

Chin J Plant Ecol ›› 2022, Vol. 46 ›› Issue (7): 823-833.DOI: 10.17521/cjpe.2021.0200
Special Issue: 菌根真菌; 生物地球化学; 微生物生态学
• Research Articles • Previous Articles Next Articles
XIA Ti-Ze1,2, LI Lu-Shuang1, YANG Han-Qi1,*( )
)
Received:2021-05-26
Accepted:2021-10-18
Online:2022-07-20
Published:2021-12-16
Contact:
YANG Han-Qi
Supported by:XIA Ti-Ze, LI Lu-Shuang, YANG Han-Qi. Soil fungal community characteristics at the upper and lower altitudinal range limits of Cephalostachyum pingbianense[J]. Chin J Plant Ecol, 2022, 46(7): 823-833.
Add to citation manager EndNote|Ris|BibTeX
URL: https://www.plant-ecology.com/EN/10.17521/cjpe.2021.0200
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| pH | 5.06 ± 0.17a | 3.65 ± 0.32c | 4.49 ± 0.18b | 4.04 ± 0.12c | 4.67 ± 0.12b |
| 有机质含量 SOM content (g·kg-1) | 79.20 ± 6.67b | 120.90 ± 7.24a | 121.84 ± 6.49a | 128.75 ± 12.61a | 128.05 ± 9.39a |
| 全氮含量 TN content (g·kg-1) | 3.89 ± 0.53d | 6.36 ± 1.84b | 4.48 ± 0.36c | 7.10 ± 0.11a | 6.55 ± 0.74b |
| 速效氮含量 AN content (g·kg-1) | 0.56 ± 0.07c | 1.08 ± 0.25a | 0.61 ± 0.04bc | 0.76 ± 0.19b | 0.74 ± 0.06b |
| 全磷含量 TP content (g·kg-1) | 0.52 ± 0.02b | 0.59 ± 0.07b | 0.68 ± 0.05b | 0.53 ± 0.04b | 0.94 ± 0.10a |
| 速效磷含量 AP content (mg·kg-1) | 12.58 ± 1.02b | 9.96 ± 0.82c | 13.03 ± 0.70b | 8.43 ± 3.41c | 18.04 ± 6.67a |
| 全钾含量 TK content (g·kg-1) | 16.59 ± 1.37a | 12.49 ± 2.40b | 16.02 ± 0.53ab | 12.46 ± 2.58b | 14.77 ± 1.33b |
| 速效钾含量 AK content (g·kg-1) | 0.16 ± 0.01b | 0.19 ± 0.01a | 0.15 ± 0.02b | 0.17 ± 0.05ab | 0.19 ± 0.02a |
Table 1 Physiochemical properties of different sites in the distribution area of Cephalostachyum pingbianense (mean ± SD)
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| pH | 5.06 ± 0.17a | 3.65 ± 0.32c | 4.49 ± 0.18b | 4.04 ± 0.12c | 4.67 ± 0.12b |
| 有机质含量 SOM content (g·kg-1) | 79.20 ± 6.67b | 120.90 ± 7.24a | 121.84 ± 6.49a | 128.75 ± 12.61a | 128.05 ± 9.39a |
| 全氮含量 TN content (g·kg-1) | 3.89 ± 0.53d | 6.36 ± 1.84b | 4.48 ± 0.36c | 7.10 ± 0.11a | 6.55 ± 0.74b |
| 速效氮含量 AN content (g·kg-1) | 0.56 ± 0.07c | 1.08 ± 0.25a | 0.61 ± 0.04bc | 0.76 ± 0.19b | 0.74 ± 0.06b |
| 全磷含量 TP content (g·kg-1) | 0.52 ± 0.02b | 0.59 ± 0.07b | 0.68 ± 0.05b | 0.53 ± 0.04b | 0.94 ± 0.10a |
| 速效磷含量 AP content (mg·kg-1) | 12.58 ± 1.02b | 9.96 ± 0.82c | 13.03 ± 0.70b | 8.43 ± 3.41c | 18.04 ± 6.67a |
| 全钾含量 TK content (g·kg-1) | 16.59 ± 1.37a | 12.49 ± 2.40b | 16.02 ± 0.53ab | 12.46 ± 2.58b | 14.77 ± 1.33b |
| 速效钾含量 AK content (g·kg-1) | 0.16 ± 0.01b | 0.19 ± 0.01a | 0.15 ± 0.02b | 0.17 ± 0.05ab | 0.19 ± 0.02a |
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| Shannon指数 Shannon index | 4.37 ± 0.13b | 3.02 ± 0.48c | 4.84 ± 0.08a | 2.97 ± 0.02c | 4.31 ± 0.21b |
| Chao1指数 Chao1 index | 670 ± 160c | 1 163 ± 152b | 1 655 ± 147a | 1 199 ± 42b | 1 321 ± 190ab |
| 可操作分类单元 OTU | 641 ± 130c | 935 ± 140b | 1 442 ± 95a | 917 ± 28b | 1 107 ± 117b |
Table 2 Comparison analysis of soil fungal alpha diversity in the distribution area of Cephalostachyum pingbianense (mean ± SD)
| 指标 Index | A | B | C | D | E |
|---|---|---|---|---|---|
| Shannon指数 Shannon index | 4.37 ± 0.13b | 3.02 ± 0.48c | 4.84 ± 0.08a | 2.97 ± 0.02c | 4.31 ± 0.21b |
| Chao1指数 Chao1 index | 670 ± 160c | 1 163 ± 152b | 1 655 ± 147a | 1 199 ± 42b | 1 321 ± 190ab |
| 可操作分类单元 OTU | 641 ± 130c | 935 ± 140b | 1 442 ± 95a | 917 ± 28b | 1 107 ± 117b |
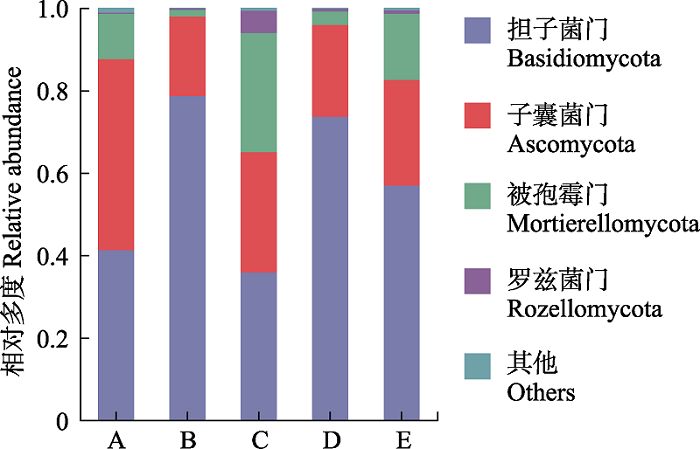
Fig. 1 Soil fungal community composition at the phylum level at different sites in the distribution area of Cephalostachyum pingbianense. A, Cyclobalanopsis kerrii forest; B, the lower altitudinal range limit of Cephalostachyum pingbianense; C, the altitudinal range center of C. pingbianense; D, the upper altitudinal range limit of C. pingbianense; E, Lithocarpus glaber forest.
