

植物生态学报 ›› 2022, Vol. 46 ›› Issue (7): 823-833.DOI: 10.17521/cjpe.2021.0200
收稿日期:2021-05-26
接受日期:2021-10-18
出版日期:2022-07-20
发布日期:2021-12-16
通讯作者:
杨汉奇
作者简介:* 杨汉奇: ORCID:0000-0002-0404-6685 (yanghanqikm@aliyun.com)基金资助:
XIA Ti-Ze1,2, LI Lu-Shuang1, YANG Han-Qi1,*( )
)
Received:2021-05-26
Accepted:2021-10-18
Online:2022-07-20
Published:2021-12-16
Contact:
YANG Han-Qi
Supported by:摘要:
物种地理分布边界的形成一直是进化生物学中的一个重要课题。植物分布区边界如何影响土壤微生物尚不清楚。屏边空竹(Cephalostachyum pingbianense)是目前已知唯一能够在自然条件下四季出笋的珍稀竹种, 仅分布于中国云南东南部, 对研究竹类中的狭域分布种具有重要意义。为探究土壤真菌群落与屏边空竹分布边界间的联系, 该研究测定了屏边空竹分布中心、分布边界以及分布区外的土壤理化性质, 并利用基于真菌内转录间隔区(ITS)序列的Illumina MiSeq高通量测序技术分析土壤真菌群落变化。主要结果: 1)屏边空竹分布边界处土壤pH与速效磷含量显著低于其他位点。2)在分布中心, 土壤真菌物种多样性最高, 被孢霉属(Moritierella)相对多度显著高于其他位点; 在分布边界处, 土壤真菌物种多样性最低, 担子菌门相对多度大于65.0%。3)土壤pH是驱动真菌群落变异的关键因素, 与外生菌根真菌的相对多度负相关, 与腐生真菌的相对多度正相关。综上, 土壤酸化与磷缺乏可能是控制屏边空竹分布区域的重要土壤属性。土壤中的被孢霉具有溶解磷、缓解土壤酸化等功能, 可能是屏边空竹重要的互利共生菌。
夏体泽, 李露双, 杨汉奇. 屏边空竹分布区海拔上下边界的土壤真菌群落特征. 植物生态学报, 2022, 46(7): 823-833. DOI: 10.17521/cjpe.2021.0200
XIA Ti-Ze, LI Lu-Shuang, YANG Han-Qi. Soil fungal community characteristics at the upper and lower altitudinal range limits of Cephalostachyum pingbianense. Chinese Journal of Plant Ecology, 2022, 46(7): 823-833. DOI: 10.17521/cjpe.2021.0200
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| pH | 5.06 ± 0.17a | 3.65 ± 0.32c | 4.49 ± 0.18b | 4.04 ± 0.12c | 4.67 ± 0.12b |
| 有机质含量 SOM content (g·kg-1) | 79.20 ± 6.67b | 120.90 ± 7.24a | 121.84 ± 6.49a | 128.75 ± 12.61a | 128.05 ± 9.39a |
| 全氮含量 TN content (g·kg-1) | 3.89 ± 0.53d | 6.36 ± 1.84b | 4.48 ± 0.36c | 7.10 ± 0.11a | 6.55 ± 0.74b |
| 速效氮含量 AN content (g·kg-1) | 0.56 ± 0.07c | 1.08 ± 0.25a | 0.61 ± 0.04bc | 0.76 ± 0.19b | 0.74 ± 0.06b |
| 全磷含量 TP content (g·kg-1) | 0.52 ± 0.02b | 0.59 ± 0.07b | 0.68 ± 0.05b | 0.53 ± 0.04b | 0.94 ± 0.10a |
| 速效磷含量 AP content (mg·kg-1) | 12.58 ± 1.02b | 9.96 ± 0.82c | 13.03 ± 0.70b | 8.43 ± 3.41c | 18.04 ± 6.67a |
| 全钾含量 TK content (g·kg-1) | 16.59 ± 1.37a | 12.49 ± 2.40b | 16.02 ± 0.53ab | 12.46 ± 2.58b | 14.77 ± 1.33b |
| 速效钾含量 AK content (g·kg-1) | 0.16 ± 0.01b | 0.19 ± 0.01a | 0.15 ± 0.02b | 0.17 ± 0.05ab | 0.19 ± 0.02a |
表1 屏边空竹分布区不同位点土壤理化性质(平均值±标准差)
Table 1 Physiochemical properties of different sites in the distribution area of Cephalostachyum pingbianense (mean ± SD)
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| pH | 5.06 ± 0.17a | 3.65 ± 0.32c | 4.49 ± 0.18b | 4.04 ± 0.12c | 4.67 ± 0.12b |
| 有机质含量 SOM content (g·kg-1) | 79.20 ± 6.67b | 120.90 ± 7.24a | 121.84 ± 6.49a | 128.75 ± 12.61a | 128.05 ± 9.39a |
| 全氮含量 TN content (g·kg-1) | 3.89 ± 0.53d | 6.36 ± 1.84b | 4.48 ± 0.36c | 7.10 ± 0.11a | 6.55 ± 0.74b |
