Aa | 2.21 ± 0.39Ab | 1.75 ± 0.28Aa | |
| 上部 Upper | 2.83 ± 0.24Ab | 3.57 ± 0.59Aa | 2.22 ± 0.20Ab | 1.71 ± 0.29Aa | |
| 干旱 Drought | 下部 Bottom | 3.81 ± 0.37Aa | 3.72 ± 0.56Aa | 3.01 ± 0.27Aa | 1.56 ± 0.07Aa |
| 中部 Middle | 3.48 ± 0.58Aa | 3.12 ± 0.59Aa | 3.01 ± 0.14Aa | 1.34 ± 0.33Aa | |
| 上部 Upper | 4.43 ± 0.49Aa | 3.65 ± 0.71Aa | 3.03 ± 0.19Aa | 1.53 ± 0.13Aa | |
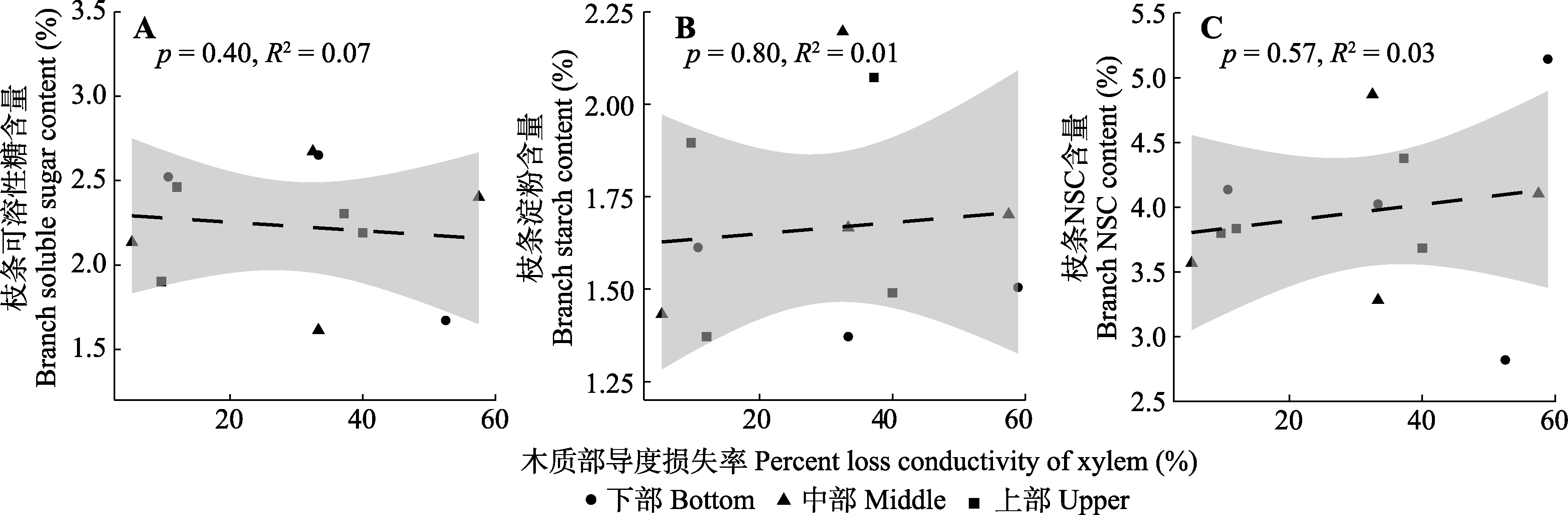
图8 干旱处理组梭梭枝条木质部导度损失率与枝条非结构性碳水化合物(NSC)含量之间的关系。
Fig. 8 Relationship between branch xylem hydraulic conductivity loss rate and nonstructural carbohydrate (NSC) content of Haloxylon ammodendron in drought treatment group.