| 显著差异的真菌 Fungi with significant differences | A | B | C | D | E |
|---|---|---|---|---|---|
| 担子菌门 Basidiomycota | 30.04 ± 4.25c | 70.11 ± 3.32a | 30.13 ± 1.47c | 66.35 ± 3.80a | 50.84 ± 2.71b |
| 子囊菌门 Ascomycota | 48.87 ± 5.07a | 20.63 ± 1.67c | 26.61 ± 1.93b | 25.42 ± 5.17bc | 27.94 ± 2.51b |
| 被孢霉门 Mortierellomycota | 8.59 ± 4.27bc | 1.52 ± 0.13c | 23.78 ± 0.96a | 2.93 ± 2.23c | 14.49 ± 0.27b |
| 罗兹菌门 Rozellomycota | 0.88 ± 0.32c | 1.08 ± 0.25a | 7.86 ± 0.89a | 2.88 ± 2.22b | 1.58 ± 0.08b |
| 被孢霉属 Mortierella | 8.38 ± 4.11bc | 1.46 ± 0.13c | 23.29 ± 0.85a | 0.53 ± 0.04c | 11.46 ± 0.21b |
| Saitozyma | 15.40 ± 7.77a | 2.52 ± 1.52b | 15.44 ± 1.48a | 4.05 ± 3.10b | 11.67 ± 3.34a |
| 红菇属 Russula | 0.51 ± 0.22c | 27.61 ± 22.57a | 0.50 ± 0.15c | 57.63 ± 3.03a | 10.02 ± 6.76ab |
| 产油菌属 Solicoccozyma | 0.16 ± 0.15b | 0.45 ± 0.41b | 5.56 ± 1.52a | 0.27 ± 0.37b | 1.78 ± 0.82b |
| 珊瑚菌属 Clavaria | 5.04 ± 3.59a | 0.03 ± 0.05b | 0.00 ± 0.00b | 0.02 ± 0.01b | 0.03 ± 0.01b |
| 绿僵菌属 Metarhizium | 2.41 ± 0.53b | 0.75 ± 0.55c | 4.60 ± 0.79a | 0.27 ± 0.19c | 1.60 ± 0.51bc |
| 刺球菌属 Chaetosphaeria | 0.48 ± 0.33ab | 0.50 ± 0.23ab | 1.16 ± 0.43a | 0.32 ± 0.06b | 1.24 ± 0.23a |
| 腾梗孢霉属 Gonytrichum | 0.94 ± 0.84b | 0.02 ± 0.01b | 2.20 ± 0.33a | 0.09 ± 0.08b | 0.26 ± 0.13b |
| 织球壳属 Plectosphaerella | 2.06 ± 0.40a | 0.03 ± 0.02b | 0.03 ± 0.09b | 0.02 ± 0.01b | 0.09 ± 0.02b |
Table 3 Fungal phyla and genera with significant difference between different sites in the distribution area of Cephalostachyum pingbianense (mean ± SD, %)
| 显著差异的真菌 Fungi with significant differences | A | B | C | D | E |
|---|---|---|---|---|---|
| 担子菌门 Basidiomycota | 30.04 ± 4.25c | 70.11 ± 3.32a | 30.13 ± 1.47c | 66.35 ± 3.80a | 50.84 ± 2.71b |
| 子囊菌门 Ascomycota | 48.87 ± 5.07a | 20.63 ± 1.67c | 26.61 ± 1.93b | 25.42 ± 5.17bc | 27.94 ± 2.51b |
| 被孢霉门 Mortierellomycota | 8.59 ± 4.27bc | 1.52 ± 0.13c | 23.78 ± 0.96a | 2.93 ± 2.23c | 14.49 ± 0.27b |