| 速效氮含量 AN content (g·kg-1) | 0.56 ± 0.07c | 1.08 ± 0.25a | 0.61 ± 0.04bc | 0.76 ± 0.19b | 0.74 ± 0.06b |
| 全磷含量 TP content (g·kg-1) | 0.52 ± 0.02b | 0.59 ± 0.07b | 0.68 ± 0.05b | 0.53 ± 0.04b | 0.94 ± 0.10a |
| 速效磷含量 AP content (mg·kg-1) | 12.58 ± 1.02b | 9.96 ± 0.82c | 13.03 ± 0.70b | 8.43 ± 3.41c | 18.04 ± 6.67a |
| 全钾含量 TK content (g·kg-1) | 16.59 ± 1.37a | 12.49 ± 2.40b | 16.02 ± 0.53ab | 12.46 ± 2.58b | 14.77 ± 1.33b |
| 速效钾含量 AK content (g·kg-1) | 0.16 ± 0.01b | 0.19 ± 0.01a | 0.15 ± 0.02b | 0.17 ± 0.05ab | 0.19 ± 0.02a |
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| Shannon指数 Shannon index | 4.37 ± 0.13b | 3.02 ± 0.48c | 4.84 ± 0.08a | 2.97 ± 0.02c | 4.31 ± 0.21b |
| Chao1指数 Chao1 index | 670 ± 160c | 1 163 ± 152b | 1 655 ± 147a | 1 199 ± 42b | 1 321 ± 190ab |
| 可操作分类单元 OTU | 641 ± 130c | 935 ± 140b | 1 442 ± 95a | 917 ± 28b | 1 107 ± 117b |
表2 屏边空竹分布区土壤真菌α多样性的比较分析(平均值±标准差)
Table 2 Comparison analysis of soil fungal alpha diversity in the distribution area of Cephalostachyum pingbianense (mean ± SD)
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| Shannon指数 Shannon index | 4.37 ± 0.13b | 3.02 ± 0.48c | 4.84 ± 0.08a | 2.97 ± 0.02c | 4.31 ± 0.21b |
| Chao1指数 Chao1 index | 670 ± 160c | 1 163 ± 152b | 1 655 ± 147a | 1 199 ± 42b | 1 321 ± 190ab |
| 可操作分类单元 OTU | 641 ± 130c | 935 ± 140b | 1 442 ± 95a | 917 ± 28b | 1 107 ± 117b |
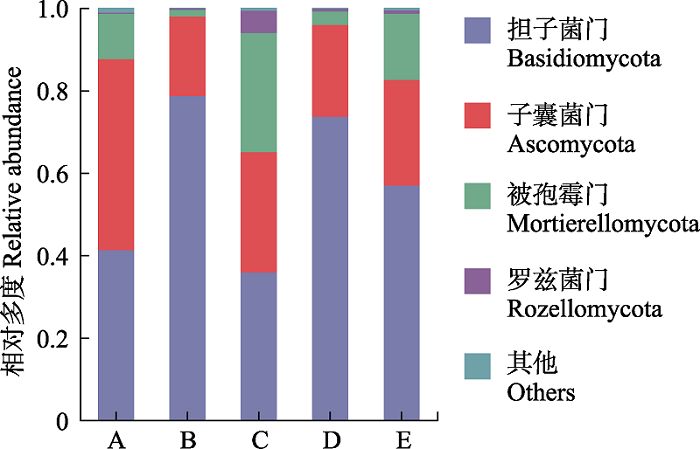
图1 屏边空竹分布区不同位点门分类水平土壤真菌群落组成。A, 毛叶青冈林; B, 屏边空竹海拔分布下限; C, 屏边空竹海拔分布中心; D, 屏边空竹海拔分布上限; E, 石栎林。
Fig. 1 Soil fungal community composition at the phylum level at different sites in the distribution area of Cephalostachyum pingbianense. A, Cyclobalanopsis kerrii forest; B, the lower altitudinal range limit of Cephalostachyum pingbianense; C, the altitudinal range center of C. pingbianense; D, the upper altitudinal range limit of C. pingbianense; E, Lithocarpus glaber forest.
| 显著差异的真菌 Fungi with significant differences | A | B | C | D | E |
|---|---|---|---|---|---|
| 担子菌门 Basidiomycota | 30.04 ± 4.25c | 70.11 ± 3.32a | 30.13 ± 1.47c | 66.35 ± 3.80a | 50.84 ± 2.71b |
| 子囊菌门 Ascomycota | 48.87 ± 5.07a | 20.63 ± 1.67c | 26.61 ± 1.93b | 25.42 ± 5.17bc | 27.94 ± 2.51b |
| 被孢霉门 Mortierellomycota | 8.59 ± 4.27bc | 1.52 ± 0.13c | 23.78 ± 0.96a | 2.93 ± 2.23c | 14.49 ± 0.27b |
| 罗兹菌门 Rozellomycota | 0.88 ± 0.32c | 1.08 ± 0.25a | 7.86 ± 0.89a | 2.88 ± 2.22b | 1.58 ± 0.08b |
| 被孢霉属 Mortierella | 8.38 ± 4.11bc | 1.46 ± 0.13c | 23.29 ± 0.85a | 0.53 ± 0.04c | 11.46 ± 0.21b |
| Saitozyma | 15.40 ± 7.77a | 2.52 ± 1.52b | 15.44 ± 1.48a | 4.05 ± 3.10b | 11.67 ± 3.34a |
| 红菇属 Russula | 0.51 ± 0.22c | 27.61 ± 22.57a | 0.50 ± 0.15c | 57.63 ± 3.03a | 10.02 ± 6.76ab |