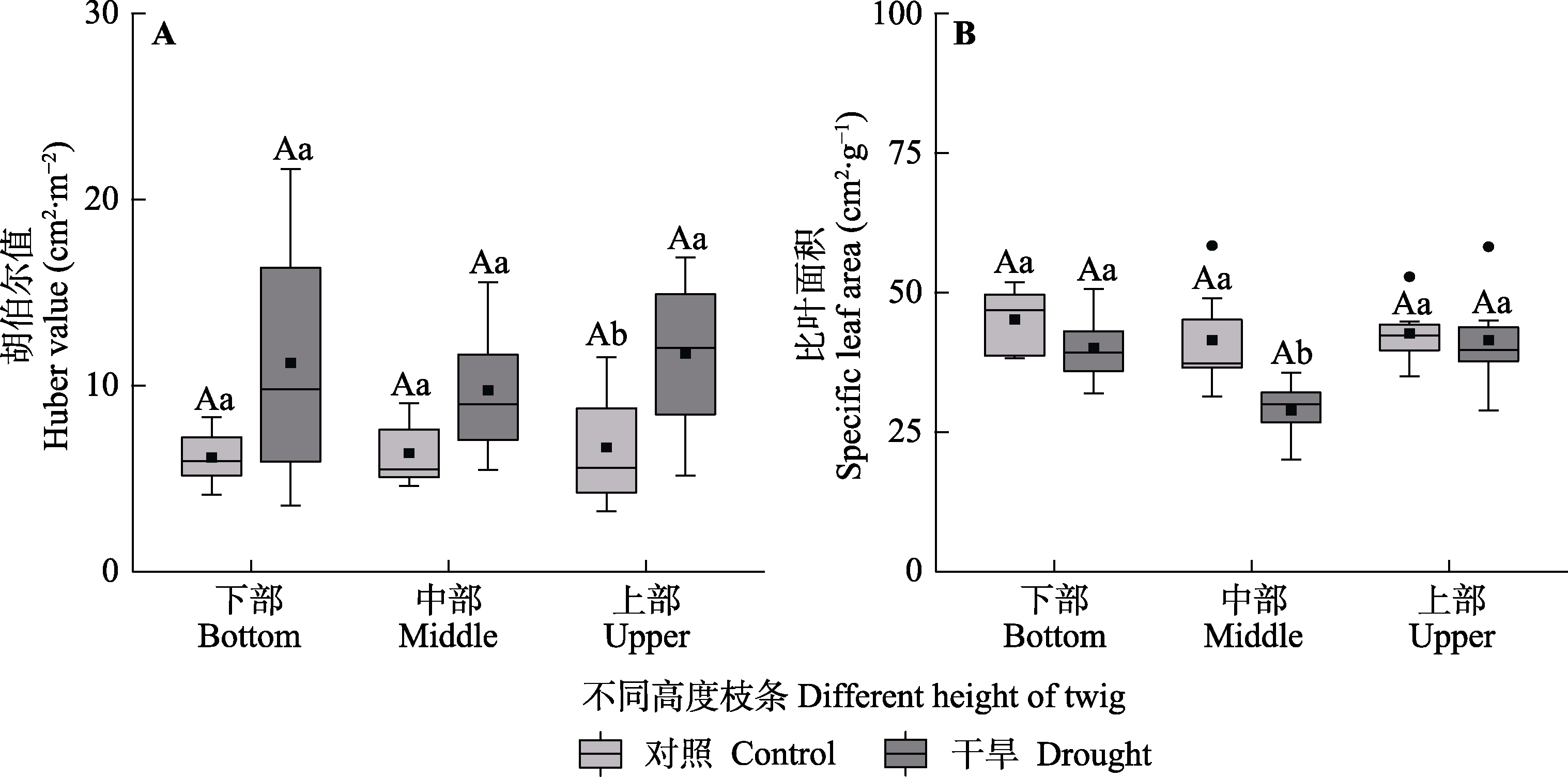
图9 对照组和干旱处理组梭梭不同高度枝条的胡伯尔值和比叶面积(平均值±标准误)。不同大写字母表示同一处理不同树高水平间存在显著差异(p < 0.05), 不同小写字母表示同一树高水平不同处理间差异显著(p < 0.05)。
Fig. 9 Huber value and specific leaf area among different heights of Haloxylon ammodendron in control and drought treatment groups (mean ± SE). Different uppercase letters indicate significant differences among different heights of the same treatment (p < 0.05) and different lowercase letters indicate significant differences between different treatments of the same height (p < 0.05).