| 罗兹菌门 Rozellomycota | 0.88 ± 0.32c | 1.08 ± 0.25a | 7.86 ± 0.89a | 2.88 ± 2.22b | 1.58 ± 0.08b |
| 被孢霉属 Mortierella | 8.38 ± 4.11bc | 1.46 ± 0.13c | 23.29 ± 0.85a | 0.53 ± 0.04c | 11.46 ± 0.21b |
| Saitozyma | 15.40 ± 7.77a | 2.52 ± 1.52b | 15.44 ± 1.48a | 4.05 ± 3.10b | 11.67 ± 3.34a |
| 红菇属 Russula | 0.51 ± 0.22c | 27.61 ± 22.57a | 0.50 ± 0.15c | 57.63 ± 3.03a | 10.02 ± 6.76ab |
| 产油菌属 Solicoccozyma | 0.16 ± 0.15b | 0.45 ± 0.41b | 5.56 ± 1.52a | 0.27 ± 0.37b | 1.78 ± 0.82b |
| 珊瑚菌属 Clavaria | 5.04 ± 3.59a | 0.03 ± 0.05b | 0.00 ± 0.00b | 0.02 ± 0.01b | 0.03 ± 0.01b |
| 绿僵菌属 Metarhizium | 2.41 ± 0.53b | 0.75 ± 0.55c | 4.60 ± 0.79a | 0.27 ± 0.19c | 1.60 ± 0.51bc |
| 刺球菌属 Chaetosphaeria | 0.48 ± 0.33ab | 0.50 ± 0.23ab | 1.16 ± 0.43a | 0.32 ± 0.06b | 1.24 ± 0.23a |
| 腾梗孢霉属 Gonytrichum | 0.94 ± 0.84b | 0.02 ± 0.01b | 2.20 ± 0.33a | 0.09 ± 0.08b | 0.26 ± 0.13b |
| 织球壳属 Plectosphaerella | 2.06 ± 0.40a | 0.03 ± 0.02b | 0.03 ± 0.09b | 0.02 ± 0.01b | 0.09 ± 0.02b |
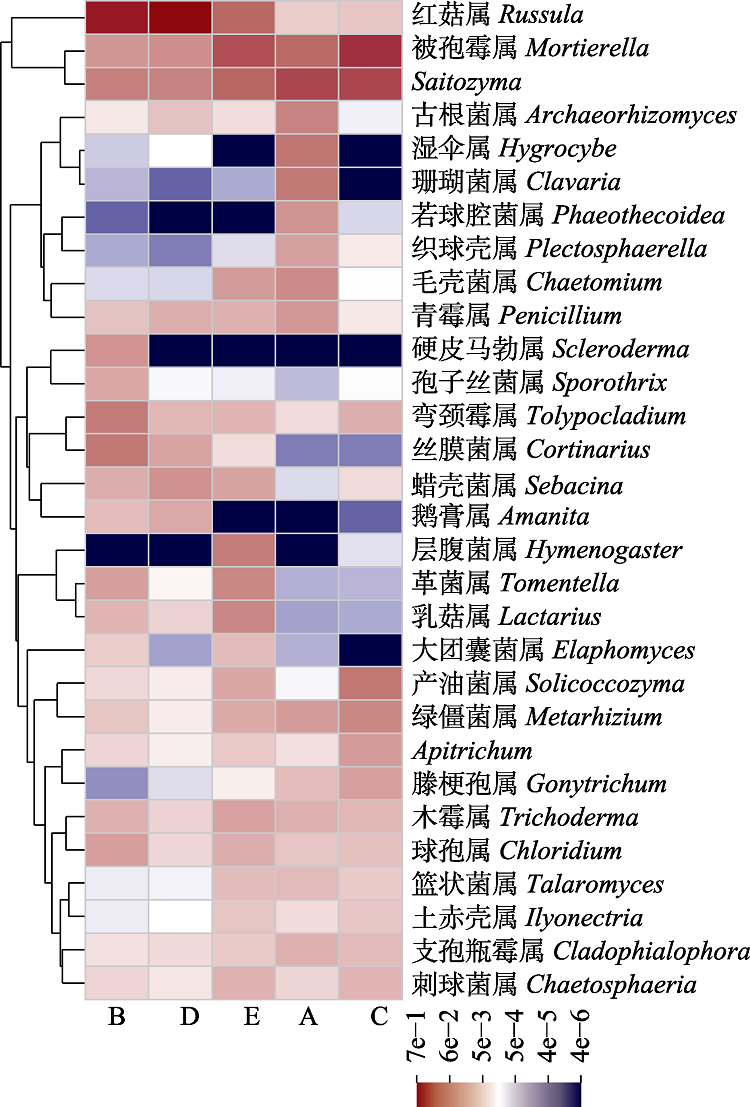
Fig. 2 Heatmap showing the top 30 abundant genera in soil at different sites in the distribution area of Cephalostachyum pingbianense. A, Cyclobalanopsis kerrii forest; B, the lower altitudinal range limit of Cephalostachyum pingbianense; C, the altitudinal range center of C. pingbianense; D, the upper altitudinal range limit of C. pingbianense; E, Lithocarpus glaber forest.
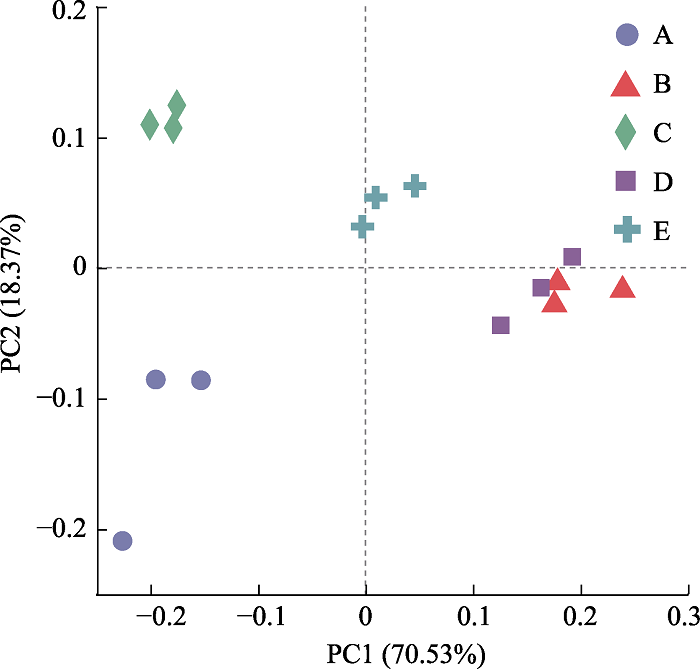
Fig. 3 Principle coordinate analysis (PCoA) based on Bray-Curtis distance method of soil fungal community. PC1, principal co-ordinates 1; PC2, principal co-ordinates 2. A, Cyclobalanopsis kerrii forest; B, the lower altitudinal range limit of Cephalostachyum pingbianense; C, the altitudinal range center of C. pingbianense; D, the upper altitudinal range limit of C. pingbianense; E, Lithocarpus glaber forest.
