

植物生态学报 ›› 2025, Vol. 49 ›› Issue (7): 1163-1176.DOI: 10.17521/cjpe.2024.0428 cstr: 32100.14.cjpe.2024.0428
• 研究论文 • 上一篇
崔冬晴1,2, 田晨2, 宋慧敏2, 鲁小名2,3, 萨其日4, 徐国庆4, 杨培志1,*( ), 白永飞2,3, 田建卿2,3,*(
), 白永飞2,3, 田建卿2,3,*( )
)
收稿日期:2024-12-02
接受日期:2025-01-20
出版日期:2025-07-20
发布日期:2025-01-20
通讯作者:
*杨培志, E-mail: yangpeizhi@126.com;基金资助:
CUI Dong-Qing1,2, TIAN Chen2, SONG Hui-Min2, LU Xiao-Ming2,3, SA Qi-Ri4, XU Guo-Qing4, YANG Pei-Zhi1,*( ), BAI Yong-Fei2,3, TIAN Jian-Qing2,3,*(
), BAI Yong-Fei2,3, TIAN Jian-Qing2,3,*( )
)
Received:2024-12-02
Accepted:2025-01-20
Online:2025-07-20
Published:2025-01-20
Supported by:摘要: 长期放牧深刻影响草地生态系统中植物生长发育的外部环境, 植物通过与根际微生物的相互作用来适应环境变化。然而, 目前关于放牧如何影响草地不同生存策略植物根际微生物多样性的研究十分有限。该研究依托内蒙古典型草原长期放牧实验平台, 选取优势植物大针茅(Stipa grandis)和糙隐子草(Cleistogenes squarrosa)作为研究对象, 利用高通量测序技术探讨不同放牧强度(对照, 轻度: 1.5 sheep·hm-2, 中度: 4.5 sheep·hm-2, 重度: 7.5 sheep·hm-2)下植物根际细菌多样性变化趋势, 解析两种优势植物根际细菌的响应差异, 及其与植物功能性状的内在联系。结果表明: (1)重度放牧显著降低大针茅根际细菌丰富度(8.97%)和Chao1指数(9.48%), 但对糙隐子草根际细菌α多样性无显著影响, 且大针茅根际细菌α多样性显著低于糙隐子草; 此外, 重度放牧显著改变了两种植物根际细菌的群落组成, 其中大针茅的变化幅度显著大于糙隐子草。(2)随着放牧强度增加, 大针茅富集了较多的根际促生菌和生物防治菌, 而糙隐子草则主要富集了根际促生菌。(3)大针茅根际细菌群落多样性和功能菌群相对丰度的变化与其较大的根系直径、较小的比叶面积和比根长等代表避牧和资源保守策略的功能性状显著相关; 而糙隐子草的根际细菌群落变化则与其较高的地上生物量碳氮比和较大的比叶面积等代表耐牧和资源消耗策略的功能性状显著相关。综上, 不同优势植物根际细菌群落对放牧压力的响应与其生存策略密切相关, 丰富了对长期放牧背景下植物与根际微生物群落协同适应机制的理解。
崔冬晴, 田晨, 宋慧敏, 鲁小名, 萨其日, 徐国庆, 杨培志, 白永飞, 田建卿. 典型草原优势植物根际细菌群落多样性和功能群组成对长期放牧的响应机制. 植物生态学报, 2025, 49(7): 1163-1176. DOI: 10.17521/cjpe.2024.0428
CUI Dong-Qing, TIAN Chen, SONG Hui-Min, LU Xiao-Ming, SA Qi-Ri, XU Guo-Qing, YANG Pei-Zhi, BAI Yong-Fei, TIAN Jian-Qing. Response mechanisms of rhizosphere bacterial community diversity and functional group composition of dominant plants in typical grasslands to long-term grazing. Chinese Journal of Plant Ecology, 2025, 49(7): 1163-1176. DOI: 10.17521/cjpe.2024.0428
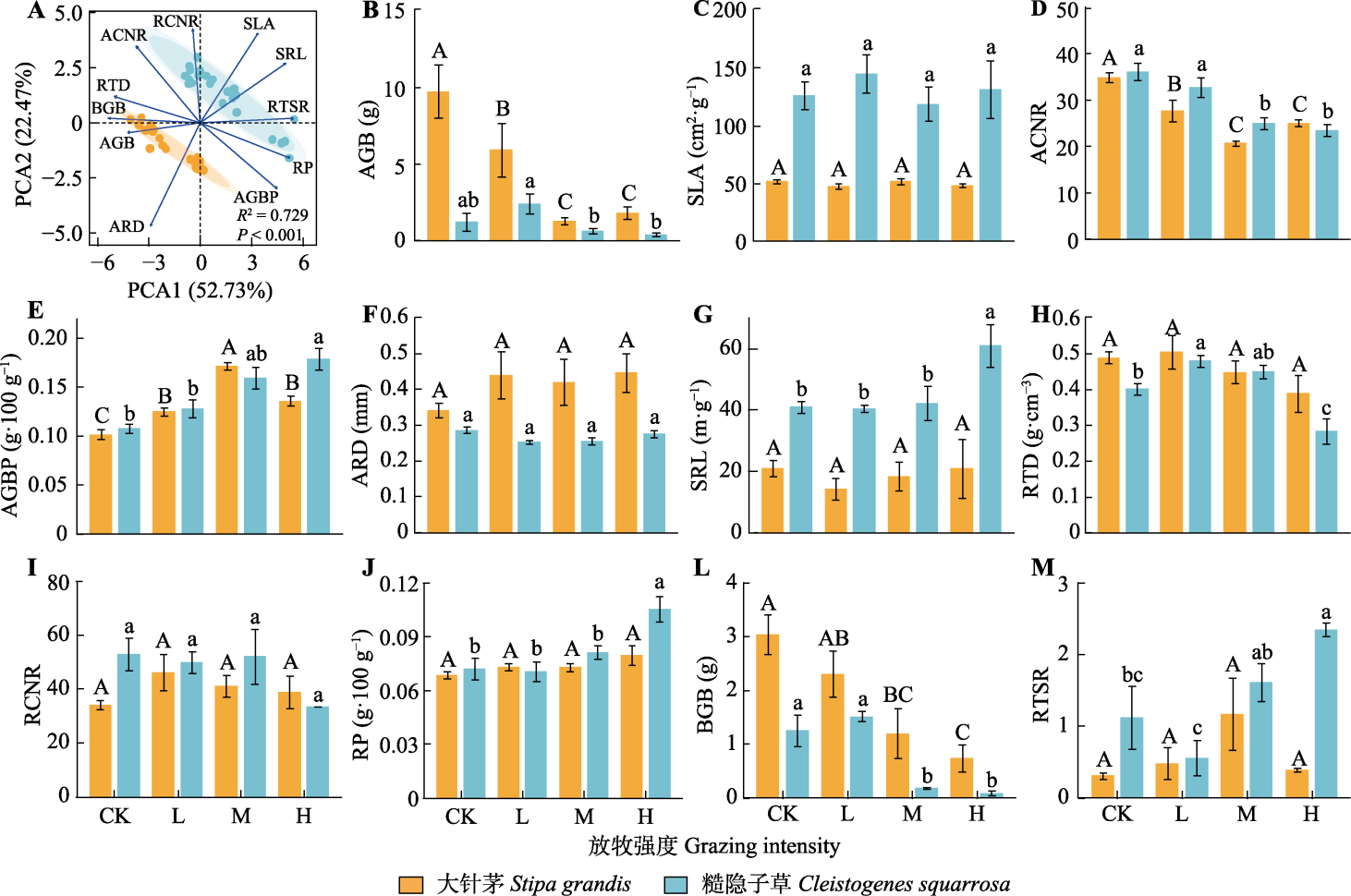
图1 大针茅和糙隐子草功能性状差异及不同放牧强度对二者功能性状的影响(平均值±标准误)。ACNR, 地上生物量碳氮比; AGB, 地上生物量; AGBP, 地上生物量磷含量; ARD, 根平均直径; BGB, 地下生物量; RCNR, 根碳氮比; RP, 根磷含量; RTD, 根组织密度; RTSR, 根冠比; SLA, 比叶面积; SRL, 比根长。CK、L、M和H分别代表对照、轻度放牧、中度放牧、重度放牧。不同大写和小写字母分别表示不同放牧强度对大针茅和糙隐子草植物功能性状影响差异显著(p < 0.05)。
Fig. 1 Difference of plant functional traits of Stipa grandis and Cleistogenes squarrosa, and the effect of different grazing intensity on functional traits of the two species (mean ± SE). ACNR, aboveground biomass carbon to nitrogen ratio; AGB, aboveground biomass; AGBP, aboveground biomass phosphorus content; ARD, average root diameter; BGB, belowground biomass; RCNR, root carbon to nitrogen ratio; RP, root phosphorus content; RTD, root tissue density; RTSR, root-to-shoot ratio; SLA, specific leaf area; SRL, specific root length. CK, L, M and H represent the control, light grazing, moderate grazing and heavy grazing intensity, respectively. Different upper- and lower-case letters indicate significant differences (p < 0.05) in the effects of grazing intensities on plant functional traits of Stipa grandis and Cleistogenes squarrosa.