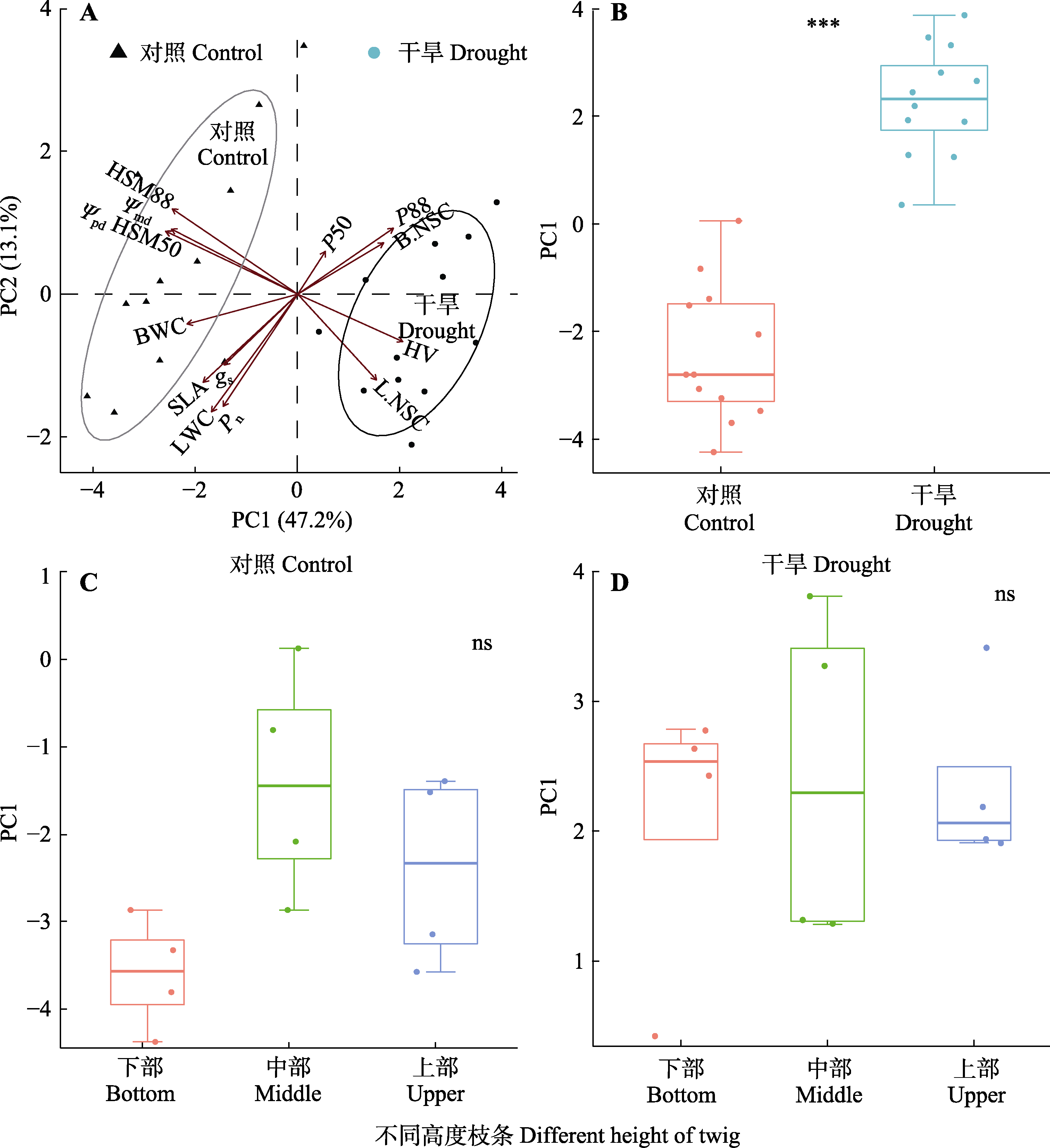
图10 梭梭抗旱策略的主成分(PC)分析。ns, 无显著差异; ***, p < 0.001。Ψmd, 正午同化枝水势; Ψpd, 黎明同化枝水势; B.NSC, 枝条非结构性碳水化合物含量; BWC, 枝条含水量; gs, 气孔导度; HSM50, Ψmd - P50; HSM88, Ψmd - P88; HV, 胡伯尔值; L.NSC, 同化枝非结构性碳水化合物含量; LWC, 同化枝含水量; P50, 导水度损失50%时的木质部水势; P88, 导水度损失88%时的木质部水势; Pn, 净光合速率; SLA, 比叶面积。
Fig. 10 Principal component (PC) analysis of each trait of drought resistance strategies for Haloxylon ammodendron. ns, no significant difference; ***, p < 0.001. Ψmd, midday assimilation twig water potential; Ψpd, predawn assimilation twig water potential; B.NSC, nonstructural carbohydrate content of branch; BWC, branch water content; gs, stomatal conductance; HSM50, Ψmd - P50; HSM88, Ψmd - P88; HV, Huber value; L.NSC, nonstructural carbohydrate content of assimilation twigs; LWC, assimilation twig water content; P50, xylem water potentials for 50% loss of maximum hydraulic conductivity; P88, xylem water potentials for 88% loss of maximum hydraulic conductivity; Pn, net photosynthetic rate; SLA, specific leaf area.