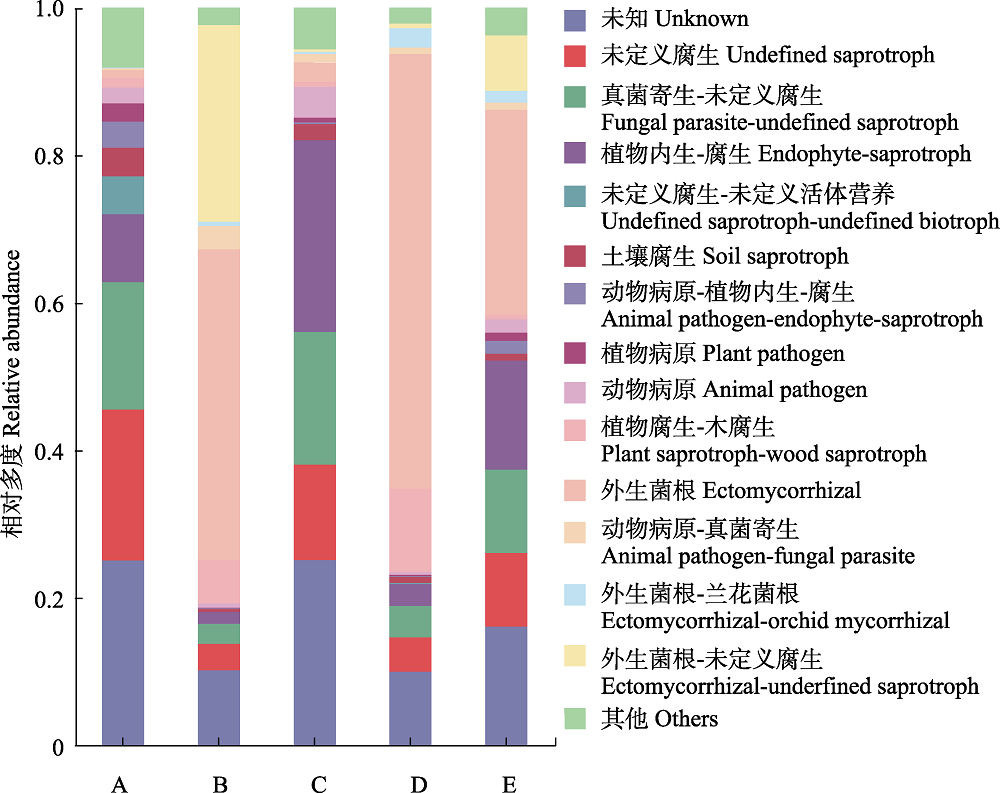
Fig. 4 Functional annotation of soil fungal community at different sites in the distribution area of Cephalostachyum pingbianense. A, Cyclobalanopsis kerrii forest; B, the lower range limit of Cephalostachyum pingbianense; C, the range center of C. pingbianense; D, the upper range limit of C. pingbianense; E, Lithocarpus glaber forest.
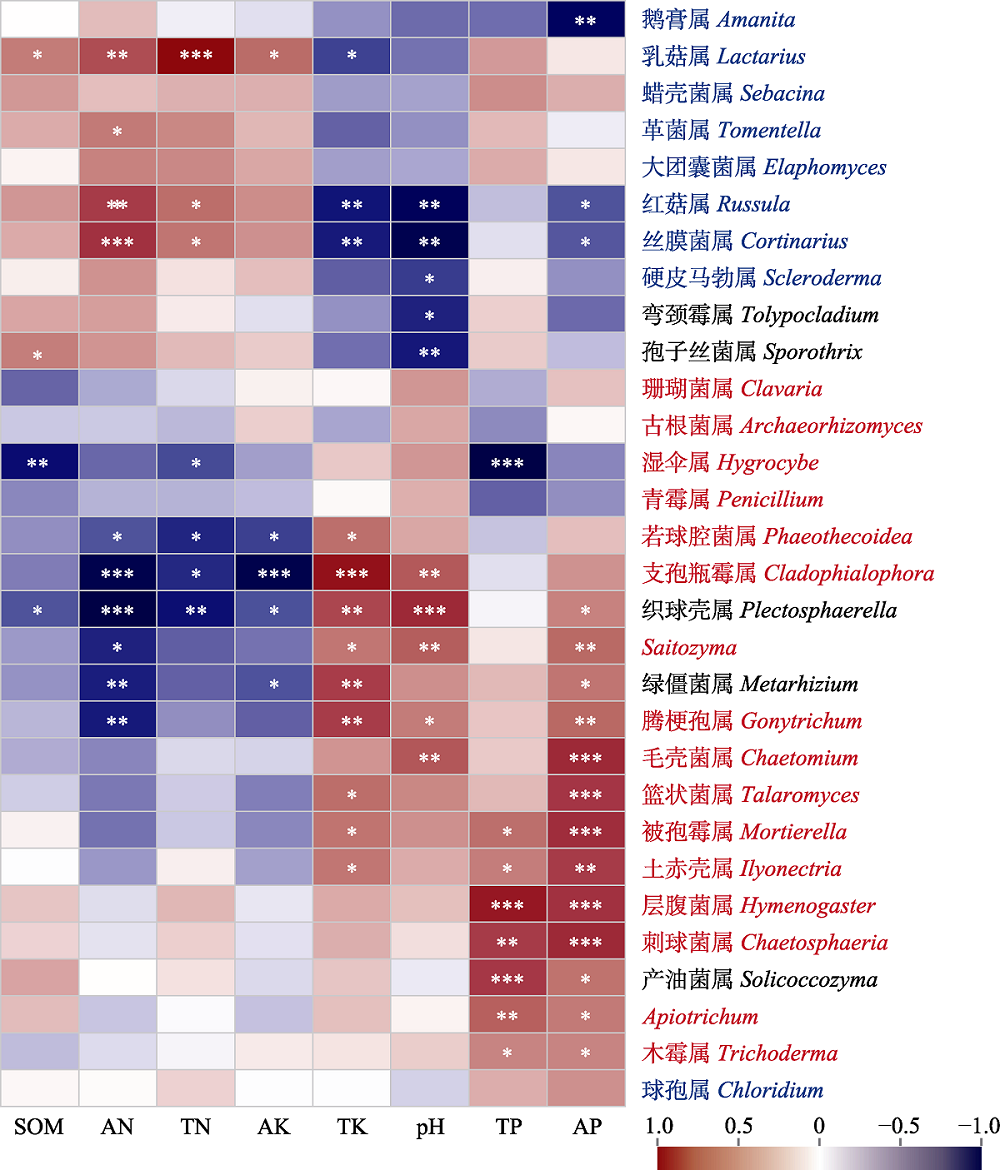
Fig. 5 Spearman correlation analysis between fungal genera and soil physicochemical properties, and functional annotation in the distribution area of Cephalostachyum pingbianense. Top 30 abundant fungal genera are listed. Based on fungal functional annotation, ectomycorrhizal fungi are marked in red, saprophytic fungi are marked in blue and other types of fungi are marked in black. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001. AK, available potassium (K) content; AN, available nitrogen (N) content; AP, available phosphorus (P) content; SOM, soil organic matter content; TK, total K content; TN, total N content; TP, total P content.
| 项目 Item | 全磷含量 TP content | 速效钾含量 AK content | 有机质含量 SOM content | 全氮含量 TN content | 全钾含量 TK content | 速效氮含量 AN content | 速效磷含量 AP content | pH |
|---|---|---|---|---|---|---|---|---|
| r | -0.004 | 0.03 | 0.14 | 0.23 | 0.26 | 0.36 | 0.30 | 0.53 |
| p | 0.963 | 0.766 | 0.125 | 0.033 | 0.014 | 0.005 | 0.004 | 0.001 |
Table 4 Mantel test results for the correlation between fungal community composition and soil environment factors in the distribution area of Cephalostachyum pingbianense
| 项目 Item | 全磷含量 TP content | 速效钾含量 AK content | 有机质含量 SOM content | 全氮含量 TN content | 全钾含量 TK content | 速效氮含量 AN content | 速效磷含量 AP content | pH |
|---|---|---|---|---|---|---|---|---|
| r | -0.004 | 0.03 | 0.14 | 0.23 | 0.26 | 0.36 | 0.30 | 0.53 |