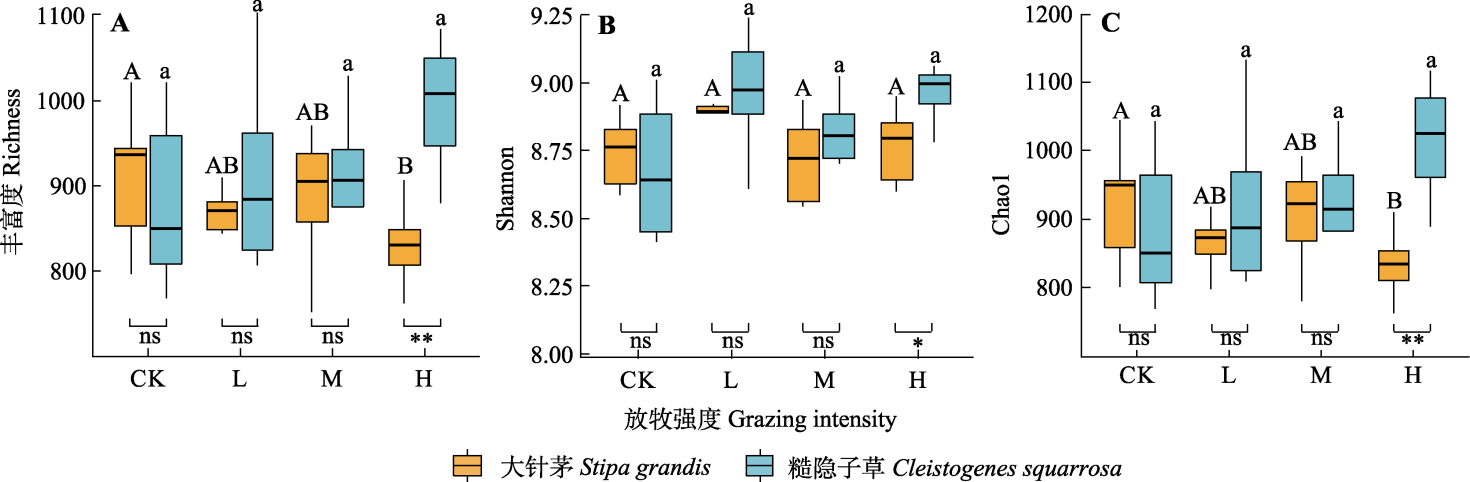
图2 不同放牧强度下大针茅和糙隐子草根际细菌α多样性指数。*, p < 0.05; **, p < 0.01; ns, p ≥ 0.05。CK、L、M和H分别代表对照、轻度放牧、中度放牧、重度放牧。不同大写和小写字母分别表示不同放牧强度对大针茅和糙隐子草根际细菌α多样性影响差异显著(p < 0.05)。
Fig 2 Alpha diversity index of rhizosphere bacteria of Stipa grandis and Cleistogenes squarrosa under different grazing intensities. *, p < 0.05; **, p < 0.01; ns, p ≥ 0.05. CK, L, M and H represent the control, light grazing, moderate grazing and heavy grazing intensity, respectively. Different upper- and lower-case letters indicate significant differences (p < 0.05) in the effects of rhizosphere bacterial alpha diversity of Stipa grandis and Cleistogenes squarrosa.
| 物种名称 Species | CK vs. L | CK vs. M | CK vs. H |
|---|---|---|---|
| 大针茅 Stipa grandis | 0.194 6** | 0.224 9** | 0.238 7** |
| 糙隐子草 Cleistogenes squarrosa | 0.146 0** | 0.156 5** | 0.163 2** |
表1 放牧与对照处理下大针茅和糙隐子草根际细菌群落组成差异
Table 1 Differences in rhizobacterial community composition of Stipa grandis and Cleistogenes squarrosa between grazing and control treatments
| 物种名称 Species | CK vs. L | CK vs. M | CK vs. H |
|---|---|---|---|
| 大针茅 Stipa grandis | 0.194 6** | 0.224 9** | 0.238 7** |
| 糙隐子草 Cleistogenes squarrosa | 0.146 0** | 0.156 5** | 0.163 2** |
| 大针茅 Stipa grandis | 糙隐子草 Cleistogenes squarrosa | ||||
|---|---|---|---|---|---|
| 植物性状 Plant traits | r | Variance (%) | 植物性状 Plant trait | r | Variance (%) |
| AGB | 0.093 | 43.89*** | AGB | 0.063 | 29.02* |
| SLA | 0.039 | 26.37 | SLA | 0.154 | 28.45* |
| ACNR | 0.028 | 27.72 | ACNR | 0.162* | 38.07*** |
| SRL | -0.112 | 36.17** | SRL | 0.062 | 25.32 |
| ARD | 0.154* | 30.71** | RTD | 0.036 | 27.95 |
| RTD | -0.015 | 27.56* | RCNR | 0.061 | 28.06 |
表2 植物功能性状对大针茅和糙隐子草根际细菌丰富度和群落组成的影响
Table 2 Effects of plant functional traits on rhizosphere soil bacterial richness and community composition of Stipa grandis and Cleistogenes squarrosa
| 大针茅 Stipa grandis | 糙隐子草 Cleistogenes squarrosa | ||||
|---|---|---|---|---|---|
| 植物性状 Plant traits | r | Variance (%) | 植物性状 Plant trait | r | Variance (%) |
| AGB | 0.093 | 43.89*** | AGB | 0.063 | 29.02* |
| SLA | 0.039 | 26.37 | SLA | 0.154 | 28.45* |
| ACNR | 0.028 | 27.72 | ACNR | 0.162* | 38.07*** |
| SRL | -0.112 | 36.17** | SRL | 0.062 | 25.32 |
| ARD | 0.154* | 30.71** | RTD | 0.036 | 27.95 |
| RTD | -0.015 | 27.56* | RCNR | 0.061 | 28.06 |
| 属名 Genus | 参考文献 Reference | |
|---|---|---|
| 根际促生菌Plant growth- promoting rhizobacteria (PGPR) | 浅野氏菌属、硝化螺旋菌属、类诺卡氏菌属、RB41菌属(未正式命名)、红色杆菌属、土壤红色杆菌属、鞘氨醇单胞菌属、苔藓杆菌属、芽单胞菌属、克罗斯氏菌属、根瘤杆菌属、候选乌达杆菌属 Asanoa, Nitrospira, Nocardioides, RB41, Rubrobacter, Solirubrobacter, Sphingomonas, Bryobacter, Gemmatimonas, Crossiella, Rhizobacter, Candidatus Udaeobacter | Chen et al., |
| 生物防治菌Biocontrol agents (BCAs) | 游动放线菌属、芽孢杆菌属、盖氏菌属、莱氏菌属、小单孢菌属、连接杆菌属 Bacillus, Gaiella, Lechevalieria, Micromonospora, Conexibacter, Actinoplanes | Cui et al., |
表3 大针茅和糙隐子草根际富集的细菌属水平功能类群划分
Table 3 Functional group classification of rhizosphere-enriched bacterial genera in Stipa grandis and Cleistogenes squarrosa
| 属名 Genus | 参考文献 Reference | |
|---|---|---|
| 根际促生菌Plant growth- promoting rhizobacteria (PGPR) | 浅野氏菌属、硝化螺旋菌属、类诺卡氏菌属、RB41菌属(未正式命名)、红色杆菌属、土壤红色杆菌属、鞘氨醇单胞菌属、苔藓杆菌属、芽单胞菌属、克罗斯氏菌属、根瘤杆菌属、候选乌达杆菌属 Asanoa, Nitrospira, Nocardioides, RB41, Rubrobacter, Solirubrobacter, Sphingomonas, Bryobacter, Gemmatimonas, Crossiella, Rhizobacter, Candidatus Udaeobacter | Chen et al., |
| 生物防治菌Biocontrol agents (BCAs) | 游动放线菌属、芽孢杆菌属、盖氏菌属、莱氏菌属、小单孢菌属、连接杆菌属 Bacillus, Gaiella, Lechevalieria, Micromonospora, Conexibacter, Actinoplanes | Cui et al., |
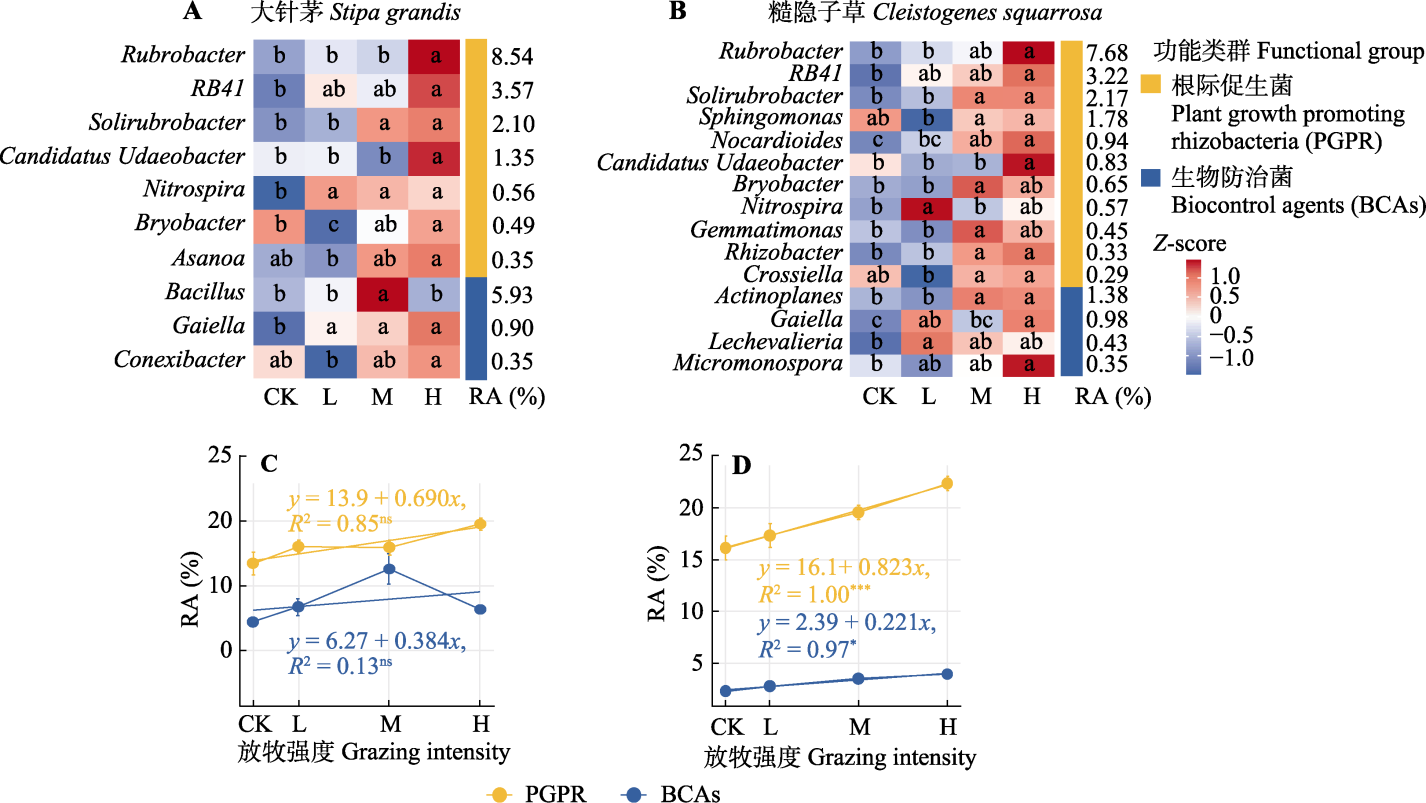
图3 不同放牧强度下大针茅和糙隐子草根际细菌相对丰度增加属热图(A、B)和功能类群相对丰度线性回归图(C、D)。A、B中不同小写字母分别表示不同放牧强度对该属的相对丰度影响差异显著(p < 0.05)。CK、L、M和H分别代表对照、轻度放牧、中度放牧、重度放牧。RA表示4个放牧强度下该属的平均相对丰度。C、D中误差棒为平均值±标准误。*, p < 0.05; ***, p < 0.001; ns, p ≥ 0.05。Actinoplanes, 游动放线菌属; Asanoa, 浅野氏菌属; Bacillus, 芽孢杆菌属; Bryobacter, 苔藓杆菌属; Candidatus Udaeobacter, 候选乌达杆菌属; Conexibacter, 连接杆菌属; Gaiella, 盖氏菌属; Crossiella, 克罗斯氏菌属; Gemmatimonas, 芽单胞菌属; Lechevalieria, 莱氏菌属; Micromonospora, 小单孢菌属; Nitrospira, 硝化螺旋菌属; Nocardioides, 类诺卡氏菌属; RB41, RB41菌属(未正式命名); Rhizobacter, 根瘤杆菌属; Rubrobacter, 红色杆菌属; Solirubrobacter, 土壤红色杆菌属; Sphingomonas, 鞘氨醇单胞菌属。
Fig. 3 Heatmaps of rhizobacterial genera with increased relative abundance (A, B) and linear regression of functional group relative abundance (C, D) for Stipa grandis and Cleistogenes squarrosa under different grazing intensities. Different lowercase letters indicate significant differences (p < 0.05) in the relative abundance of genera influenced by varying grazing intensities in A and B. CK, L, M, and H represent control, light grazing, moderate grazing, and heavy grazing, respectively. RA denotes the average relative abundance of the genus under the four grazing intensities. Error bars in C and D represent mean ± SE. *, p < 0.05; ***, p < 0.001; ns, p ≥ 0.05.