| [1] |
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, 259, 660-684.
DOI URL |
| [2] |
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012). The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proceedings of the National Academy of Sciences of the United States of America, 109, 233-237.
DOI PMID |
| [3] |
Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, Jansen S (2016). Meta-analysis reveals that hydraulic traits explain cross-species patterns of droughtinduced tree mortality across the globe. Proceedings of the National Academy of Sciences of the United States of America, 113, 5024-5029.
DOI PMID |
| [4] |
Anderegg WRL, Konings AG, Trugman AT, Yu KL, Bowling DR, Gabbitas R, Karp DS, Pacala S, Sperry JS, Sulman BN, Zenes N (2018). Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature, 561, 538-541.
DOI |
| [5] | Anest A, Charles-Dominique T, Maurin O, Millan M, Edelin C, Tomlinson KW (2021). Evolving the structure: climatic and developmental constraints on the evolution of plant architecture. A case study in Euphorbia. New Phytologist, 231, 1278-1295. |
| [6] | Beikircher B, Mayr S (2008). The hydraulic architecture of Juniperus communis L. ssp. communis: shrubs and trees compared. Plant, Cell & Environment, 31, 1545-1556. |
| [7] |
Bond WJ, Midgley JJ (2001). Ecology of sprouting in woody plants: the persistence niche. Trends in Ecology & Evolution, 16, 45-51.
DOI URL |
| [8] |
Breshears DD, Myers OB, Meyer CW, Barnes FJ, Zou CB, Allen CD, McDowell NG, Pockman WT (2009). Tree die-off in response to global change-type drought: mortality insights from a decade of plant water potential measurements. Frontiers in Ecology and the Environment, 7, 185-189.
DOI URL |
| [9] |
Brodribb TJ, Cochard H (2009). Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiology, 149, 575-584.
DOI PMID |
| [10] | Brodribb TJ, Feild TS (2000). Stem hydraulic supply is linked to leaf photosynthetic capacity: evidence from New Caledonian and Tasmanian rainforests. Plant, Cell & Environment, 23, 1381-1388. |
| [11] |
Carter JL, White DA (2009). Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth. Tree Physiology, 29, 1407-1418.
DOI PMID |
| [12] |
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002). How plants cope with water stress in the field. Photosynthesis and growth. Annals of Botany, 89, 907-916.
DOI URL |
| [13] |
Chen YJ, Choat B, Sterck F, Maenpuen P, Katabuchi M, Zhang SB, Tomlinson KW, Oliveira RS, Zhang YJ, Shen JX, Cao KF, Jansen S (2021). Hydraulic prediction of drought-induced plant dieback and top-kill depends on leaf habit and growth form. Ecology Letters, 24, 2350-2363.
DOI URL |
| [14] |
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martínez-Vilalta J, Mayr S, et al. (2012). Global convergence in the vulnerability of forests to drought. Nature, 491, 752-755.
DOI |
| [15] |
Cooper DJ, D’Amico DR, Scott ML (2003). Physiological and morphological response patterns of Populus deltoides to alluvial groundwater pumping. Environmental Management, 31, 215-226.
PMID |
| [16] |
Dai AG (2013). Increasing drought under global warming in observations and models. Nature Climate Change, 3, 52-58.