| p | 0.963 | 0.766 | 0.125 | 0.033 | 0.014 | 0.005 | 0.004 | 0.001 |
| [1] |
Adams RI, Miletto M, Taylor JW, Bruns TD (2013). Dispersal in microbes: fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. The ISME Journal, 7, 1262-1273.
DOI URL |
| [2] |
Ali A, Lin SL, He JK, Kong FM, Yu JH, Jiang HS (2019). Climatic water availability is the main limiting factor of biotic attributes across large-scale elevational gradients in tropical forests. Science of the Total Environment, 647, 1211-1221.
DOI URL |
| [3] |
Benning JW, Moeller DA (2021). Microbes, mutualism, and range margins: testing the fitness consequences of soil microbial communities across and beyond a native plantʼs range. New Phytologist, 229, 2886-2900.
DOI PMID |
| [4] |
Bojórquez-Quintal E, Escalante-Magaña C, Echevarría-Machado I, Martínez-Estévez M (2017). Aluminum, a friend or foe of higher plants in acid soils. Frontiers in Plant Science, 8, 1767. DOI: 10.3389/fpls.2017.01767.
DOI PMID |
| [5] |
Bonfante P, Venice F (2020). Mucoromycota: going to the roots of plant-interacting fungi. Fungal Biology Reviews, 34, 100-113.
DOI URL |
| [6] | Bruelheide H, Scheidel U (1999). Slug herbivory as a limiting factor for the geographical range of Arnica montana. Journal of Ecology, 87, 839-848. |
| [7] |
Burns JH, Anacker BL, Strauss SY, Burke DJ (2015). Soil microbial community variation correlates most strongly with plant species identity, followed by soil chemistry, spatial location and plant genus. AoB PLANTS, 7, plv030. DOI: 10.1093/aobpla/plv030.
DOI |
| [8] |
Corsaro D, Walochnik J, Venditti D, Hauröder B, Michel R (2020). Solving an old enigma: Morellospora saccamoebae gen. nov., sp. nov. (Rozellomycota), a Sphaerita-like parasite of free-living amoebae. Parasitology Research, 119, 925-934.