| 产油菌属 Solicoccozyma | 0.16 ± 0.15b | 0.45 ± 0.41b | 5.56 ± 1.52a | 0.27 ± 0.37b | 1.78 ± 0.82b |
| 珊瑚菌属 Clavaria | 5.04 ± 3.59a | 0.03 ± 0.05b | 0.00 ± 0.00b | 0.02 ± 0.01b | 0.03 ± 0.01b |
| 绿僵菌属 Metarhizium | 2.41 ± 0.53b | 0.75 ± 0.55c | 4.60 ± 0.79a | 0.27 ± 0.19c | 1.60 ± 0.51bc |
| 刺球菌属 Chaetosphaeria | 0.48 ± 0.33ab | 0.50 ± 0.23ab | 1.16 ± 0.43a | 0.32 ± 0.06b | 1.24 ± 0.23a |
| 腾梗孢霉属 Gonytrichum | 0.94 ± 0.84b | 0.02 ± 0.01b | 2.20 ± 0.33a | 0.09 ± 0.08b | 0.26 ± 0.13b |
| 织球壳属 Plectosphaerella | 2.06 ± 0.40a | 0.03 ± 0.02b | 0.03 ± 0.09b | 0.02 ± 0.01b | 0.09 ± 0.02b |
表3 屏边空竹分布区不同位点间显著差异的土壤真菌门和属(平均值±标准差, %)
Table 3 Fungal phyla and genera with significant difference between different sites in the distribution area of Cephalostachyum pingbianense (mean ± SD, %)
| 显著差异的真菌 Fungi with significant differences | A | B | C | D | E |
|---|---|---|---|---|---|
| 担子菌门 Basidiomycota | 30.04 ± 4.25c | 70.11 ± 3.32a | 30.13 ± 1.47c | 66.35 ± 3.80a | 50.84 ± 2.71b |
| 子囊菌门 Ascomycota | 48.87 ± 5.07a | 20.63 ± 1.67c | 26.61 ± 1.93b | 25.42 ± 5.17bc | 27.94 ± 2.51b |
| 被孢霉门 Mortierellomycota | 8.59 ± 4.27bc | 1.52 ± 0.13c | 23.78 ± 0.96a | 2.93 ± 2.23c | 14.49 ± 0.27b |
| 罗兹菌门 Rozellomycota | 0.88 ± 0.32c | 1.08 ± 0.25a | 7.86 ± 0.89a | 2.88 ± 2.22b | 1.58 ± 0.08b |
| 被孢霉属 Mortierella | 8.38 ± 4.11bc | 1.46 ± 0.13c | 23.29 ± 0.85a | 0.53 ± 0.04c | 11.46 ± 0.21b |
| Saitozyma | 15.40 ± 7.77a | 2.52 ± 1.52b | 15.44 ± 1.48a | 4.05 ± 3.10b | 11.67 ± 3.34a |
| 红菇属 Russula | 0.51 ± 0.22c | 27.61 ± 22.57a | 0.50 ± 0.15c | 57.63 ± 3.03a | 10.02 ± 6.76ab |
| 产油菌属 Solicoccozyma | 0.16 ± 0.15b | 0.45 ± 0.41b | 5.56 ± 1.52a | 0.27 ± 0.37b | 1.78 ± 0.82b |
| 珊瑚菌属 Clavaria | 5.04 ± 3.59a | 0.03 ± 0.05b | 0.00 ± 0.00b | 0.02 ± 0.01b | 0.03 ± 0.01b |
| 绿僵菌属 Metarhizium | 2.41 ± 0.53b | 0.75 ± 0.55c | 4.60 ± 0.79a | 0.27 ± 0.19c | 1.60 ± 0.51bc |
| 刺球菌属 Chaetosphaeria | 0.48 ± 0.33ab | 0.50 ± 0.23ab | 1.16 ± 0.43a | 0.32 ± 0.06b | 1.24 ± 0.23a |
| 腾梗孢霉属 Gonytrichum | 0.94 ± 0.84b | 0.02 ± 0.01b | 2.20 ± 0.33a | 0.09 ± 0.08b | 0.26 ± 0.13b |
| 织球壳属 Plectosphaerella | 2.06 ± 0.40a | 0.03 ± 0.02b | 0.03 ± 0.09b | 0.02 ± 0.01b | 0.09 ± 0.02b |
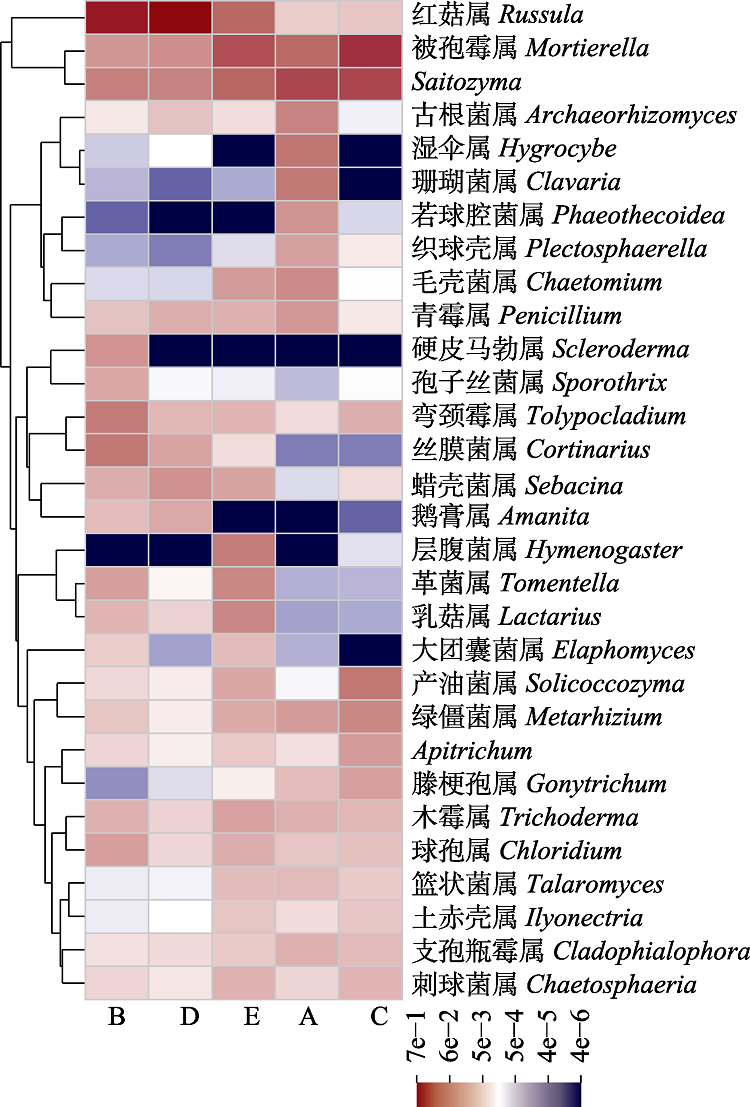
图2 屏边空竹分布区不同位点土壤相对多度前30真菌属的聚类热图。A, 毛叶青冈林; B, 屏边空竹海拔分布下限; C, 屏边空竹海拔分布中心; D, 屏边空竹海拔分布上限; E, 石栎林。
Fig. 2 Heatmap showing the top 30 abundant genera in soil at different sites in the distribution area of Cephalostachyum pingbianense. A, Cyclobalanopsis kerrii forest; B, the lower altitudinal range limit of Cephalostachyum pingbianense; C, the altitudinal range center of C. pingbianense; D, the upper altitudinal range limit of C. pingbianense; E, Lithocarpus glaber forest.
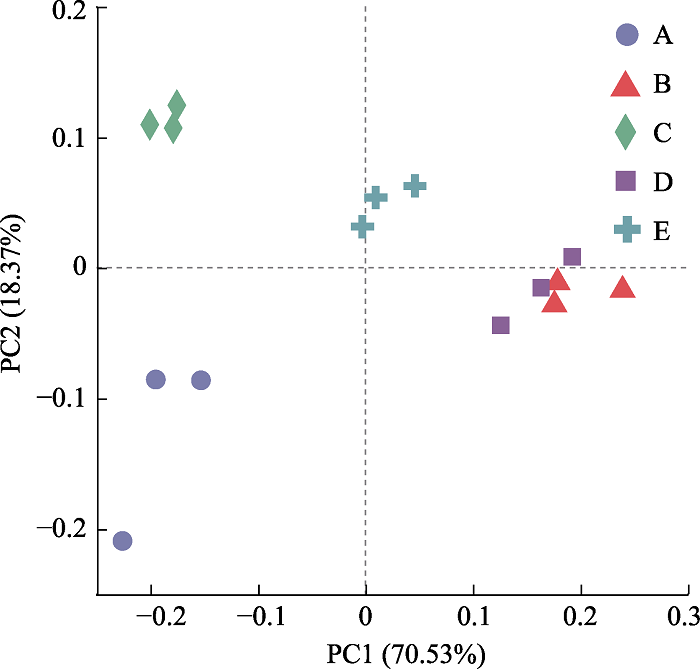
图3 基于Bray-Curtis距离算法的土壤真菌群落主坐标分析(PCoA)。 PC1, 主坐标1; PC2, 主坐标2。A, 毛叶青冈林; B, 屏边空竹海拔分布下限; C, 屏边空竹海拔分布中心; D, 屏边空竹海拔分布上限; E, 石栎林。
Fig. 3 Principle coordinate analysis (PCoA) based on Bray-Curtis distance method of soil fungal community. PC1, principal co-ordinates 1; PC2, principal co-ordinates 2. A, Cyclobalanopsis kerrii forest; B, the lower altitudinal range limit of Cephalostachyum pingbianense; C, the altitudinal range center of C. pingbianense; D, the upper altitudinal range limit of C. pingbianense; E, Lithocarpus glaber forest.
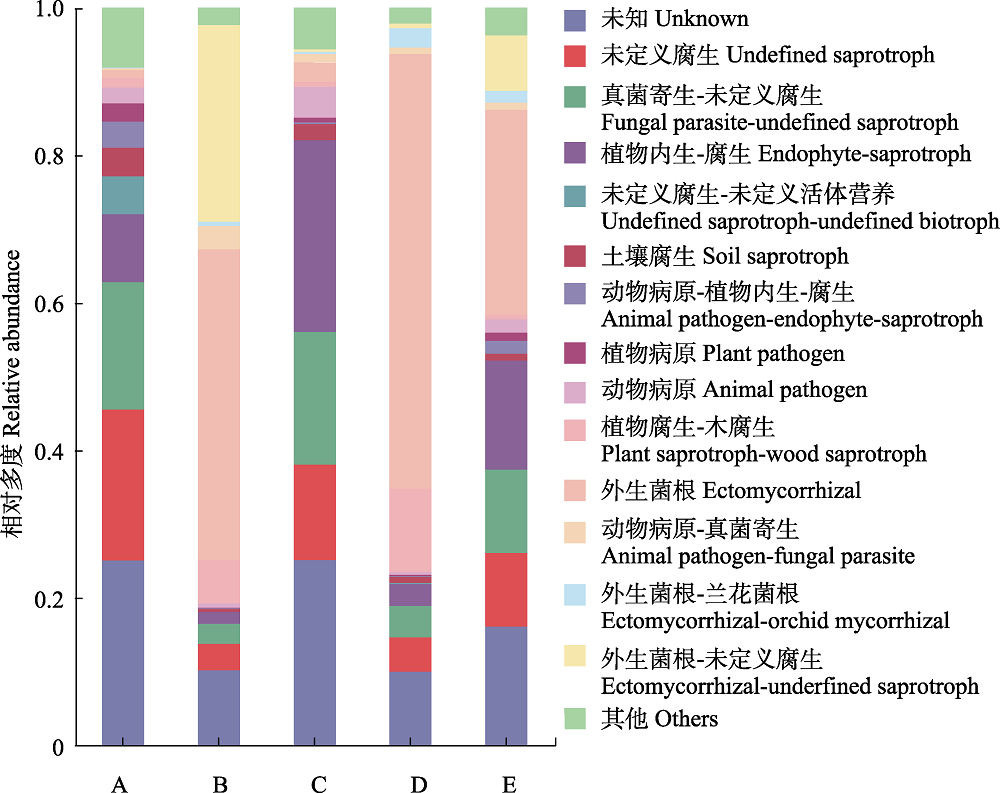
图4 屏边空竹分布区不同位点土壤真菌群落功能注释。A, 毛叶青冈林; B, 屏边空竹海拔分布下限; C, 屏边空竹海拔分布中心; D, 屏边空竹海拔分布上限; E, 石栎林。
Fig. 4 Functional annotation of soil fungal community at different sites in the distribution area of Cephalostachyum pingbianense. A, Cyclobalanopsis kerrii forest; B, the lower range limit of Cephalostachyum pingbianense; C, the range center of C. pingbianense; D, the upper range limit of C. pingbianense; E, Lithocarpus glaber forest.
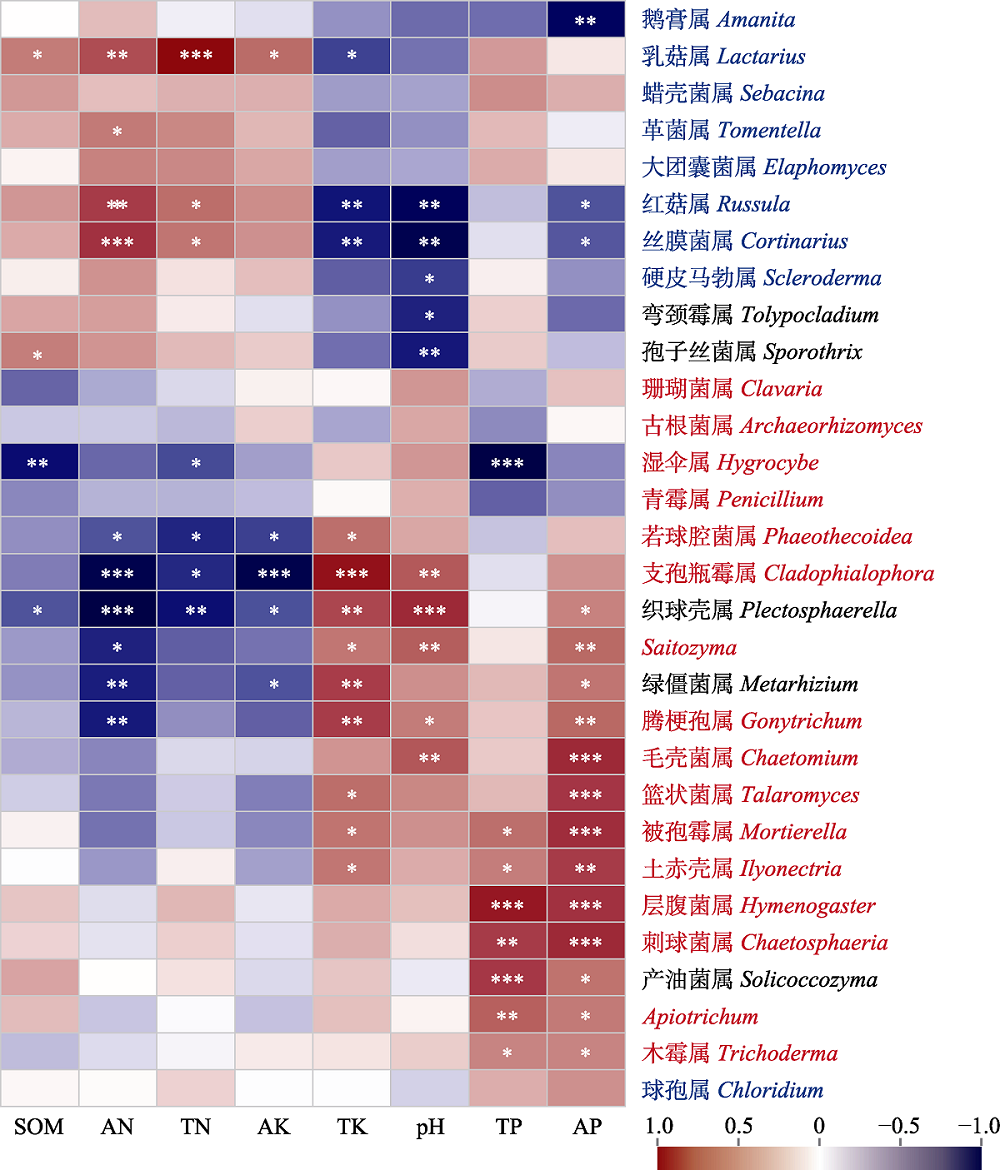
图5 屏边空竹分布区土壤真菌属水平与土壤理化性质的Spearman相关性分析及功能注释。图中所列为相对多度前30的真菌属, 根据真菌功能注释, 外生菌根真菌用蓝色标出, 腐生真菌用红色标出, 其他类型真菌用黑色标出。*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001。AK, 速效钾含量; AN, 全氮含量; AP, 速效磷含量; SOM, 有机质含量; TK, 全钾含量; TN, 全氮含量; TP, 全磷含量。
Fig. 5 Spearman correlation analysis between fungal genera and soil physicochemical properties, and functional annotation in the distribution area of Cephalostachyum pingbianense. Top 30 abundant fungal genera are listed. Based on fungal functional annotation, ectomycorrhizal fungi are marked in red, saprophytic fungi are marked in blue and other types of fungi are marked in black. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. AK, available potassium (K) content; AN, available nitrogen (N) content; AP, available phosphorus (P) content; SOM, soil organic matter content; TK, total K content; TN, total N content; TP, total P content.
| 项目 Item | 全磷含量 TP content | 速效钾含量 AK content | 有机质含量 SOM content | 全氮含量 TN content | 全钾含量 TK content | 速效氮含量 AN content | 速效磷含量 AP content | pH |
|---|---|---|---|---|---|---|---|---|
| r | -0.004 | 0.03 | 0.14 | 0.23 | 0.26 | 0.36 | 0.30 | 0.53 |
| p | 0.963 | 0.766 | 0.125 | 0.033 | 0.014 | 0.005 | 0.004 | 0.001 |
表4 Mantel检验屏边空竹分布区微生物群落结构与土壤环境因子的相关性
Table 4 Mantel test results for the correlation between fungal community composition and soil environment factors in the distribution area of Cephalostachyum pingbianense
| 项目 Item | 全磷含量 TP content | 速效钾含量 AK content | 有机质含量 SOM content | 全氮含量 TN content | 全钾含量 TK content | 速效氮含量 AN content | 速效磷含量 AP content | pH |
|---|---|---|---|---|---|---|---|---|
| r | -0.004 | 0.03 | 0.14 | 0.23 | 0.26 | 0.36 | 0.30 | 0.53 |
| p | 0.963 | 0.766 | 0.125 | 0.033 | 0.014 | 0.005 | 0.004 | 0.001 |
| [1] |
Adams RI, Miletto M, Taylor JW, Bruns TD (2013). Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. The ISME Journal, 7, 1262-1273.
DOI URL |
| [2] |
Ali A, Lin SL, He JK, Kong FM, Yu JH, Jiang HS (2019). Climatic water availability is the main limiting factor of biotic attributes across large-scale elevational gradients in tropical forests. Science of the Total Environment, 647, 1211-1221.
DOI URL |
| [3] |
Benning JW, Moeller DA (2021). Microbes, mutualism, and range margins: testing the fitness consequences of soil microbial communities across and beyond a native plantʼs range. New Phytologist, 229, 2886-2900.
DOI PMID |
| [4] |
Bojórquez-Quintal E, Escalante-Magaña C, Echevarría-Machado I, Martínez-Estévez M (2017). Aluminum, a friend or foe of higher plants in acid soils. Frontiers in Plant Science, 8, 1767. DOI: 10.3389/fpls.2017.01767.
DOI PMID |
| [5] |
Bonfante P, Venice F (2020). Mucoromycota: going to the roots of plant-interacting fungi. Fungal Biology Reviews, 34, 100-113.
DOI URL |
| [6] | Bruelheide H, Scheidel U (1999). Slug herbivory as a limiting factor for the geographical range of Arnica montana. Journal of Ecology, 87, 839-848. |
| [7] |
Burns JH, Anacker BL, Strauss SY, Burke DJ (2015). Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AoB PLANTS, 7, plv030. DOI: 10.1093/aobpla/plv030.
DOI |
| [8] |
Corsaro D, Walochnik J, Venditti D, Hauröder B, Michel R (2020). Solving an old enigma: Morellospora saccamoebae gen. nov., sp. nov. (Rozellomycota), a Sphaerita-like parasite of free-living amoebae. Parasitology Research, 119, 925-934.
DOI URL |
| [9] |
de Wit R, Bouvier T (2006). “Everything is everywhere, but, the environment selects”; What did Baas Becking and Beijerinck really say? Environmental Microbiology, 8, 755-758.
DOI URL |
| [10] |
Emamverdian A, Ding Y, Ranaei F, Ahmad Z (2020). Application of bamboo plants in nine aspects. The Scientific World Journal, 7284203. DOI: 10.1155/2020/7284203.
DOI |
| [11] |
Feng HF, Liu LY, Xue L (2019). Effects of nitrogen and phosphorus additions and stand density on soil chemical property in Acacia auriculiformis stands. Chinese Journal of Plant Ecology, 43, 1010-1020.
DOI URL |
|
[冯慧芳, 刘落鱼, 薛立 (2019). 氮磷添加及林分密度对大叶相思林土壤化学性质的影响. 植物生态学报, 43, 1010-1020.]
DOI |
|
| [12] |
Fierer N, Bradford MA, Jackson RB (2007). Toward an ecological classification of soil bacteria. Ecology, 88, 1354-1364.
PMID |
| [13] |
Genre A, Lanfranco L, Perotto S, Bonfante P (2020). Unique and common traits in mycorrhizal symbioses. Nature Reviews Microbiology, 18, 649-660.
DOI URL |
| [14] |
Hannula SE, Kielak AM, Steinauer K, Huberty M, Jongen R, de Long JR, Heinen R, Bezemer TM (2019). Time after time: temporal variation in the effects of grass and forb species on soil bacterial and fungal communities. mBio, 10, e02635-19. DOI: 10.1128/mBio.02635-19.
DOI |
| [15] |
Hargreaves AL, Weiner JL, Eckert CG (2015). High-elevation range limit of an annual herb is neither caused nor reinforced by declining pollinator service. Journal of Ecology, 103, 572-584.
DOI URL |
| [16] |
Jones DL, Cooledge EC, Hoyle FC, Griffiths RI, Murphy DV (2019). pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biology & Biochemistry, 138, 107584. DOI: 10.1016/j.soilbio.2019.107584.
DOI URL |
| [17] |
Kaushal R, Singh I, Thapliyal SD, Gupta AK, Mandal D, Tomar JMS, Kumar A, Alam NM, Kadam D, Singh DV, Mehta H, Dogra P, Ojasvi PR, Reza S, Durai J (2020). Rooting behaviour and soil properties in different bamboo species of Western Himalayan Foothills, India. Scientific Reports, 10, 4966. DOI: 10.1038/s41598-020-61418-z.
DOI PMID |
| [18] |
Kirkpatrick M, Barton NH (1997). Evolution of a speciesʼ range. The American Naturalist, 150, 1-23.
DOI URL |
| [19] |
Kochian LV, Hoekenga OA, Pineros MA (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology, 55, 459-493.
PMID |
| [20] |
Kochian LV, Piñeros MA, Liu J, Magalhaes JV (2015). Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology, 66, 571-598.
DOI PMID |
| [21] |
Lankau RA, Keymer DP (2016). Ectomycorrhizal fungal richness declines towards the host species' range edge. Molecular Ecology, 25, 3224-3241.
DOI URL |
| [22] |
Li P, Li W, Dumbrell AJ, Liu M, Li G, Wu M, Jiang C, Li Z (2020). Spatial variation in soil fungal communities across paddy fields in subtropical China. mSystems, 5, e00704-19. DOI: 10.1128/mSystems.00704-19.
DOI |
| [23] |
Liao HL, Bonito G, Rojas JA, Hameed K, Wu S, Schadt CW, Labbé J, Tuskan GA, Martin F, Grigoriev IV, Vilgalys R (2019). Fungal endophytes of Populus trichocarpa alter host phenotype, gene expression, and rhizobiome composition. Molecular Plant-Microbe Interactions, 32, 853-864.
DOI URL |
| [24] |
Liu D, Wang H, An S, Bhople P, Davlatbekov F (2019). Geographic distance and soil microbial biomass carbon drive biogeographical distribution of fungal communities in Chinese Loess Plateau soils. Science of the Total Environment, 660, 1058-1069.
DOI URL |
| [25] |
Liu WY, Wang F, Sun YM, Yang L, Chen HH, Liu WJ, Zhu B, Hui CM, Wang SW (2020). Influence of dragon bamboo with different planting patterns on microbial community and physicochemical property of soil on sunny and shady slopes. Journal of Microbiology, 58, 906-914.
DOI URL |
| [26] |
Locosselli GM, Krottenthaler S, Pitsch P, Anhuf D, Ceccantini G (2019). Impact of temperature on the growth of a Neotropical tree species (Hymenaea courbaril, Fabaceae) at its southern distribution limit. International Journal of Biometeorology, 63, 1683-1692.
DOI PMID |
| [27] | Lu RK (2000). Analysis Methods of Soil Agrochemistry. China Agricultural Science and Technology Press, Beijing. |
| [鲁如坤 (2000). 土壤农业化学分析方法. 中国农业科技出版社, 北京.] | |
| [28] |
Lu T, Ke M, Lavoie M, Jin Y, Fan X, Zhang Z, Fu Z, Sun L, Gillings M, Peñuelas J, Qian H, Zhu YG (2018). Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome, 6, 231. DOI: 10.1186/s40168-018-0615-0.
DOI URL |
| [29] |
Moeller DA, Geber MA, Eckhart VM, Tiffin P (2012). Reduced pollinator service and elevated pollen limitation at the geographic range limit of an annual plant. Ecology, 93, 1036-1048.
PMID |
| [30] |
Nadimi M, Beaudet D, Forget L, Hijri M, Lang BF (2012). Group I intron-mediated trans-splicing in mitochondria of Gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with Mortierellales. Molecular Biology and Evolution, 29, 2199-2210.
DOI URL |
| [31] | Ning Q, Chen L, Li F, Zhang CZ, Ma DH, Cai ZJ, Zhang JB (2020). Effects of Mortierella on nutrient availability and straw decomposition in soil. Acta Pedologica Sinica, 1-12. |
| [宁琪, 陈林, 李芳, 张丛志, 马东豪, 蔡泽江, 张佳宝 (2020). 被孢霉对土壤养分有效性和秸秆降解的影响. 土壤学报, 1-12.] | |
| [32] |
Onweremadu EU (2007). Predicting phosphorus sorption characteristics in highly weathered soils of south-eastern Nigeria. Research Journal of Environmental Sciences, 1, 47-55.
DOI URL |
| [33] |
Osorio NW, Habte M (2013). Phosphate desorption from the surface of soil mineral particles by a phosphate-solubilizing fungus. Biology and Fertility of Soils, 49, 481-486.
DOI URL |
| [34] |
Pan L, Mou P, Bai SB, Gu M (2015). Impact of Phyllostachys heterocycla ‘Pubescens' expansion on mycorrhizal associations of the adjacent forests. Chinese Journal of Plant Ecology, 39, 371-382.
DOI URL |
|
[潘璐, 牟溥, 白尚斌, 古牧 (2015). 毛竹林扩张对周边森林群落菌根系统的影响. 植物生态学报, 39, 371-382.]
DOI |
|
| [35] |
Salvioli Di Fossalunga AM, Novero M (2019). To trade in the field: the molecular determinants of arbuscular mycorrhiza nutrient exchange. Chemical and Biological Technologies in Agriculture, 6, 12. DOI: 10.1186/s40538-019-0150-7.
DOI URL |
| [36] | Song QN (2017). The Effects for Phyllostachys pubscens Expansion on Nitrogen and Phosphorus Distribution Pattern and Process of Evergreen Broadleaved Forest. PhD dissertation, Tsinghua University, Beijing. 63-64. |
| [宋庆妮 (2017). 毛竹扩张对常绿阔叶林氮磷分配格局与过程的影响. 博士学位论文, 清华大学, 北京. 63-64.] | |
| [37] | Song QN, Ouyang M, Yang QP, Lu H, Yang GY, Chen FS, Shi JM (2016). Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant & Soil, 404, 113-124. |
| [38] |
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, OʼDonnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE (2016). A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia, 108, 1028- 1046.
PMID |
| [39] |
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020). Plant-microbiome interactions: from community assembly to plant health. Nature Reviews Microbiology, 18, 607-621.
DOI URL |
| [40] |
Walthert L, Graf Pannatier E, Meier ES (2013). Shortage of nutrients and excess of toxic elements in soils limit the distribution of soil-sensitive tree species in temperate forests. Forest Ecology and Management, 297, 94-107.
DOI URL |
| [41] |
Wani ZA, Kumar A, Sultan P, Bindu K, Riyaz-Ul-Hassan S, Ashraf N (2017). Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Scientific Reports, 7, 8598. DOI: 10.1038/s41598-017-08974-z.
DOI URL |
| [42] |
Xun WB, Li W, Xiong W, Ren Y, Liu YP, Miao YZ, Xu ZH, Zhang N, Shen QR, Zhang RF (2019). Diversity-triggered deterministic bacterial assembly constrains community functions. Nature Communications, 10, 3833. DOI: 10.1038/s41467-019-11787-5.
DOI |
| [43] | Yang HQ, Li DZ (2015). Revision on Cephalostachyum Munro (Poaceae: Bambusoideae) in China. Plant Diversity and Resources, 37, 546-550. |
| [杨汉奇, 李德铢 (2015). 中国竹亚科空竹属的整理. 植物分类与资源学报, 37, 546- 550.] | |
| [44] | Yang YM, Xue JR (1990). A preliminary study on the natural bamboo forests in the Dawei mountain of southeastern Yunnan. Journal of Southwest Forestry College, 10(1), 21-30. |
| [杨宇明, 薛纪如 (1990). 云南大围山地区天然竹林的初步研究. 西南林业大学学报(自然科学), 10(1), 21-30.] | |
| [45] |
Yao F, Yang S, Wang Z, Wang X, Ye J, Wang X, DeBruyn JM, Feng X, Jiang Y, Li H (2017). Microbial taxa distribution is associated with ecological trophic cascades along an elevation gradient. Frontiers in Microbiology, 8, 2071. DOI: 10.3389/fmicb.2017.02071.
DOI |
| [46] |
Zhang J, Zhang BG, Liu Y, Guo YQ, Shi P, Wei GH (2018). Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Science of the Total Environment, 644, 791-800.
DOI URL |
| [47] | Zhang XP, Gao GB, Wu ZZ, Wen X, Zhong H, Zhong ZZ, Yang CB, Bian FY, Gai X (2020). Responses of soil nutrients and microbial communities to intercropping medicinal plants in moso bamboo plantations in subtropical China. Environmental Science and Pollution Research International, 27, 2301-2310. |
| [48] | Zhao TX, Mao XW, Cheng M, Chen JH, Qin H, Li YC, Liang CF, Xu QF (2017). Effects of Phyllostachys edulis cultiv-ation on soil bacterial and fungal community structure and diversity. Chinese Journal of Applied Ecology, 28, 3740-3750. |
|
[赵天心, 毛新伟, 程敏, 陈俊辉, 秦华, 李永春, 梁辰飞, 徐秋芳 (2017). 毛竹种植对土壤细菌和真菌群落结构及多样性的影响. 应用生态学报, 28, 3740-3750.]
DOI |
|
| [49] | Zheng XQ, Cui YZ, Chen LN, Yang HQ (2018). Study on bamboo shooting and shoot growth of Cephalostachyum pingbianense. Forest Research, 31(5), 131-136. |
| [郑祥亁, 崔永忠, 陈凌娜, 杨汉奇 (2018). 屏边空竹四季出笋及幼竹生长发育规律研究. 林业科学研究, 31(5), 131-136.] | |
| [50] |
Zhou ZH, Wang CK, Luo YQ (2020). Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nature Communications, 11, 3072. DOI: 10.1038/s41467-020-16881-7.
DOI URL |
| [1] | 陈科宇 邢森 唐玉 孙佳慧 任世杰 张静 纪宝明. 不同草地型土壤丛枝菌根真菌群落特征及其驱动因素[J]. 植物生态学报, 2024, 48(5): 660-674. |
| [2] | 徐子怡 金光泽. 阔叶红松林不同菌根类型幼苗细根功能性状的变异与权衡[J]. 植物生态学报, 2024, 48(5): 612-622. |
| [3] | 胡蝶 蒋欣琪 戴志聪 陈戴一 张雨 祁珊珊 杜道林. 丛枝菌根真菌提高入侵杂草南美蟛蜞菊对除草剂的耐受性[J]. 植物生态学报, 2024, 48(5): 651-659. |
| [4] | 陈保冬, 付伟, 伍松林, 朱永官. 菌根真菌在陆地生态系统碳循环中的作用[J]. 植物生态学报, 2024, 48(1): 1-20. |
| [5] | 任悦, 高广磊, 丁国栋, 张英, 赵珮杉, 柳叶. 不同生长期樟子松外生菌根真菌群落物种组成及其驱动因素[J]. 植物生态学报, 2023, 47(9): 1298-1309. |
| [6] | 郭敏, 罗林, 梁进, 王彦杰, 赵春章. 冻融变化对西南亚高山森林优势种云杉和华西箭竹根区土壤理化性质与酶活性的影响[J]. 植物生态学报, 2023, 47(6): 882-894. |
| [7] | 李冠军, 陈珑, 余雯静, 苏亲桂, 吴承祯, 苏军, 李键. 固体培养内生真菌对土壤盐胁迫下木麻黄幼苗渗透调节和抗氧化系统的影响[J]. 植物生态学报, 2023, 47(6): 804-821. |
| [8] | 何斐, 李川, Faisal SHAH, 卢谢敏, 王莹, 王梦, 阮佳, 魏梦琳, 马星光, 王卓, 姜浩. 丛枝菌根菌丝桥介导刺槐-魔芋间碳转运和磷吸收[J]. 植物生态学报, 2023, 47(6): 782-791. |
| [9] | 杨佳绒, 戴冬, 陈俊芳, 吴宪, 刘啸林, 刘宇. 丛枝菌根真菌多样性对植物群落构建和稀有种维持的研究进展[J]. 植物生态学报, 2023, 47(6): 745-755. |
| [10] | 胡同欣, 李蓓, 李光新, 任玥霄, 丁海磊, 孙龙. 火烧黑碳对生长季兴安落叶松林外生菌根真菌群落物种组成的影响[J]. 植物生态学报, 2023, 47(6): 792-803. |
| [11] | 冯可, 刘冬梅, 张琦, 安菁, 何双辉. 旅游干扰对松山油松林土壤微生物多样性及群落结构的影响[J]. 植物生态学报, 2023, 47(4): 584-596. |
| [12] | 罗来聪, 赖晓琴, 白健, 李爱新, 方海富, Nasir SHAD, 唐明, 胡冬南, 张令. 氮添加背景下土壤真菌和细菌对不同种源入侵植物乌桕生长特征的影响[J]. 植物生态学报, 2023, 47(2): 206-215. |
| [13] | 赵榕江, 陈焘, 董丽佳, 郭辉, 马海鲲, 宋旭, 王明刚, 薛伟, 杨强. 植物-土壤反馈及其在生态学中的研究进展[J]. 植物生态学报, 2023, 47(10): 1333-1355. |
| [14] | 张慧, 曾文静, 龚新桃, 马泽清. 亚热带典型树种根毛特征及其与共生真菌的关系[J]. 植物生态学报, 2023, 47(1): 88-100. |
| [15] | 秦江环, 张春雨, 赵秀海. 基于温带针阔混交林植物-土壤反馈的Janzen- Connell假说检验[J]. 植物生态学报, 2022, 46(6): 624-631. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19