DOI |
| [17] |
Dai Y, Zheng XJ, Tang LS, Li Y (2015). Stable oxygen isotopes reveal distinct water use patterns of two Haloxylon species in the Gurbantonggut Desert. Plant and Soil, 389, 73-87.
DOI URL |
| [18] |
Davis SD, Ewers FW, Sperry JS, Portwood KA, Crocker MC, Adams GC (2002). Shoot dieback during prolonged drought in Ceanothus (Rhamnaceae) chaparral of California: a possible case of hydraulic failure. American Journal of Botany, 89, 820-828.
DOI URL |
| [19] | Duursma R, Choat B (2017). fitplc-an R package to fit hydraulic vulnerability curves. Journal of Plant Hydraulics, 4, e002. DOI: 10.20870/jph.2017.e002. |
| [20] | Fang LD, Ning QR, Guo JJ, Gong XW, Zhu JJ, Hao GY (2021). Hydraulic limitation underlies the dieback of Populus pseudosimonii trees in water-limited areas of Northern China. Forest Ecology and Management, 483, 118764. DOI: 10.1016/j.foreco.2020.118764. |
| [21] |
Fu PL, Jiang YJ, Wang AY, Brodribb TJ, Zhang JL, Zhu SD, Cao KF (2012). Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an Asian tropical dry karst forest. Annals of Botany, 110, 189-199.
DOI URL |
| [22] | Garcia-Forner N, Adams HD, Sevanto S, Collins AD, Dickman LT, Hudson PJ, Zeppel MJB, Jenkins M, Powers H, Martinez-Vilalta J, McDowell NG (2016). Responses of two semiarid conifer tree species to reduced precipitation and warming reveal new perspectives for stomatal regulation. Plant, Cell & Environment, 39, 38-49. |
| [23] |
Giorgi F, Coppola E, Raffaele F (2014). A consistent picture of the hydroclimatic response to global warming from multiple indices: models and observations. Journal of Geophysical Research: Atmospheres, 119, 11695-11708.
DOI URL |
| [24] |
Gong CM, Wang JJ, Hu CX, Wang JH, Ning PB, Bai J (2015). Interactive response of photosynthetic characteristics in Haloxylon ammodendron and Hedysarum scoparium exposed to soil water and air vapor pressure deficits. Journal of Environmental Sciences (China), 34, 184-196.
DOI URL |
| [25] |
Gong XW, Guo JJ, Fang LD, Bucci SJ, Goldstein G, Hao GY (2021). Hydraulic dysfunction due to root-exposure- initiated water stress is responsible for the mortality of Salix gordejevii shrubs on the windward slopes of active sand dunes. Plant and Soil, 459, 185-201.
DOI |
| [26] |
Grossiord C, Sevanto S, Dawson TE, Adams HD, Collins AD, Dickman LT, Newman BD, Stockton EA, McDowell NG (2017). Warming combined with more extreme precipitation regimes modifies the water sources used by trees. New Phytologist, 213, 584-596.
DOI PMID |
| [27] |
Hartmann H, Moura CF, Anderegg WRL, Ruehr NK, Salmon Y, Allen CD, Arndt SK, Breshears DD, Davi H, Galbraith D, Ruthrof KX, Wunder J, Adams HD, Bloemen J, Cailleret M, et al. (2018). Research frontiers for improving our understanding of drought induced tree and forest mortality. New Phytologist, 218, 15-28.
DOI PMID |
| [28] |
Hartmann H, Trumbore S (2016). Understanding the roles of nonstructural carbohydrates in forest trees-from what we can measure to what we want to know. New Phytologist, 211, 386-403.
DOI PMID |
| [29] |
Hochberg U, Windt CW, Ponomarenko A, Zhang YJ, Gersony J, Rockwell FE, Holbrook NM (2017). Stomatal closure, basal leaf embolism, and shedding protect the hydraulic integrity of grape stems. Plant Physiology, 174, 764-775.
DOI PMID |
| [30] |
Hoover DL, Duniway MC, Belnap J (2015). Pulse-drought atop press-drought: unexpected plant responses and implications for dryland ecosystems. Oecologia, 179, 1211-1221.
DOI PMID |
| [31] |
Huang JP, Ma JR, Guan XD, Li Y, He YL (2019). Progress in semi-arid climate change studies in China. Advances in Atmospheric Sciences, 36, 922-937.
DOI |
| [32] |
Huang JP, Yu HP, Guan XD, Wang GY, Guo RX (2016). Accelerated dryland expansion under climate change. Nature Climate Change, 6, 166-171.
DOI |
| [33] | Johnson DM, McCulloh KA, Meinzer FC, Woodruff DR, Eissenstat DM, Phillips N (2011). Hydraulic patterns and safety margins, from stem to stomata, in three eastern US tree species. Tree Physiology, 31, 659-668. |
| [34] |
Koch GW, Sillett SC, Jennings GM, Davis SD (2004). The limits to tree height. Nature, 428, 851-854.
DOI |
| [35] |
Li BF, Chen YN, Shi X, Chen ZS, Li WH (2013). Temperature and precipitation changes in different environments in the arid region of northwest China. Theoretical and Applied Climatology, 112, 589-596.
DOI URL |
| [36] |
Li JY, Chang H, Liu T, Zhang C (2019). The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agricultural and Forest Meteorology, 275, 243-254.
DOI URL |
| [37] | Li Y, Zheng XJ, Wang YG, Xu GQ, Liu R (2021). Experiment and simulation platform for oasis-desert symbiotic relationship (ODP). Bulletin of Chinese Academy of Sciences, 36, 1506-1514. |
| [李彦, 郑新军, 王玉刚, 徐贵青, 刘冉 (2021). 绿洲-荒漠共生关系实验模拟平台(绿洲-荒漠平台). 中国科学院院刊, 36, 1506-1514.] | |
| [38] | Liu H, Gleason SM, Hao GY, Hua L, He PC, Goldstein G, Ye Q (2019). Hydraulic traits are coordinated with maximum plant height at the global scale. Science Advances, 5, eaav1332. DOI: 10.1126/sciadv.aav1332. |
| [39] |
López R, Cano FJ, Martin-StPaul NK, Cochard H, Choat B (2021). Coordination of stem and leaf traits define different strategies to regulate water loss and tolerance ranges to aridity. New Phytologist, 230, 497-509.
DOI PMID |
| [40] |
Lü XP, Gao HJ, Zhang L, Wang YP, Shao KZ, Zhao Q, Zhang JL (2019). Dynamic responses of Haloxylon ammodendron to various degrees of simulated drought stress. Plant Physiology and Biochemistry, 139, 121-131.
DOI URL |
| [41] | Martínez-Vilalta J, Garcia-Forner N (2017). Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell & Environment, 40, 962-976. |
| [42] |
Martínez-Vilalta J, Sala AN, Asensio D, Galiano L, Hoch G, Palacio S, Piper FI, Lloret F (2016). Dynamics of non-structural carbohydrates in terrestrial plants: a global synthesis. Ecological Monographs, 86, 495-516.
DOI URL |
| [43] |
Mayr S, Beikircher B, Obkircher MA, Schmid P (2010). Hydraulic plasticity and limitations of alpine Rhododendron species. Oecologia, 164, 321-330.
DOI URL |
| [44] |
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008). Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytologist, 178, 719-739.
DOI PMID |
| [45] |
McDowell NG (2011). Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology, 155, 1051-1059.
DOI PMID |
| [46] |
Noy-Meir I (1973). Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics, 4, 25-51.
DOI URL |
| [47] |
Powers JS, Vargas GG, Brodribb TJ, Schwartz NB, Pérez- Aviles D, Smith-Martin CM, Becknell JM, Aureli F, Blanco R, Calderón-Morales E, Calvo-Alvarado JC, Calvo- Obando AJ, Chavarría MM, Carvajal-Vanegas D, Jiménez-Rodríguez CD, et al. (2020). A catastrophic tropical drought kills hydraulically vulnerable tree species. Global Change Biology, 26, 3122-3133.
DOI PMID |
| [48] |
Rosner S, Heinze B, Savi T, Dalla-Salda G (2019). Prediction of hydraulic conductivity loss from relative water loss: new insights into water storage of tree stems and branches. Physiologia Plantarum, 165, 843-854.
DOI URL |
| [49] |
Rosner S, Klein A, Wimmer R, Karlsson B (2006). Extraction of features from ultrasound acoustic emissions: a tool to assess the hydraulic vulnerability of Norway spruce trunkwood? New Phytologist, 171, 105-116.
DOI PMID |
| [50] |
Sage RF, Sultmanis S (2016). Why are there no C4 forests? Journal of Plant Physiology, 203, 55-68.
DOI URL |
| [51] |
Schenk HJ, Espino S, Goedhart CM, Nordenstahl M, Cabrera HIM, Jones CS (2008). Hydraulic integration and shrub growth form linked across continental aridity gradients. Proceedings of the National Academy of Sciences of the United States of America, 105, 11248-11253.
DOI PMID |
| [52] |
Scholander PF, Bradstreet ED, Hemmingsen EA, Hammel HT (1965). Sap pressure in vascular plants. Science, 148, 339-346.
PMID |
| [53] | Sevanto S, McDowell NG, Dickman LT, Pangle R, Pockman WT (2014). How do trees Die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell & Environment, 37, 153-161. |
| [54] | Song CW, Li CJ, Halik Ü, Xu XW, Lei JQ, Zhou ZB, Fan JL (2021). Spatial distribution and structural characteristics for Haloxylon ammodendron plantation on the southwestern edge of the gurbantünggüt desert. Forests, 12, 633. DOI: 10.3390/f12050633. |
| [55] | Stovall AEL, Shugart H, Yang X (2019). Tree height explains mortality risk during an intense drought. Nature Communications, 10, 4385. DOI: 10.1038/s41467-019-12380-6. |
| [56] |
Tiemuerbieke B, Min XJ, Zang YX, Xing P, Ma JY, Sun W (2018). Water use patterns of co-occurring C3 and C4shrubs in the Gurbantonggut Desert in northwestern China. Science of the Total Environment, 634, 341-354.
DOI URL |
| [57] |
Trugman AT, Anderegg LDL, Anderegg WRL, Das AJ, Stephenson NL (2021). Why is tree drought mortality so hard to predict? Trends in Ecology & Evolution, 36, 520-532.
DOI URL |
| [58] |
Werner C, Correia O, Beyschlag W (1999). Two different strategies of Mediterranean macchia plants to avoid photoinhibitory damage by excessive radiation levels during summer drought. Acta Oecologica, 20, 15-23.
DOI URL |
| [59] |
Wu X, Zheng XJ, Li Y, Xu GQ (2019). Varying responses of two Haloxylon species to extreme drought and groundwater depth. Environmental and Experimental Botany, 158, 63-72.
DOI URL |
| [60] |
Xu GQ, Li Y (2008). Rooting depth and leaf hydraulic conductance in the xeric tree Haloxyolon ammodendron growing at sites of contrasting soil texture. Functional Plant Biology, 35, 1234-1242.
DOI PMID |
| [61] |
Xu GQ, McDowell NG, Li Y (2016). A possible link between life and death of a xeric tree in desert. Journal of Plant Physiology, 194, 35-44.
DOI URL |
| [62] |
Xu GQ, Yu DD, Li Y (2017). Patterns of biomass allocation in Haloxylon persicum woodlands and their understory herbaceous layer along a groundwater depth gradient. Forest Ecology and Management, 395, 37-47.
DOI URL |
| [63] | Yang XY, Lu MQ, Wang YF, Wang YR, Liu ZJ, Chen S (2021). Response mechanism of plants to drought stress. Horticulturae, 7, 50. DOI: 10.3390/horticulturae7030050. |
| [64] |
Yu X, Lei JQ, Gao X (2022). An over review of desertification in Xinjiang, Northwest China. Journal of Arid Land, 14, 1181-1195.
DOI |
| [65] |
Zhang HY, Wang CK, Wang XC (2014). Spatial variations in non-structural carbohydrates in stems of twelve temperate tree species. Trees, 28, 77-89.
DOI URL |
| [66] | Zhang L, Liu L, Zhao H, Jiang ZM, Cai J (2020). Differences in near isohydric and anisohydric behavior of contrasting poplar hybrids (I-101 (Populus alba L.) × 84K (Populus alba L. × Populus glandulosa Uyeki)) under drought- rehydration treatments. Forests, 11, 402. DOI: 10.3390/f11040402. |
| [67] | Zhang T, Cao Y, Chen YM, Liu GB (2015). Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees, 29, 1837-1849. |
| [68] |
Zolfaghar S, Villalobos-Vega R, Zeppel M, Eamus D (2015). The hydraulic architecture of Eucalyptus trees growing across a gradient of depth-to-groundwater. Functional Plant Biology, 42, 888-898.
DOI PMID |
| [1] | 俞庆水 倪晓凤 吉成均 朱江玲 唐志尧 方精云. 10年氮磷添加对海南尖峰岭两种热带雨林优势植物叶片非结构性碳水化合物的影响[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 陈雨婷, 马松梅, 张丹, 张林, 王春成. 新疆同域分布梭梭和白梭梭多样性格局及其形成机制[J]. 植物生态学报, 2024, 48(1): 56-67. |
| [3] | 苏炜, 陈平, 吴婷, 刘岳, 宋雨婷, 刘旭军, 刘菊秀. 氮添加与干季延长对降香黄檀幼苗非结构性碳水化合物、养分与生物量的影响[J]. 植物生态学报, 2023, 47(8): 1094-1104. |
| [4] | 余海霞, 曲鲁平, 汤行昊, 刘南, 张子雷, 王浩, 王艺璇, 邵长亮, 董刚, 胡亚林. 闽楠和木荷非结构性碳水化合物对不同模式热浪的差异性响应[J]. 植物生态学报, 2023, 47(2): 249-261. |
| [5] | 李变变, 张凤华, 赵亚光, 孙秉楠. 不同刈割程度对油莎豆非结构性碳水化合物代谢及生物量的影响[J]. 植物生态学报, 2023, 47(1): 101-113. |
| [6] | 周洁, 杨晓东, 王雅芸, 隆彦昕, 王妍, 李浡睿, 孙启兴, 孙楠. 梭梭和骆驼刺对干旱的适应策略差异[J]. 植物生态学报, 2022, 46(9): 1064-1076. |
| [7] | 伍敏, 田雨, 樊大勇, 张祥雪. 干旱胁迫下毛白杨和元宝槭的水力学调控[J]. 植物生态学报, 2022, 46(9): 1086-1097. |
| [8] | 董涵君, 王兴昌, 苑丹阳, 柳荻, 刘玉龙, 桑英, 王晓春. 温带不同材性树种树干非结构性碳水化合物的径向分配差异[J]. 植物生态学报, 2022, 46(6): 722-734. |
| [9] | 李思源, 张照鑫, 饶良懿. 桑苗非结构性碳水化合物和生长激素对水淹胁迫的响应[J]. 植物生态学报, 2022, 46(3): 311-320. |
| [10] | 秦慧君, 焦亮, 周怡, 薛儒鸿, 柒常亮, 杜达石. 祁连山优势树木碳水化合物资源分配的海拔和树种效应[J]. 植物生态学报, 2022, 46(2): 208-219. |
| [11] | 林夏珍, 刘林, 董婷婷, 方琦博, 郭庆学. 非结构性碳水化合物与氮分配对美洲黑杨和青杨耐盐能力的影响[J]. 植物生态学报, 2021, 45(9): 961-971. |
| [12] | 任金培, 李俊鹏, 王卫锋, 代永欣, 王林. 八个树种叶水力性状对水分条件的响应及其驱动因素[J]. 植物生态学报, 2021, 45(9): 942-951. |
| [13] | 罗丹丹, 王传宽, 金鹰. 木本植物水力系统对干旱胁迫的响应机制[J]. 植物生态学报, 2021, 45(9): 925-941. |
| [14] | 吴秋霞, 吴福忠, 胡仪, 康自佳, 张耀艺, 杨静, 岳楷, 倪祥银, 杨玉盛. 亚热带同质园11个树种新老叶非结构性碳水化合物含量比较[J]. 植物生态学报, 2021, 45(7): 771-779. |
| [15] | 刘丽燕, 冯锦霞, 刘文鑫, 万贤崇. 干旱胁迫对转PtPIP2;8基因84K杨苗木光合、生长和根系结构的影响[J]. 植物生态学报, 2020, 44(6): 677-686. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2026 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
![]()