DOI URL |
| [9] |
de Wit R, Bouvier T (2006). “Everything is everywhere, but, the environment selects”; What did Baas Becking and Beijerinck really say? Environmental Microbiology, 8, 755-758.
DOI URL |
| [10] |
Emamverdian A, Ding Y, Ranaei F, Ahmad Z (2020). Application of bamboo plants in nine aspects. The Scientific World Journal, 7284203. DOI: 10.1155/2020/7284203.
DOI |
| [11] |
Feng HF, Liu LY, Xue L (2019). Effects of nitrogen and phosphorus additions and stand density on soil chemical property in Acacia auriculiformis stands. Chinese Journal of Plant Ecology, 43, 1010-1020.
DOI URL |
|
[冯慧芳, 刘落鱼, 薛立 (2019). 氮磷添加及林分密度对大叶相思林土壤化学性质的影响. 植物生态学报, 43, 1010-1020.]
DOI |
|
| [12] |
Fierer N, Bradford MA, Jackson RB (2007). Toward an ecological classification of soil bacteria. Ecology, 88, 1354-1364.
PMID |
| [13] |
Genre A, Lanfranco L, Perotto S, Bonfante P (2020). Unique and common traits in mycorrhizal symbioses. Nature Reviews Microbiology, 18, 649-660.
DOI URL |
| [14] |
Hannula SE, Kielak AM, Steinauer K, Huberty M, Jongen R, de Long JR, Heinen R, Bezemer TM (2019). Time after time: temporal variation in the effects of grass and forb species on soil bacterial and fungal communities. mBio, 10, e02635-19. DOI: 10.1128/mBio.02635-19.
DOI |
| [15] |
Hargreaves AL, Weiner JL, Eckert CG (2015). High-elevation range limit of an annual herb is neither caused nor reinforced by declining pollinator service. Journal of Ecology, 103, 572-584.
DOI URL |
| [16] |
Jones DL, Cooledge EC, Hoyle FC, Griffiths RI, Murphy DV (2019). pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biology & Biochemistry, 138, 107584. DOI: 10.1016/j.soilbio.2019.107584.
DOI URL |
| [17] |
Kaushal R, Singh I, Thapliyal SD, Gupta AK, Mandal D, Tomar JMS, Kumar A, Alam NM, Kadam D, Singh DV, Mehta H, Dogra P, Ojasvi PR, Reza S, Durai J (2020). Rooting behaviour and soil properties in different bamboo species of Western Himalayan Foothills, India. Scientific Reports, 10, 4966. DOI: 10.1038/s41598-020-61418-z.
DOI PMID |
| [18] |
Kirkpatrick M, Barton NH (1997). Evolution of a speciesʼ range. The American Naturalist, 150, 1-23.
DOI URL |
| [19] |
Kochian LV, Hoekenga OA, Pineros MA (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology, 55, 459-493.
PMID |
| [20] |
Kochian LV, Piñeros MA, Liu J, Magalhaes JV (2015). Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annual Review of Plant Biology, 66, 571-598.
DOI PMID |
| [21] |
Lankau RA, Keymer DP (2016). Ectomycorrhizal fungal richness declines towards the host species' range edge. Molecular Ecology, 25, 3224-3241.
DOI URL |
| [22] |
Li P, Li W, Dumbrell AJ, Liu M, Li G, Wu M, Jiang C, Li Z (2020). Spatial variation in soil fungal communities across paddy fields in subtropical China. mSystems, 5, e00704-19. DOI: 10.1128/mSystems.00704-19.
DOI |
| [23] |
Liao HL, Bonito G, Rojas JA, Hameed K, Wu S, Schadt CW, Labbé J, Tuskan GA, Martin F, Grigoriev IV, Vilgalys R (2019). Fungal endophytes of Populus trichocarpa alter host phenotype, gene expression, and rhizobiome composition. Molecular Plant-Microbe Interactions, 32, 853-864.
DOI URL |
| [24] |
Liu D, Wang H, An S, Bhople P, Davlatbekov F (2019). Geographic distance and soil microbial biomass carbon drive biogeographical distribution of fungal communities in Chinese Loess Plateau soils. Science of the Total Environment, 660, 1058-1069.
DOI URL |
| [25] |
Liu WY, Wang F, Sun YM, Yang L, Chen HH, Liu WJ, Zhu B, Hui CM, Wang SW (2020). Influence of dragon bamboo with different planting patterns on microbial community and physicochemical property of soil on sunny and shady slopes. Journal of Microbiology, 58, 906-914.
DOI URL |
| [26] |
Locosselli GM, Krottenthaler S, Pitsch P, Anhuf D, Ceccantini G (2019). Impact of temperature on the growth of a Neotropical tree species (Hymenaea courbaril, Fabaceae) at its southern distribution limit. International Journal of Biometeorology, 63, 1683-1692.
DOI PMID |
| [27] | Lu RK (2000). Analysis Methods of Soil Agrochemistry. China Agricultural Science and Technology Press, Beijing. |
| [鲁如坤 (2000). 土壤农业化学分析方法. 中国农业科技出版社, 北京.] | |
| [28] |
Lu T, Ke M, Lavoie M, Jin Y, Fan X, Zhang Z, Fu Z, Sun L, Gillings M, Peñuelas J, Qian H, Zhu YG (2018). Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome, 6, 231. DOI: 10.1186/s40168-018-0615-0.
DOI URL |
| [29] |
Moeller DA, Geber MA, Eckhart VM, Tiffin P (2012). Reduced pollinator service and elevated pollen limitation at the geographic range limit of an annual plant. Ecology, 93, 1036-1048.
PMID |
| [30] |
Nadimi M, Beaudet D, Forget L, Hijri M, Lang BF (2012). Group I intron-mediated trans-splicing in mitochondria of Gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi with Mortierellales. Molecular Biology and Evolution, 29, 2199-2210.