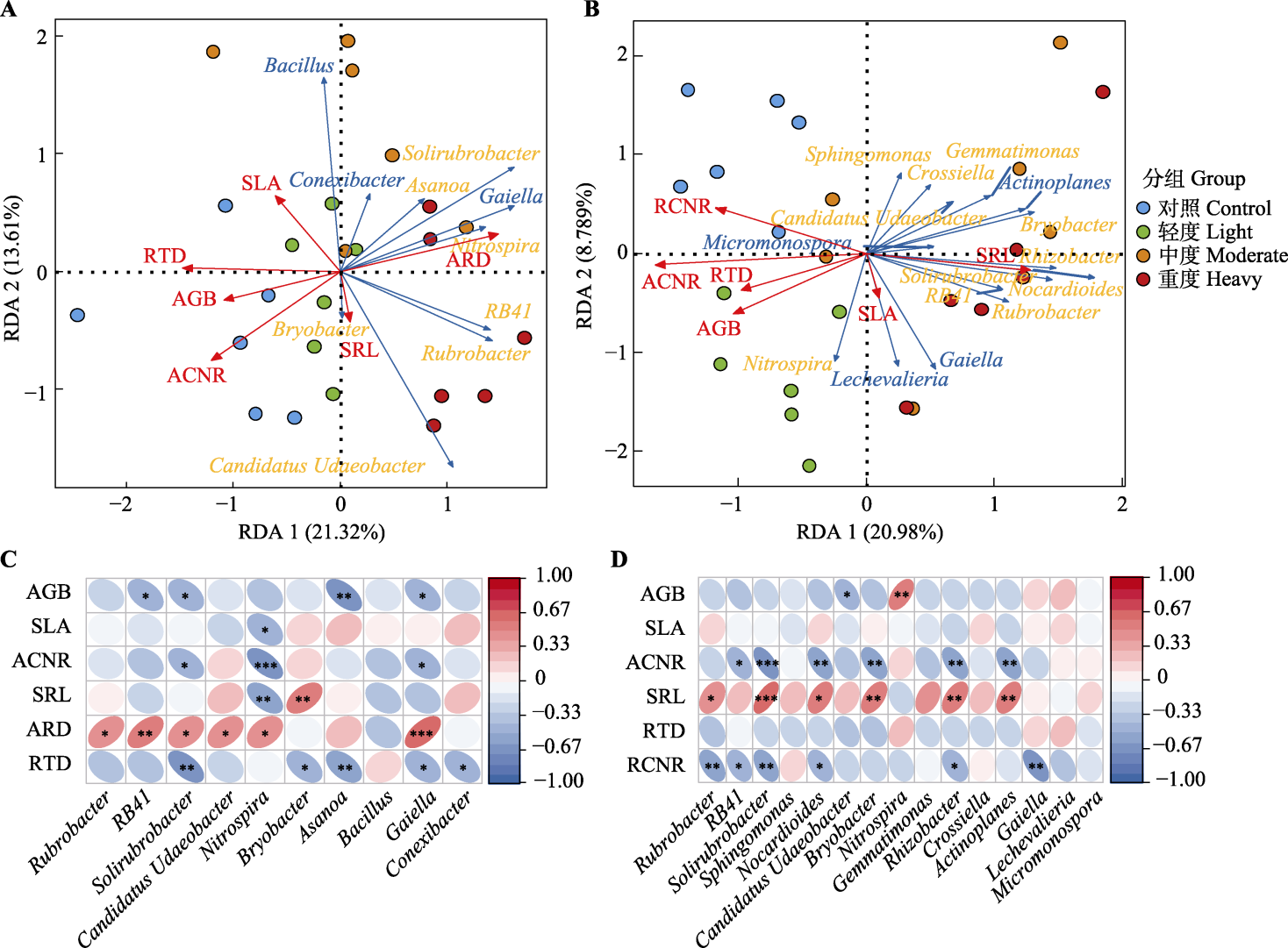
图4 不同放牧强度下大针茅(A、C)和糙隐子草(B、D)根际富集细菌属与植物功能性状的冗余分析(RDA)结果及相关性热图。*, p < 0.05; **, p < 0.01; ***, p < 0.001。AGB, 地上生物量; SLA, 比叶面积; ACNR, 地上生物量碳氮比; SRL, 比根长; ARD, 根平均直径; RTD, 根组织密度; RCNR, 根碳氮比。Actinoplanes, 游动放线菌属; Asanoa, 浅野氏菌属; Bacillus, 芽孢杆菌属; Bryobacter, 苔藓杆菌属; Candidatus Udaeobacter, 候选乌达杆菌属; Conexibacter, 连接杆菌属; Crossiella, 克罗斯氏菌属; Gaiella, 盖氏菌属; Gemmatimonas, 芽单胞菌属; Lechevalieria, 莱氏菌属; Micromonospora, 小单孢菌属; Nitrospira, 硝化螺旋菌属; Nocardioides, 类诺卡氏菌属; RB41, RB41菌属(未正式命名); Rhizobacter, 根瘤杆菌属; Rubrobacter, 红色杆菌属; Solirubrobacter, 土壤红色杆菌属; Sphingomonas, 鞘氨醇单胞菌属。
Fig. 4 Redundancy analysis (RDA) results and correlation heatmaps of rhizosphere enriched bacterial genera with plant functional traits of Stipa grandis (A, C) and Cleistogenes squarrosa (B, D) under different grazing intensities. *, p < 0.05; **, p < 0.01; ***, p < 0.001。AGB, aboveground biomass; SLA, specific leaf area; ACNR, aboveground biomass carbon to nitrogen ratio; SRL, specific root length; ARD, average root diameter; RTD, root tissue density; RCNR, root carbon to nitrogen ratio.
| [1] | Ahkami AH, White III RA, Handakumbura PP, Jansson C (2017). Rhizosphere engineering: enhancing sustainable plant ecosystem productivity. Rhizosphere, 3, 233-243. |
| [2] | An JY, Li XL, Ding Y, Li F, Guo FH, Ma HL, Gao SB, Li YH (2021). Effects of different grazing intensities on functional traits of Cleistogenes squarrosa. Chinese Journal of Grassland, 43(1), 50-57. |
| [安景源, 李西良, 丁勇, 李芳, 郭丰辉, 马晖玲, 高韶勃, 李元恒 (2021). 不同放牧强度对糙隐子草功能性状的影响. 中国草地学报, 43(1), 50-57.] | |
| [3] | Anderson MJ (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26, 32-46. |
| [4] |
Bai B, Liu WD, Qiu XY, Zhang J, Zhang JY, Bai Y (2022). The root microbiome: community assembly and its contributions to plant fitness. Journal of Integrative Plant Biology, 64, 230-243.
DOI |
| [5] |
Bai YF, Cotrufo MF (2022). Grassland soil carbon sequestration: current understanding, challenges, and solutions. Science, 377, 603-608.
DOI PMID |
| [6] | Bai YF, Wu JG, Clark CM, Naeem S, Pan QM, Huang JH, Zhang LX, Han XG (2010). Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from Inner Mongolia grasslands. Global Change Biology, 16, 358-372. |
| [7] | Bardgett RD, Mommer L, de Vries FT (2014). Going underground: root traits as drivers of ecosystem processes. Trends in Ecology & Evolution, 29, 692-699. |
| [8] |
Berendsen RL, Pieterse CM, Bakker PA (2012). The rhizosphere microbiome and plant health. Trends in Plant Science, 17, 478-486.
DOI PMID |
| [9] | Blee K, Hein J, Wolfe GV (2013). Molecular Microbial Ecology of the Rhizosphere. Wiley-Blackwell, Hoboken, USA. |
| [10] | Bradford MA, Wieder WR, Bonan GB, Fierer N, Raymond PA, Crowther TW (2016). Managing uncertainty in soil carbon feedbacks to climate change. Nature Climate Change, 6, 751-758. |
| [11] | Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N, Gordon JI, Knight R (2011). Moving pictures of the human microbiome. Genome Biology, 12, R50. DOI: 10.1186/gb-2011-12-5-r50. |
| [12] | Carvalhais LC, Dennis PG, Fedoseyenko D, Hajirezaei MR, Borriss R, von Wirén N (2011). Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. Journal of Plant Nutrition and Soil Science, 174, 3-11. |
| [13] | Chai JL, Xu CL, Zhang DG, Xiao H, Pan TT, Yu XJ (2019). Effects of simulated trampling and rainfall on soil nutrients and enzyme activity in an alpine meadow. Acta Ecologica Sinica, 39, 333-344. |
| [柴锦隆, 徐长林, 张德罡, 肖红, 潘涛涛, 鱼小军 (2019). 模拟践踏和降水对高寒草甸土壤养分和酶活性的影响. 生态学报, 39, 333-344.] | |
| [14] | Chen LL, Wang KX, Baoyin TGT (2021). Effects of grazing and mowing on vertical distribution of soil nutrients and their stoichiometry (C:N:P) in a semi-arid grassland of North China. Catena, 206, 105507. DOI: 10.1016/j.catena.2021.105507. |
| [15] | Chen MY, Wu SH, Lin GH, Lu CP, Lin YT, Chang WC, Tsay SS (2004). Rubrobacter taiwanensis sp. nov., a novel thermophilic, radiation-resistant species isolated from hot springs. International Journal of Systematic and Evolutionary Microbiology, 54, 1849-1855. |
| [16] | Cheng JH, Chu PF, Chen DM, Bai YF (2015). Functional correlations between specific leaf area and specific root length along a regional environmental gradient in Inner Mongolia grasslands. Functional Ecology, 30, 985-997. |
| [17] | Cole DN (1995). Experimental trampling of vegetation. II. Predictors of resistance and resilience. Journal of Applied Ecology, 32, 215-224. |
| [18] | Cui L, Guo F, Zhang JL, Yang S, Wang JG, Meng JJ, Geng Y, Li XG, Wan SB (2019). Improvement of continuous microbial environment in peanut rhizosphere soil by Funneliformis mosseae. Chinese Journal of Plant Ecology, 43, 718-728. |
|
[崔利, 郭峰, 张佳蕾, 杨莎, 王建国, 孟静静, 耿耘, 李新国, 万书波 (2019). 摩西斗管囊霉改善连作花生根际土壤的微环境. 植物生态学报, 43, 718-728.]
DOI |
|
| [19] | Delgado-Baquerizo M, Guerra CA, Cano-Díaz C, Egidi E, Wang JT, Eisenhauer N, Singh BK, Maestre FT (2020). The proportion of soil-borne pathogens increases with warming at the global scale. Nature Climate Change, 10, 550-554. |
| [20] | Deng Y, Yu LY, Zhang YQ (2021). Research progress on the family Micromonosporaceae. Biotic Resources, 43, 583-596. |
| [邓阳, 余利岩, 张玉琴 (2021). 小单孢菌科放线菌的研究进展. 生物资源, 43, 583-596.] | |
| [21] | Deng ZY, Wang YC, Xiao CC, Zhang DX, Feng G, Long WX (2022). Effects of plant fine root functional traits and soil nutrients on the diversity of rhizosphere microbial communities in tropical cloud forests in a dry season. Forests, 13, 421. DOI: 10.3390/f13030421. |
| [22] | Dhungana I, Kantar MB, Nguyen NH (2023). Root exudate composition from different plant species influences the growth of rhizosphere bacteria. Rhizosphere, 25, 100645. DOI: 10.1016/j.rhisph.2022.100645. |
| [23] | Díaz S, Noy-Meir I, Cabido M (2001). Can grazing response of herbaceous plants be predicted from simple vegetative traits? Journal of Applied Ecology, 38, 497-508. |
| [24] | Dwivedi D, Riley WJ, Torn MS, Spycher N, Maggi F, Tang JY (2017). Mineral properties, microbes, transport, and plant-input profiles control vertical distribution and age of soil carbon stocks. Soil Biology & Biochemistry, 107, 244-259. |
| [25] | Foesel BU, Rohde M, Overmann J (2013). Blastocatella fastidiosa gen. nov., sp. nov., isolated from semiarid savanna soil—The first described species of Acidobacteria subdivision 4. Systematic and Applied Microbiology, 36, 82-89. |
| [26] | Garbeva P, van Elsas JD, van Veen JA (2008). Rhizosphere microbial community and its response to plant species and soil history. Plant and Soil, 302, 19-32. |
| [27] | He PC, Ye Q (2019). Plant functional traits: from individual plant to global scale. Journal of Tropical and Subtropical Botany, 27, 523-533. |
| [贺鹏程, 叶清 (2019). 基于植物功能性状的生态学研究进展: 从个体水平到全球尺度. 热带亚热带植物学报, 27, 523-533.] | |
| [28] |
Hiruma K, Gerlach N, Sacristán S, Nakano RT, Hacquard S, Kracher B, Neumann U, Ramírez D, Bucher M, O’Connell RJ, Schulze-Lefert P (2016). Root endophyte colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell, 165, 464-474.
DOI PMID |
| [29] | Hu HT, Zhu ZG, Yang JZ, Cao AC, Yan DD (2020). Effect of treatments on bacterial wilt of ginger at high mountain area and soil bacterial community. Microbiology China, 47, 1763-1775. |
| [胡洪涛, 朱志刚, 杨靖钟, 曹坳程, 颜冬冬 (2020). 不同处理对高山凤头姜姜瘟病的防效及土壤细菌群落结构和功能的影响. 微生物学通报, 47, 1763-1775.] | |
| [30] | Hu JP, Zhang MX, Lü ZL, He YY, Yang XX, Khan A, Xiong YC, Fang XL, Dong QM, Zhang JL (2023). Grazing practices affect phyllosphere and rhizosphere bacterial communities of Kobresia humilis by altering their network stability. Science of the Total Environment, 900, 165814. DOI: 10.1016/j.scitotenv.2023.165814. |
| [31] | Huang XF, Chaparro JM, Reardon KF, Zhang RF, Shen QR, Vivanco JM (2014). Rhizosphere interactions: root exudates, microbes, and microbial communities. Botany, 92, 267-275. |
| [32] |
Hufkens K, Keenan TF, Flanagan LB, Scott RL, Bernacchi CJ, Joo E, Brunsell NA, Verfaillie J, Richardson AD (2016). Productivity of North American grasslands is increased under future climate scenarios despite rising aridity. Nature Climate Change, 6, 710-714.
DOI |
| [33] | Jia LJ, Wang Z, Ji L, de Neve S, Struik PC, Yao YQ, Lv JJ, Zhou T, Jin K (2022). Keystone microbiome in the rhizosphere soil reveals the effect of long-term conservation tillage on crop growth in the Chinese Loess Plateau. Plant and Soil, 473, 457-472. |
| [34] | Jiao S, Xu YQ, Zhang J, Hao X, Lu YH (2019). Core microbiota in agricultural soils and their potential associations with nutrient cycling. mSystems, 4, e00313-18. DOI: 10.1128/mSystems.00313-18. |
| [35] |
Jiménez S, Ollat N, Deborde C, Maucourt M, Rellán-Álvarez R, Moreno MÁ, Gogorcena Y (2011). Metabolic response in roots of Prunus rootstocks submitted to iron chlorosis. Journal of Plant Physiology, 168, 415-423.
DOI PMID |
| [36] | Jing JY, Bezemer TM, van der Putten WH (2015). Complementarity and selection effects in early and mid-successional plant communities are differentially affected by plant-soil feedback. Journal of Ecology, 103, 641-647. |
| [37] | Kaštovská E, Edwards K, Picek T, Šantrůčková H (2015). A larger investment into exudation by competitive versus conservative plants is connected to more coupled plant-microbe N cycling. Biogeochemistry, 122, 47-59. |
| [38] | Kaźmierczak M, Błońska E, Kempf M, Zarek M, Lasota J (2024). Rhizosphere effect: microbial and enzymatic dynamics in the rhizosphere of various shrub species. Plant and Soil, 511, 245-262. |
| [39] | Lan ZC, Bai YF (2012). Testing mechanisms of N-enrichment-induced species loss in a semiarid Inner Mongolia grassland: critical thresholds and implications for long-term ecosystem responses. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 3125-3134. |
| [40] | Li Q, Zhao CZ, Zhao LC, Wang JW, Wen J (2019). The correlation analysis between specific leaf area and photosynthetic efficiency of Phragmites australis in salt marshes of Qinwangchuan. Acta Ecologica Sinica, 39, 7124-7133. |
| [李群, 赵成章, 赵连春, 王继伟, 文军 (2019). 秦王川盐沼湿地芦苇叶片比叶面积与光合效率的关联分析. 生态学报, 39, 7124-7133.] | |
| [41] | Li WH, Zheng SX, Bai YF (2014). Effects of grazing intensity and topography on species abundance distribution in a typical steppe of Inner Mongolia. Chinese Journal of Plant Ecology, 38, 178-187. |
|
[李文怀, 郑淑霞, 白永飞 (2014). 放牧强度和地形对内蒙古典型草原物种多度分布的影响. 植物生态学报, 38, 178-187.]
DOI |
|
| [42] | Lin QH, Tian D, Gao F, Ge XF, Ding YH, Ma SH, Hong JM, Ren D (2022). Effects of returning farmland to forest and grassland on soil bacteria: a case study in Bashang area, China. Soils, 54, 307-313. |
| [林权虹, 田地, 高菲, 葛星霏, 丁月泓, 马素辉, 洪剑明, 任东 (2022). 坝上地区退耕还林还草措施对土壤细菌的影响. 土壤, 54, 307-313.] | |
| [43] | Ling N, Wang TT, Kuzyakov Y (2022). Rhizosphere bacteriome structure and functions. Nature Communications, 13, 836. DOI: 10.1038/s41467-022-28448-9. |
| [44] | Lucke M, Correa MG, Levy A (2020). The role of secretion systems, effectors, and secondary metabolites of beneficial rhizobacteria in interactions with plants and microbes. Frontiers in Plant Science, 11, 589416. DOI: 10.3389/fpls.2020.589416. |
| [45] | Lv B, Ding L, Guo C, Chen F, Zhou HP, Wang XS, Dong XL, Xiang FY (2024). Effects of compound microbial fertilizer on soil nutrients and rhizosphere bacterial community in cotton field. Crops, 40(4), 209-215. |
| [吕博, 丁亮, 过聪, 陈锋, 周海平, 汪雪松, 董小林, 向发云 (2024). 复合微生物肥对棉田土壤养分及根际细菌群落的影响. 作物杂志, 40(4), 209-215.] | |
| [46] | Ma XD (2023). Assembly, Turnover and Function of Rhizosphere Microbial Communities of Seven Zonal Stipa species in Inner Mongolia Steppe. PhD dissertation, Inner Mongolia University, Hohhot. 54-67. |
| [马晓丹 (2023). 内蒙古草原7种地带性针茅根际微生物群落的构建、更替及功能探究. 博士学位论文, 内蒙古大学, 呼和浩特. 54-67.] | |
| [47] | Maitra P, Hrynkiewicz K, Szuba A, Jagodziński AM, Al-Rashid J, Mandal D, Mucha J (2024). Metabolic niches in the rhizosphere microbiome: dependence on soil horizons, root traits and climate variables in forest ecosystems. Frontiers in Plant Science, 15, 1344205. DOI: 10.3389/fpls.2024.1344205. |
| [48] |
Meier IC, Tuckmantel T, Heitkotter J, Muller K, Preusser S, Wrobel TJ, Kandeler E, Marschner B, Leuschner C (2020). Root exudation of mature beech forests across a nutrient availability gradient: the role of root morphology and fungal activity. New Phytologist, 226, 583-594.
DOI PMID |
| [49] | Molefe RR, Amoo AE, Babalola OO (2023). Communication between plant roots and the soil microbiome; involvement in plant growth and development. Symbiosis, 90, 231-239. |
| [50] |
Moreau D, Pivato B, Bru D, Busset H, Deau F, Faivre C, Matejicek A, Strbik F, Philippot L, Mougel C (2015). Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology, 96, 2300-2310.
PMID |
| [51] | Muscarella SM, Alduina R, Badalucco L, Capri FC, Di Leto Y, Gallo G, Laudicina VA, Paliaga S, Mannina G (2024). Water reuse of treated domestic wastewater in agriculture: Effects on tomato plants, soil nutrient availability and microbial community structure. Science of the Total Environment, 928, 172259. DOI: 10.1016/j.scitotenv.2024.172259. |
| [52] | Nabais C, Labuto G, Gonçalves S, Buscardo E, Semensatto D, Nogueira ARA, Freitas H (2011). Effect of root age on the allocation of metals, amino acids and sugars in different cell fractions of the perennial grass Paspalum notatum (bahiagrass). Plant Physiology and Biochemistry, 49, 1442-1447. |
| [53] | Nan J, Chao LM, Ma XD, Xu DL, Mo L, Zhang XD, Zhao XP, Bao YY (2020). Microbial diversity in the rhizosphere soils of three Stipa species from the eastern Inner Mongolian grasslands. Global Ecology and Conservation, 22, e00992. DOI: 10.1016/j.gecco.2020.e00992. |
| [54] |
Ortiz-López FJ, Oves-Costales D, Carretero-Molina D, Martín J, Díaz C, de la Cruz M, Román-Hurtado F, Álvarez-Arévalo M, Jørgensen TS, Reyes F, Weber T, Genilloud O (2023). Crossiellidines A-F, unprecedented pyrazine-alkylguanidine metabolites with broad-spectrum antibacterial activity from Crossiella sp. Organic Letters, 25, 3502-3507.
DOI PMID |
| [55] |
Rathore N, Hanzelková V, Dostálek T, Semerád J, Schnablová R, Cajthaml T, Münzbergová Z (2023). Species phylogeny, ecology, and root traits as predictors of root exudate composition. New Phytologist, 239, 1212-1224.
DOI PMID |
| [56] | Revillini D, David AS, Reyes AL, Knecht LD, Vigo C, Allen P, Searcy CA, Afkhami ME (2023). Allelopathy-selected microbiomes mitigate chemical inhibition of plant performance. New Phytologist, 240, 2007-2019. |
| [57] | Saeed Q, Wang XK, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, Naseem M, Kintl A, Ejaz M, Naveed M, Brtnicky M, Mustafa A (2021). Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: a comprehensive review of effects and mechanisms. International Journal of Molecular Sciences, 22, 10529. DOI: 10.3390/ijms221910529. |
| [58] | Sivaram AK, Subashchandrabose SR, Logeshwaran P, Lockington R, Naidu R, Megharaj M (2020). Rhizodegradation of PAHs differentially altered by C3 and C4 plants. Scientific Reports, 10, 16109. DOI: 10.1038/s41598-020-72844-4. |
| [59] | Sparks DL, Page AL, Loeppert PA, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME (1996). Methods of Soil Analysis Part 3: Chemical Methods. Soil Science Society of America and American Society of Agronomy, Madison. |
| [60] | Sweeney CJ, de Vries FT, van Dongen BE, Bardgett RD (2021). Root traits explain rhizosphere fungal community composition among temperate grassland plant species. New Phytologist, 229, 1492-1507. |
| [61] | Wan HW, Bai YF, Schönbach P, Gierus M, Taube F (2011). Effects of grazing management system on plant community structure and functioning in a semiarid steppe: scaling from species to community. Plant and Soil, 340, 215-226. |
| [62] | Wang CN, Li X, Lu XM, Wang Y, Bai YF (2023). Intraspecific trait variation governs grazing-induced shifts in plant community above- and below-ground functional trait composition. Agriculture, Ecosystems & Environment, 346, 108357. DOI: 10.1016/j.agee.2023.108357. |
| [63] | Wang SW, Li WH, Li YL, Yan H, Li YH (2022). Effects of different livestock types on plant diversity and community structure of a typical steppe in Nei Mongol, China. Chinese Journal of Plant Ecology, 46, 941-950. |
|
[王姝文, 李文怀, 李艳龙, 严慧, 李永宏 (2022). 放牧家畜类型对内蒙古典型草原植物多样性和群落结构的影响. 植物生态学报, 46, 941-950.]
DOI |
|
| [64] | Wang Z (2021). Effects of Different Grazing Types on Functional Traits of Dominant Species in Typical Steppe of Inner Mongolia. Master degree dissertation, Inner Mongolia University, Hohhot. |
| [王铮 (2021). 不同放牧方式对内蒙古典型草原优势种植物功能性状的影响. 硕士学位论文, 内蒙古大学, 呼和浩特.] | |
| [65] | Wei CF, Liu ST, Li Q, He J, Sun ZJ, Pan XY (2023). Diversity analysis of vineyards soil bacterial community in different planting years at eastern foot of Helan Mountain, Ningxia. Rhizosphere, 25, 100650. DOI: 10.1016/j.rhisph.2022.100650. |
| [66] |
Wen T, Zhao ML, Yuan J, Kowalchuk GA, Shen QR (2021). Root exudates mediate plant defense against foliar pathogens by recruiting beneficial microbes. Soil Ecology Letters, 3, 42-51.
DOI |
| [67] | Willms IM, Rudolph AY, Göschel I, Bolz SH, Schneider D, Penone C, Poehlein A, Schöning I, Nacke H (2020). Globally abundant “Candidatus Udaeobacter” benefits from release of antibiotics in soil and potentially performs trace gas scavenging. mSphere, 5, e00186-20. DOI: 10.1128/mSphere.00186-20. |
| [68] |
Wu LK, Lin XM, Lin WX (2014). Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chinese Journal of Plant Ecology, 38, 298-310.
DOI |
|
[吴林坤, 林向民, 林文雄 (2014). 根系分泌物介导下植物-土壤-微生物互作关系研究进展与展望. 植物生态学报, 38, 298-310.]
DOI |
|
| [69] | Wu SY, Baoyin T, Xu HB, Zhang L (2021). Effects of grazing intensities on functional traits of Cleistogenes squarrosa in a typical grassland of Inner Mongolia, China. Chinese Journal of Applied Ecology, 32, 392-398. |
|
[吴思雨, 宝音陶格涛, 许宏斌, 张璐 (2021). 放牧强度对内蒙古典型草原糙隐子草功能性状的影响. 应用生态学报, 32, 392-398.]
DOI |
|
| [70] | Xia XY, Wei QH, Wu HX, Chen XY, Xiao CX, Ye YP, Liu CT, Yu HY, Guo YW, Sun WX, Liu WD (2024). Bacillus species are core microbiota of resistant maize cultivars that induce host metabolic defense against corn stalk rot. Microbiome, 12, 156. DOI: 10.1186/s40168-024-01887-w. |
| [71] | Xiao T, Li P, Fei WB, Wang JD (2024). Effects of vegetation roots on the structure and hydraulic properties of soils: a perspective review. Science of the Total Environment, 906, 167524. DOI: 10.1016/j.scitotenv.2023.167524. |
| [72] | Xu K, Lu JH, Li X, Zhang JD, Luo JF, Zheng XR (2024). Composition and functions of soil bacterial communities of wild Glycyrrhiza uralensis Fisch. in habitats with different degrees of salinization. Acta Microbiologica Sinica, 64, 1550-1566. |
| [徐可, 陆嘉惠, 李新, 张迦得, 罗加粉, 郑雪荣 (2024). 不同盐渍化生境野生乌拉尔甘草土壤细菌群落结构及功能预测分析. 微生物学报, 64, 1550-1566.] | |
| [73] | Xun WB, Liu YP, Li W, Ren Y, Xiong W, Xu ZH, Zhang N, Miao YZ, Shen QR, Zhang RF (2021). Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome, 9, 35. DOI: 10.1186/s40168-020-00985-9. |
| [74] | Yang CB, Zhang XP, Ni HJ, Gai X, Huang ZC, Du XH, Zhong ZK (2021). Soil carbon and associated bacterial community shifts driven by fine root traits along a chronosequence of Moso bamboo (Phyllostachys edulis) plantations in subtropical China. Science of the Total Environment, 752, 142333. DOI: 10.1016/j.scitotenv.2020.142333. |
| [75] | Yang Y, Zhang H, Liu W, Sun JM, Zhao ML, Han GD, Pan QM (2023). Effects of grazing intensity on diversity and composition of rhizosphere and non-rhizosphere microbial communities in a desert grassland. Ecology and Evolution, 13, e10300. DOI: 10.1002/ece3.10300. |
| [76] | Yu FM, Jayawardena RS, Thongklang N, Lv ML, Zhu XT, Zhao Q (2022). Morel production associated with soil nitrogen-fixing and nitrifying microorganisms. Journal of Fungi, 8, 299. DOI: 10.3390/jof8030299. |
| [77] | Yu ZY, Yan YJ, Liu CX, Huang JP, Xiang WS, Huang SX (2021). Secondary metabolites and genetic system of the rare actinobacteria Lechevalieria rhizosphaerae NEAU-A2. Microbiology China, 48, 2318-2328. |
| [余志银, 颜一军, 刘重喜, 黄建萍, 向文胜, 黄胜雄 (2021). 稀有放线菌Lechevalieria rhizosphaerae NEAU-A2的次级代谢产物研究及遗传操作系统的建立. 微生物学通报, 48, 2318-2328.] | |
| [78] |
Yuan J, Zhao J, Wen T, Zhao ML, Li R, Goossens P, Huang QW, Bai Y, Vivanco JM, Kowalchuk GA, Berendsen RL, Shen QR (2018). Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome, 6, 156. DOI: 10.1186/s40168-018-0537-x.
PMID |
| [79] | Zeng WA, Wang ZH, Xiao YS, Teng K, Cao ZH, Cai HL, Liu YJ, Yin HQ, Cao PJ, Tao JM (2022). Insights into the interactions between root phenotypic traits and the rhizosphere bacterial community. Current Microbiology, 79, 176. DOI: 10.1007/s00284-022-02870-0. |
| [80] |
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi SJ, Cho H, Karaoz U, Loqué D, Bowen BP, Firestone MK, Northen TR, Brodie EL (2018). Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nature Microbiology, 3, 470-480.
DOI PMID |
| [81] | Zhang JH, He NP, Liu CC, Xu L, Chen Z, Li Y, Wang RM, Yu GR, Sun W, Xiao CW, Chen HYH, Reich PB (2020a). Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Global Change Biology, 26, 2534-2543. |
| [82] | Zhang JY, Liu YX, Zhang N, Hu B, Jin T, Xu HR, Qin Y, Yan PX, Zhang XN, Guo XX, Hui J, Cao SY, Wang X, Wang C, Wang H, et al. (2019). NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nature Biotechnology, 37, 676-684. |
| [83] | Zhang YT, Gao XL, Hao XY, Alexander TW, Shi XJ, Jin L, Thomas BW (2020b). Heavy grazing over 64 years reduced soil bacterial diversity in the foothills of the Rocky Mountains, Canada. Applied Soil Ecology, 147, 103361. DOI: 10.1016/j.apsoil.2019.09.011. |
| [84] | Zhang ZW, Gao B, Lin CC, Wang Q, Liu JL, Yang N, Su DR, Ping XY (2021). Effects of grazing intensity on biomass allocation patterns of six plant species in a typical grassland. Acta Agrestia Sinica, 29, 149-155. |
|
[张紫薇, 高斌, 林长存, 王青, 刘佳乐, 杨娜, 苏德荣, 平晓燕 (2021). 放牧强度对典型草原6个物种生物量分配格局的影响. 草地学报, 29, 149-155.]
DOI |
|
| [85] |
Zhao K, Han GD (2023). Responses of the functional traits of dominant species to different grazing intensities in desert steppe. Acta Agrestia Sinica, 31, 649-656.
DOI |
|
[赵坤, 韩国栋 (2023). 荒漠草原优势种功能性状对不同放牧强度的响应. 草地学报, 31, 649-656.]
DOI |
|
| [86] | Zhao Y, Peth S, Reszkowska A, Gan L, Krümmelbein J, Peng XH, Horn R (2011). Response of soil moisture and temperature to grazing intensity in a Leymus chinensis steppe, Inner Mongolia. Plant and Soil, 340, 89-102. |
| [87] | Zheng SX, Li WH, Lan ZC, Ren HY, Wang KB (2015). Functional trait responses to grazing are mediated by soil moisture and plant functional group identity. Scientific Reports, 5, 18163. DOI: 10.1038/srep18163. |
| [88] | Zhou YX, Chen J, Li Y, Hou ZA, Min W (2022). Effects of cotton stalk returning on soil enzyme activity and bacterial community structure diversity in cotton field with long-term saline water irrigation. Environmental Science, 43, 2192-2203. |
| [周永学, 陈静, 李远, 侯振安, 闵伟 (2022). 棉秆还田对咸水滴灌棉田土壤酶活性和细菌群落结构多样性的影响. 环境科学, 43, 2192-2203.] |
| [1] | 童金莲, 张博纳, 汤璐瑶, 叶琳峰, 李姝雯, 谢江波, 李彦, 王忠媛. C4植物狗尾草功能性状网络沿降水梯度带的区域分异规律[J]. 植物生态学报, 2025, 49(预发表): 1-. |
| [2] | 郑子仪, 陈江慧, 刘慧颖. 气候变暖增加了青藏高原高寒草甸优势物种的根系分泌速率[J]. , 2025, 49(地上地下生态过程关联): 0-. |
| [3] | 马腾飞, 郝杰, 刁华杰, 宁亚楠, $\boxed{\hbox{王常慧}}$, 董宽虎. 晋北农牧交错带草地土壤无机氮含量的季节变化及其对放牧强度的响应[J]. 植物生态学报, 2025, 49(6): 965-974. |
| [4] | 杜英杰, 范爱连, 王雪, 闫晓俊, 陈廷廷, 贾林巧, 姜琦, 陈光水. 亚热带天然常绿阔叶林乔木树种与林下灌木树种根-叶功能性状协调性及差异[J]. 植物生态学报, 2025, 49(4): 585-595. |
| [5] | 秦嘉晨, 王欢, 朱江, 王扬, 田晨, 白永飞, 杨培志, 郑淑霞. 基于种内与种间性状变异的放牧过滤作用及其尺度效应[J]. 植物生态学报, 2024, 48(7): 858-871. |
| [6] | 付粱晨, 丁宗巨, 唐茂, 曾辉, 朱彪. 北京东灵山白桦和蒙古栎的根际效应及其季节动态[J]. 植物生态学报, 2024, 48(4): 508-522. |
| [7] | 萨其拉, 张霞, 朱琳, 康萨如拉. 长期不同放牧强度下荒漠草原优势种无芒隐子草叶片解剖结构变化[J]. 植物生态学报, 2024, 48(3): 331-340. |
| [8] | 王思琦, 金光泽. 五角槭不同生活史阶段叶枝根性状的变异与权衡[J]. 植物生态学报, 2024, 48(11): 1510-1523. |
| [9] | 胡楚婷, 杨柳依依, 石绍林, 周琰, 陈婷婷, 郑博瀚, 杨暘, 卢小玲, 王陈玲, 倪健. 浙江金华典型人工植被的植物功能性状[J]. 植物生态学报, 2024, 48(10): 1336-1350. |
| [10] | 李红琴, 张法伟, 仪律北. 高寒草甸表层土壤和优势植物叶片的化学计量特征对降水改变和氮添加的响应[J]. 植物生态学报, 2023, 47(7): 922-931. |
| [11] | 张仲富, 王四海, 杨卫, 陈剑. 蒜头果根际细菌群落结构与功能特征对其健康状态的响应[J]. 植物生态学报, 2023, 47(7): 1020-1031. |
| [12] | 汤璐瑶, 方菁, 钱海蓉, 张博纳, 上官方京, 叶琳峰, 李姝雯, 童金莲, 谢江波. 落羽杉和池杉功能性状随高度的变异与协同[J]. 植物生态学报, 2023, 47(11): 1561-1575. |
| [13] | 张义, 程杰, 苏纪帅, 程积民. 长期封育演替下典型草原植物群落生产力与多样性关系[J]. 植物生态学报, 2022, 46(2): 176-187. |
| [14] | 罗源林, 马文红, 张芯毓, 苏闯, 史亚博, 赵利清. 内蒙古锦鸡儿属植物地理替代分布种的功能性状沿环境梯度的变化[J]. 植物生态学报, 2022, 46(11): 1364-1375. |
| [15] | 祁鲁玉, 陈浩楠, 库丽洪·赛热别力, 籍天宇, 孟高德, 秦慧颖, 王宁, 宋逸欣, 刘春雨, 杜宁, 郭卫华. 基于植物功能性状的暖温带5种灌木幼苗生长策略[J]. 植物生态学报, 2022, 46(11): 1388-1399. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19