DOI URL |
| [31] | Ning Q, Chen L, Li F, Zhang CZ, Ma DH, Cai ZJ, Zhang JB (2020). Effects of Mortierella on nutrient availability and straw decomposition in soil. Acta Pedologica Sinica, 1-12. |
| [宁琪, 陈林, 李芳, 张丛志, 马东豪, 蔡泽江, 张佳宝 (2020). 被孢霉对土壤养分有效性和秸秆降解的影响. 土壤学报, 1-12.] | |
| [32] |
Onweremadu EU (2007). Predicting phosphorus sorption characteristics in highly weathered soils of south-eastern Nigeria. Research Journal of Environmental Sciences, 1, 47-55.
DOI URL |
| [33] |
Osorio NW, Habte M (2013). Phosphate desorption from the surface of soil mineral particles by a phosphate-solubilizing fungus. Biology and Fertility of Soils, 49, 481-486.
DOI URL |
| [34] |
Pan L, Mou P, Bai SB, Gu M (2015). Impact of Phyllostachys heterocycla ‘Pubescens' expansion on mycorrhizal associations of the adjacent forests. Chinese Journal of Plant Ecology, 39, 371-382.
DOI URL |
|
[潘璐, 牟溥, 白尚斌, 古牧 (2015). 毛竹林扩张对周边森林群落菌根系统的影响. 植物生态学报, 39, 371-382.]
DOI |
|
| [35] |
Salvioli Di Fossalunga AM, Novero M (2019). To trade in the field: the molecular determinants of arbuscular mycorrhiza nutrient exchange. Chemical and Biological Technologies in Agriculture, 6, 12. DOI: 10.1186/s40538-019-0150-7.
DOI URL |
| [36] | Song QN (2017). The Effects for Phyllostachys pubscens Expansion on Nitrogen and Phosphorus Distribution Pattern and Process of Evergreen Broadleaved Forest. PhD dissertation, Tsinghua University, Beijing. 63-64. |
| [宋庆妮 (2017). 毛竹扩张对常绿阔叶林氮磷分配格局与过程的影响. 博士学位论文, 清华大学, 北京. 63-64.] | |
| [37] | Song QN, Ouyang M, Yang QP, Lu H, Yang GY, Chen FS, Shi JM (2016). Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant & Soil, 404, 113-124. |
| [38] |
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, OʼDonnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE (2016). A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia, 108, 1028- 1046.
PMID |
| [39] |
Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK (2020). Plant-microbiome interactions: from community assembly to plant health. Nature Reviews Microbiology, 18, 607-621.
DOI URL |
| [40] |
Walthert L, Graf Pannatier E, Meier ES (2013). Shortage of nutrients and excess of toxic elements in soils limit the distribution of soil-sensitive tree species in temperate forests. Forest Ecology and Management, 297, 94-107.
DOI URL |
| [41] |
Wani ZA, Kumar A, Sultan P, Bindu K, Riyaz-Ul-Hassan S, Ashraf N (2017). Mortierella alpina CS10E4, an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in the host plant. Scientific Reports, 7, 8598. DOI: 10.1038/s41598-017-08974-z.
DOI URL |
| [42] |
Xun WB, Li W, Xiong W, Ren Y, Liu YP, Miao YZ, Xu ZH, Zhang N, Shen QR, Zhang RF (2019). Diversity-triggered deterministic bacterial assembly constrains community functions. Nature Communications, 10, 3833. DOI: 10.1038/s41467-019-11787-5.
DOI |
| [43] | Yang HQ, Li DZ (2015). Revision on Cephalostachyum Munro (Poaceae: Bambusoideae) in China. Plant Diversity and Resources, 37, 546-550. |
| [杨汉奇, 李德铢 (2015). 中国竹亚科空竹属的整理. 植物分类与资源学报, 37, 546- 550.] | |
| [44] | Yang YM, Xue JR (1990). A preliminary study on the natural bamboo forests in the Dawei mountain of southeastern Yunnan. Journal of Southwest Forestry College, 10(1), 21-30. |
| [杨宇明, 薛纪如 (1990). 云南大围山地区天然竹林的初步研究. 西南林业大学学报(自然科学), 10(1), 21-30.] | |
| [45] |
Yao F, Yang S, Wang Z, Wang X, Ye J, Wang X, DeBruyn JM, Feng X, Jiang Y, Li H (2017). Microbial taxa distribution is associated with ecological trophic cascades along an elevation gradient. Frontiers in Microbiology, 8, 2071. DOI: 10.3389/fmicb.2017.02071.
DOI |
| [46] |
Zhang J, Zhang BG, Liu Y, Guo YQ, Shi P, Wei GH (2018). Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Science of the Total Environment, 644, 791-800.
DOI URL |
| [47] | Zhang XP, Gao GB, Wu ZZ, Wen X, Zhong H, Zhong ZZ, Yang CB, Bian FY, Gai X (2020). Responses of soil nutrients and microbial communities to intercropping medicinal plants in moso bamboo plantations in subtropical China. Environmental Science and Pollution Research International, 27, 2301-2310. |
| [48] | Zhao TX, Mao XW, Cheng M, Chen JH, Qin H, Li YC, Liang CF, Xu QF (2017). Effects of Phyllostachys edulis cultiv-ation on soil bacterial and fungal community structure and diversity. Chinese Journal of Applied Ecology, 28, 3740-3750. |
|
[赵天心, 毛新伟, 程敏, 陈俊辉, 秦华, 李永春, 梁辰飞, 徐秋芳 (2017). 毛竹种植对土壤细菌和真菌群落结构及多样性的影响. 应用生态学报, 28, 3740-3750.]
DOI |
|
| [49] | Zheng XQ, Cui YZ, Chen LN, Yang HQ (2018). Study on bamboo shooting and shoot growth of Cephalostachyum pingbianense. Forest Research, 31(5), 131-136. |
| [郑祥亁, 崔永忠, 陈凌娜, 杨汉奇 (2018). 屏边空竹四季出笋及幼竹生长发育规律研究. 林业科学研究, 31(5), 131-136.] | |
| [50] |
Zhou ZH, Wang CK, Luo YQ (2020). Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nature Communications, 11, 3072. DOI: 10.1038/s41467-020-16881-7.
DOI URL |
| [1] | Ke-Yu CHEN Sen Xing Yu Tang Sun JiaHui Shijie Ren Bao-Ming JI. Arbuscular mycorrhizal fungal community characteristics and driving factors in different grassland types [J]. Chin J Plant Ecol, 2024, 48(5): 660-674. |
| [2] | Xu Zi-Yi Guang-Ze JIN. Variation and trade-offs in fine root functional traits of seedlings of different mycorrhizal types in mixed broadleaved-Korean pine forests [J]. Chin J Plant Ecol, 2024, 48(5): 612-622. |
| [3] | Die Hu Xinqi Jiang DAI Zhicong Daiyi Chen Yu Zhang Shan-Shan Qi. Arbuscular mycorrhizal fungi enhance the herbicide tolerance of an invasive weed Sphagneticola trilobata [J]. Chin J Plant Ecol, 2024, 48(5): 651-659. |
| [4] | CHEN Bao-Dong, FU Wei, WU Song-Lin, ZHU Yong-Guan. Involvements of mycorrhizal fungi in terrestrial ecosystem carbon cycling [J]. Chin J Plant Ecol, 2024, 48(1): 1-20. |
| [5] | REN Yue, GAO Guang-Lei, DING Guo-Dong, ZHANG Ying, ZHAO Pei-Shan, LIU Ye. Species composition and driving factors of the ectomycorrhizal fungal community associated with Pinus sylvestris var. mongolica at different growth periods [J]. Chin J Plant Ecol, 2023, 47(9): 1298-1309. |
| [6] | LI Guan-Jun, CHEN Long, YU Wen-Jing, SU Qin-Gui, WU Cheng-Zhen, SU Jun, LI Jian. Effects of solid culture endophytic fungi on osmotic adjustment and antioxidant system of Casuarina equisetifolia seedlings under soil salt stress [J]. Chin J Plant Ecol, 2023, 47(6): 804-821. |
| [7] | YANG Jia-Rong, DAI Dong, CHEN Jun-Fang, WU Xian, LIU Xiao-Lin, LIU Yu. Insight into recent studies on the diversity of arbuscular mycorrhizal fungi in shaping plant community assembly and maintaining rare species [J]. Chin J Plant Ecol, 2023, 47(6): 745-755. |
| [8] | HE Fei, LI Chuan, Faisal SHAH, LU Xie-Min, WANG Ying, WANG Meng, RUAN Jia, WEI Meng-Lin, MA Xing-Guang, WANG Zhuo, JIANG Hao. Carbon transport and phosphorus uptake in an intercropping system of Robinia pseudoacacia and Amorphophallus konjac mediated by arbuscular mycorrhizal hyphal networks [J]. Chin J Plant Ecol, 2023, 47(6): 782-791. |
| [9] | HU Tong-Xin, LI Bei, LI Guang-Xin, REN Yue-Xiao, DING Hai-Lei, SUN Long. Effects of fire originated black carbon on species composition of ectomycorrhizal fungi in a Larix gmelinii forest in growing season [J]. Chin J Plant Ecol, 2023, 47(6): 792-803. |
| [10] | LUO Lai-Cong, LAI Xiao-Qin, BAI Jian, LI Ai-Xin, FANG Hai-Fu, Nasir SHAD, TANG Ming, HU Dong-Nan, ZHANG Ling. Effects of soil bacteria and fungi on growth of invasive plant Triadica sebifera with different provenances under nitrogen addition [J]. Chin J Plant Ecol, 2023, 47(2): 206-215. |
| [11] | ZHAO Rong-Jiang, CHEN Tao, DONG Li-Jia, GUO Hui, MA Hai-Kun, SONG Xu, WANG Ming-Gang, XUE Wei, YANG Qiang. Progress of plant-soil feedback in ecology studies [J]. Chin J Plant Ecol, 2023, 47(10): 1333-1355. |
| [12] | ZHANG Hui, ZENG Wen-Jing, GONG Xin-Tao, MA Ze-Qing. Relationships between root hairs and mycorrhizal fungi across typical subtropical tree species [J]. Chin J Plant Ecol, 2023, 47(1): 88-100. |
| [13] | QIN Jiang-Huan, ZHANG Chun-Yu, ZHAO Xiu-Hai. Testing Janzen-Connell hypothesis based on plant-soil feedbacks in a temperate coniferous and broadleaved mixed forest [J]. Chin J Plant Ecol, 2022, 46(6): 624-631. |
| [14] | XIE Wei, HAO Zhi-Peng, ZHANG Xin, CHEN Bao-Dong. Research progress and prospect of signal transfer among plants mediated by arbuscular mycorrhizal networks [J]. Chin J Plant Ecol, 2022, 46(5): 493-515. |
| [15] | SHAN Ting-Ting, CHEN Tong-Yao, CHEN Xiao-Mei, GUO Shun-Xing, WANG Ai-Rong. Advance on the association between mycorrhizal fungi and Orchidaceae in nitrogen nutrition [J]. Chin J Plant Ecol, 2022, 46(5): 516-528. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Chinese Journal of Plant Ecology
Tel: 010-62836134, 62836138, E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn