

植物生态学报 ›› 2016, Vol. 40 ›› Issue (1): 60-68.DOI: 10.17521/cjpe.2015.0273
出版日期:2016-01-01
发布日期:2016-01-28
通讯作者:
谷加存
作者简介:# 共同第一作者
基金资助:中央高校基本科研业务费专项资金项目(2572015AA21)和国家自然科学基金(31100470)。
WANG Na, ZHANG Yun, QIAN Wen-Li, WANG Zheng-Quan, GU Jia-Cun*
Online:2016-01-01
Published:2016-01-28
Contact:
Jia-Cun GU
About author:# Co-first authors
摘要:
大气CO2浓度升高对植物的影响是目前植物生态学研究中普遍关注的问题。以往的研究主要关注植物地上部分叶解剖结构及生理功能的改变, 而对根解剖结构和生理功能变化以及根与叶变化之间潜在联系的研究较少。该文以三年生红松(Pinus koraiensis)幼苗为研究对象, 通过CO2浓度倍增(从350 µmol·mol-1增加到700 µmol·mol-1)试验, 研究当年生针叶和根尖解剖结构及生理功能的变化。结果表明: (1) CO2浓度倍增处理的红松幼苗, 气孔密度显著降低, 叶肉组织面积、木质部及韧皮部面积明显增加; (2) CO2浓度倍增导致红松幼苗根尖直径增粗, 皮层厚度和层数显著增加, 管胞直径变小; (3)高CO2浓度处理下, 叶气孔导度和蒸腾速率降低, 光合速率和水分利用效率提高, 同时根尖的导水率显著下降, 但管胞的抗栓塞能力显著提高。这些结果显示, 叶和根解剖结构及生理功能在CO2浓度升高条件下具有一致的响应。未来研究中应该同时关注全球气候变化对植物地上和地下器官结构与功能的影响。
王娜, 张韫, 钱文丽, 王政权, 谷加存. CO2浓度倍增对红松幼苗根尖和叶解剖结构及生理功能的影响. 植物生态学报, 2016, 40(1): 60-68. DOI: 10.17521/cjpe.2015.0273
WANG Na, ZHANG Yun, QIAN Wen-Li, WANG Zheng-Quan, GU Jia-Cun. Effects of elevated CO2 concentration on root and needle anatomy and physiological functions in Pinus koraiensis seedlings. Chinese Journal of Plant Ecology, 2016, 40(1): 60-68. DOI: 10.17521/cjpe.2015.0273
| CO2浓度 CO2 concentration | p | ||
|---|---|---|---|
| 350 µmol·mol-1 | 700 µmol·mol-1 | ||
| 气孔密度 Stomatal density (No.·mm-2) | 66.78 ± 1.85 | 56.83 ± 1.61 | < 0.01 |
| 叶横截面积 Needle cross section area (mm2) | 0.37 ± 0.02 | 0.44 ± 0.01 | < 0.01 |
| 叶肉组织面积 Mesophyll tissue area (mm2) | 0.26 ± 0.01 | 0.30 ± 0.01 | < 0.05 |
| 叶肉细胞面积 Mesophyll cell area (µm2) | 2 306.63 ± 69.97 | 2 501.67 ± 46.92 | < 0.05 |
| 木质部面积 Xylem area (µm2) | 3 817.96 ± 101.06 | 4 412.13 ± 126.86 | < 0.01 |
| 韧皮部面积 Phloem area (µm2) | 4 031.57 ± 152.95 | 5 534.15 ± 275.95 | < 0.01 |
| 中柱直径 Central cylinder diameter (µm) | 254.14 ± 8.31 | 277.55 ± 6.35 | < 0.05 |
| 管胞数 Tracheid number (No.) | 47.87 ± 1.85 | 50.60 ± 1.20 | 0.23 |
| 管胞直径 Tracheid diameter (µm) | 6.53 ± 0.09 | 6.62 ± 0.14 | 0.62 |
| 管胞密度 Tracheid density (No.·µm-2) | 0.001 ± 0.000 06 | 0.000 8 ± 0.000 05 | < 0.05 |
| 管胞壁厚度 Tracheid wall thickness (µm) | 0.68 ± 0.01 | 0.87 ± 0.02 | < 0.01 |
| 表皮和下皮厚度 Epidermis plus hypodermis thickness (µm) | 1.54 ± 0.03 | 1.67 ± 0.04 | < 0.05 |
表1 不同CO2浓度处理对红松幼苗叶解剖结构的影响(平均值±标准误差)
Table 1 Effects of different CO2 concentration treatments on the needle anatomy of Pinus koraiensis seedlings (mean ± SE)
| CO2浓度 CO2 concentration | p | ||
|---|---|---|---|
| 350 µmol·mol-1 | 700 µmol·mol-1 | ||
| 气孔密度 Stomatal density (No.·mm-2) | 66.78 ± 1.85 | 56.83 ± 1.61 | < 0.01 |
| 叶横截面积 Needle cross section area (mm2) | 0.37 ± 0.02 | 0.44 ± 0.01 | < 0.01 |
| 叶肉组织面积 Mesophyll tissue area (mm2) | 0.26 ± 0.01 | 0.30 ± 0.01 | < 0.05 |
| 叶肉细胞面积 Mesophyll cell area (µm2) | 2 306.63 ± 69.97 | 2 501.67 ± 46.92 | < 0.05 |
| 木质部面积 Xylem area (µm2) | 3 817.96 ± 101.06 | 4 412.13 ± 126.86 | < 0.01 |
| 韧皮部面积 Phloem area (µm2) | 4 031.57 ± 152.95 | 5 534.15 ± 275.95 | < 0.01 |
| 中柱直径 Central cylinder diameter (µm) | 254.14 ± 8.31 | 277.55 ± 6.35 | < 0.05 |
| 管胞数 Tracheid number (No.) | 47.87 ± 1.85 | 50.60 ± 1.20 | 0.23 |
| 管胞直径 Tracheid diameter (µm) | 6.53 ± 0.09 | 6.62 ± 0.14 | 0.62 |
| 管胞密度 Tracheid density (No.·µm-2) | 0.001 ± 0.000 06 | 0.000 8 ± 0.000 05 | < 0.05 |
| 管胞壁厚度 Tracheid wall thickness (µm) | 0.68 ± 0.01 | 0.87 ± 0.02 | < 0.01 |
| 表皮和下皮厚度 Epidermis plus hypodermis thickness (µm) | 1.54 ± 0.03 | 1.67 ± 0.04 | < 0.05 |
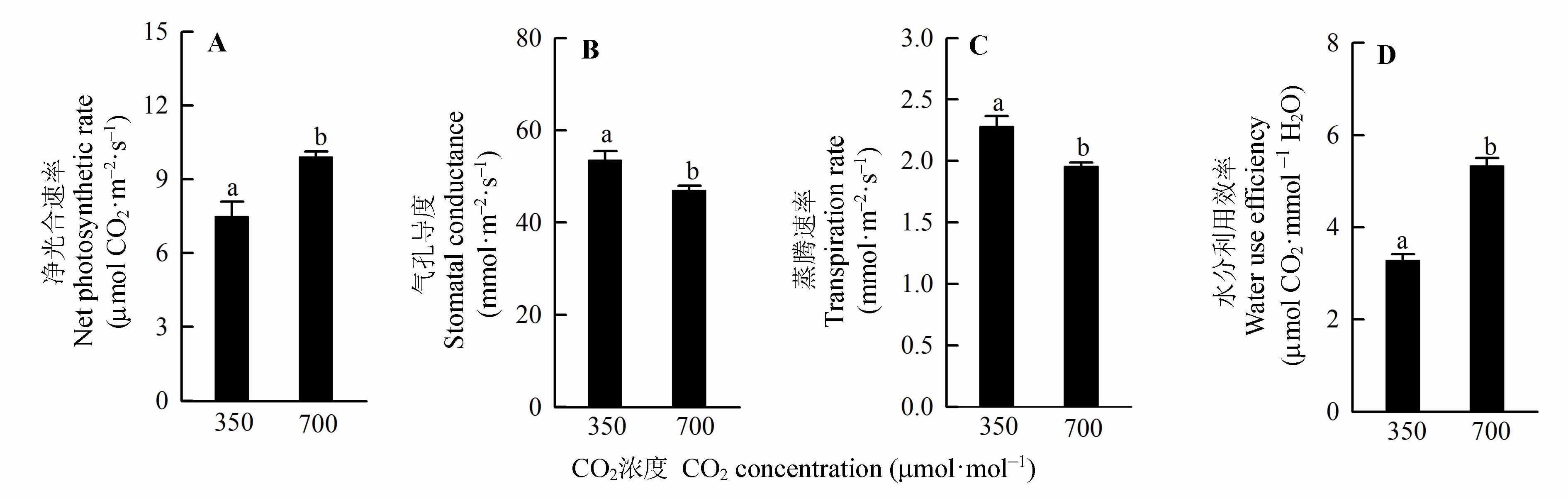
图2 不同CO2浓度处理对红松幼苗叶净光合速率(A)、气孔导度(B)、蒸腾速率(C)和水分利用效率(D)的影响(平均值±标准误差)。不同小写字母表示不同CO2浓度处理间差异显著(p < 0.05)。
Fig. 2 Effects of different CO2 concentration treatments on needle net photosynthetic rate (A), stomatal conductance (B), transpiration rate (C) and water use efficiency (D) of Pinus koraiensis seedlings (mean ± SE). Different lowercase letters indicate significant difference between two CO2 concentration treatments.
| CO2浓度 CO2 concentration | p | ||
|---|---|---|---|
| 350 µmol·mol-1 | 700 µmol·mol-1 | ||
| 根尖直径 Root tips diameter (µm) | 400.68 ± 10.90 | 453.79 ± 15.94 | < 0.01 |
| 中柱直径 Stele diameter (µm) | 216.23 ± 7.59 | 228.83 ± 12.13 | 0.38 |
| 皮层层数 Cortical layer number (No.) | 4.77 ± 0.11 | 5.87 ± 0.12 | < 0.01 |
| 皮层厚度 Cortical thickness (µm) | 79.39 ± 2.11 | 99.19 ± 2.88 | < 0.01 |
| 皮层细胞面积 Cortical cell area (µm2) | 464.17 ± 18.06 | 525.72 ± 21.36 | < 0.05 |
| 管胞数 Tracheid number (No.) | 25.23 ± 0.76 | 24.03 ± 0.81 | 0.28 |
| 管胞直径 Tracheid diameter (µm) | 12.18 ± 0.21 | 11.41 ± 0.21 | < 0.05 |
| 管胞密度 Tracheid density (No.·µm -2) | 0.0008 ± 0.00006 | 0.0007 ± 0.00006 | 0.09 |
| 管胞壁厚度 Tracheid wall thickness (µm) | 0.92 ± 0.02 | 1.08 ± 0.03 | < 0.01 |
表2 不同CO2浓度处理对红松幼苗根尖解剖结构的影响(平均值±标准误差)
Table 2 Effects of different CO2 concentration treatments on root tip anatomy of Pinus koraiensis seedlings (mean ± SE)
| CO2浓度 CO2 concentration | p | ||
|---|---|---|---|
| 350 µmol·mol-1 | 700 µmol·mol-1 | ||
| 根尖直径 Root tips diameter (µm) | 400.68 ± 10.90 | 453.79 ± 15.94 | < 0.01 |
| 中柱直径 Stele diameter (µm) | 216.23 ± 7.59 | 228.83 ± 12.13 | 0.38 |
| 皮层层数 Cortical layer number (No.) | 4.77 ± 0.11 | 5.87 ± 0.12 | < 0.01 |
| 皮层厚度 Cortical thickness (µm) | 79.39 ± 2.11 | 99.19 ± 2.88 | < 0.01 |
| 皮层细胞面积 Cortical cell area (µm2) | 464.17 ± 18.06 | 525.72 ± 21.36 | < 0.05 |
| 管胞数 Tracheid number (No.) | 25.23 ± 0.76 | 24.03 ± 0.81 | 0.28 |
| 管胞直径 Tracheid diameter (µm) | 12.18 ± 0.21 | 11.41 ± 0.21 | < 0.05 |
| 管胞密度 Tracheid density (No.·µm -2) | 0.0008 ± 0.00006 | 0.0007 ± 0.00006 | 0.09 |
| 管胞壁厚度 Tracheid wall thickness (µm) | 0.92 ± 0.02 | 1.08 ± 0.03 | < 0.01 |
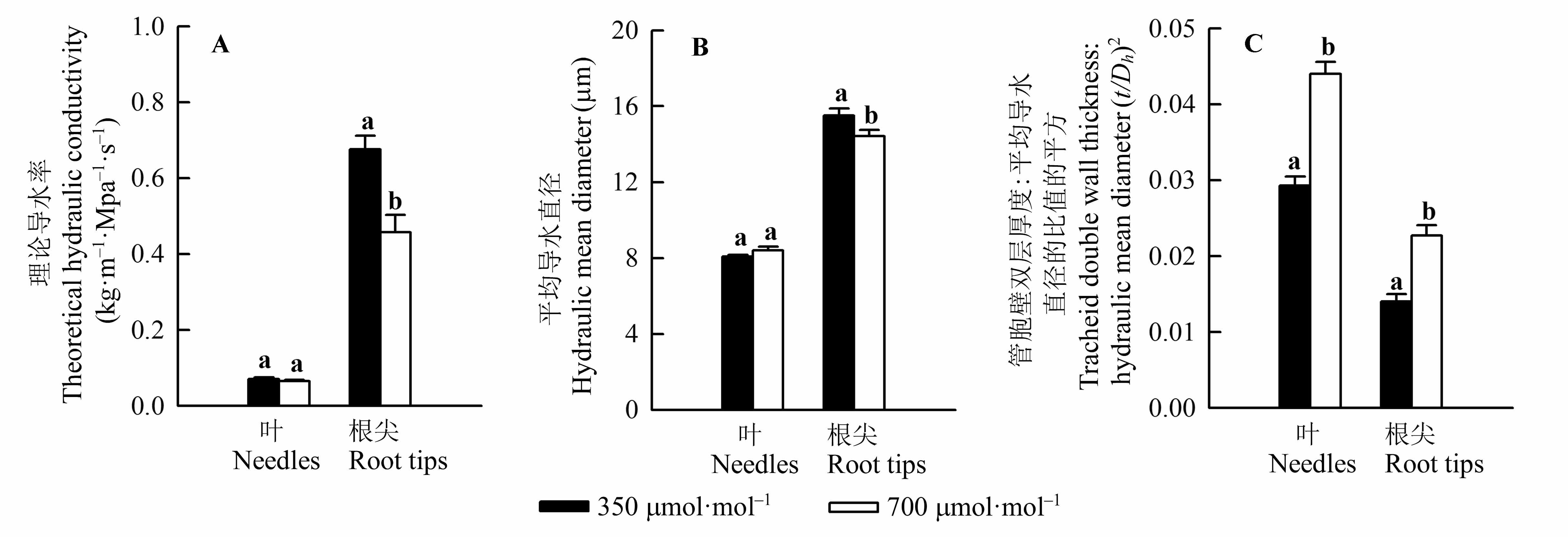
图3 不同CO2浓度处理对红松幼苗叶及根尖理论导水率(A)、平均导水直径(B)、管胞壁双层厚度与平均导水直径的比值的平方(C)的影响(平均值±标准误差)。不同小写字母表示不同CO2浓度处理间差异显著(p < 0.05)。
Fig. 3 Effect of different CO2 concentration treatments on theoretical hydraulic conductivity (A), hydraulic mean diameter (B) and the ratio of double wall thickness to hydraulic mean diameter (t/Dh)2 (C) in needles and root tips of Pinus koraiensis seedlings (mean ± SE). Different lowercase letters indicate significant difference between two CO2 concentration treatments.
| 1 | Ainsworth EA, Long SP (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2.New Phytologist, 165, 351-372. |
| 2 | Bader M, Hiltbrunner E, Körner C (2009). Fine root responses of mature deciduous forest trees to free air carbon dioxide enrichment (FACE).Functional Ecology, 23, 913-921. |
| 3 | Brodribb TJ, Mcadam SA, Jordan GJ, Feild TS (2009). Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants.New Phytologist, 183, 839-847. |
| 4 | Crookshanks M, Taylor G, Dolan L (1998). A model system to study the effects of elevated CO2 on the developmental physiology of roots: The use of Arabidopsis thaliana.Journal of Experimental Botany, 49, 593-597. |
| 5 | de Boer HJ, Lammertsma EI, Wagner-Cremer F, Dilcher DL, Wassen MJ, Dekker SC (2011). Climate forcing due to optimization of maximal leaf conductance in subtropical vegetation under rising CO2.Proceedings of the National Academy of Sciences of the United States of America, 108, 4041-4046. |
| 6 | Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000). Building roots in a changing environment: Implications for root longevity. New Phytologist, 147, 33-42. |
| 7 | Else MA, Coupland D, Dutton L, Jackson MB (2001). Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap.Physiologia Plantarum, 111, 46-54. |
| 8 | Esau K (1977). Anatomy of Seed Plants. 2nd ed. Wiley, New York. 215-255. |
| 9 | Finzi AC, Norby RJ, Calfapietra C, Gallet-Budynek A, Gielen B, Holmes WE, Hoosbeek MR, Iversen CM, Jackson RB, Kubiske ME (2007). Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2.Proceedings of the National Academy of Sciences of the United States of America, 104, 14014-14019. |
| 10 | Gu JC, Xu Y, Dong XY, Wang HF, Wang ZQ (2014). Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiology, 34, 415-425. |
| 11 | Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001). Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure.Oecologia, 126, 457-461. |
| 12 | Han M, Ji CJ, Zuo WY, He JS (2006). Interactive effects of elevated CO2 and temperature on the leaf anatomical characteristics of eleven species.Acta Ecologica Sinica, 26, 326-333. |
| (in Chinese with English abstract) [韩梅, 吉成均, 左闻韵, 贺金生 (2006). CO2浓度和温度升高对11种植物叶片解剖特征的影响. 生态学报, 26, 326-333.] | |
| 13 | Handa IT, Hagedorn F, Hättenschwiler S (2008). No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline.Functional Ecology, 22, 348-358. |
| 14 | Hou Y (2013). Recent advances in effects of elevated CO2 and temperature on plant morphology. Ecological Science, 32, 253-258. |
| (in Chinese with English abstract) [侯颖 (2013). CO2浓度和气温升高对植物形态结构影响的研究进展. 生态科学, 32, 253-258.] | |
| 15 | Iversen CM (2010). Digging deeper: Fine-root responses to rising atmospheric CO2 concentration in forested ecosystems.New Phytologist, 186, 346-357. |
| 16 | Li CR, Gan LJ, Xia K, Zhou X, Hew CS (2002). Responses of carboxylating enzymes, sucrose metabolizing enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment.Plant, Cell & Environment, 25, 369-377. |
| 17 | Lin J, Jach ME, Ceulemans R (2001). Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytologist, 150, 665-674. |
| 18 | Mao ZJ, Jia GM, Liu LX, Zhao M (2010). Combined effects of elevated temperature, elevated [CO2] and nitrogen supply on non-structural carbohydrate accumulation and allocation in Quercus mongolica seedlings.Chinese Journal of Plant Ecology, 34, 1174-1184. |
| (in Chinese with English abstract) [毛子军, 贾桂梅, 刘林馨, 赵甍 (2010). 温度增高、CO2浓度升高、施氮对蒙古栎幼苗非结构碳水化合物积累及其分配的综合影响. 植物生态学报, 34, 1174-1184.] | |
| 19 | McCormack ML, Adams TS, Smithwick EAH, Eissenstat DM (2012). Predicting fine root lifespan from plant functional traits in temperate trees.New Phytologist, 195, 823-831. |
| 20 | Nie M, Lu M, Bell J, Raut S, Pendall E (2013). Altered root traits due to elevated CO2: A meta-analysis.Global Ecology and Biogeography, 22, 1095-1105. |
| 21 | Overdieck D, Ziche D, Böttcher-Jungclaus K (2007). Temperature responses of growth and wood anatomy in European beech saplings grown in different carbon dioxide concentrations.Tree Physiology, 27, 261-268. |
| 22 | Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002). Fine root architecture of nine north American trees.Ecological Monographs, 72, 293-309. |
| 23 | Rico C, Pittermann J, Polley HW, Aspinwall MJ, Fay PA (2013). The effect of subambient to elevated atmospheric CO2 concentration on vascular function in Helianthus annuus: Implications for plant response to climate change.New Phytologist, 199, 956-965. |
| 24 | Rieger M, Litvin P (1999). Root system hydraulic conductivity in species with contrasting root anatomy.Journal of Experimental Botany, 50, 201-209. |
| 25 | Rodríguez-Gamir J, Intrigliolo DS, Primo-Millo E, Forner- Giner M (2010). Relationships between xylem anatomy, root hydraulic conductivity, leaf/root ratio and transpiration in citrus trees on different rootstocks.Physiologia plantarum, 139, 159-169. |
| 26 | Rogers HH, Peterson CM, McCrimmon JN, Cure JD (1992). Response of plant roots to elevated atmospheric carbon dioxide. Plant, Cell & Environment, 15, 749-752. |
| 27 | Rua MA, Umbanhowar J, Hu S, Burkey KO, Mitchell CE (2013). Elevated CO2 spurs reciprocal positive effects between a plant virus and an arbuscular mycorrhizal fungus.New Phytologist, 199, 541-549. |
| 28 | Sperry JS, Hacke UG, Pittermann J (2006). Size and function in conifer tracheids and angiosperm vessels.American Journal of Botany, 93, 1490-1500. |
| 29 | Tingey DT, Phillips DL, Johnson MG (2000). Elevated CO2 and conifer roots: Effects on growth, life span and turnover.New Phytologist, 147, 87-103. |
| 30 | Treseder KK (2004). A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies.New Phytologist, 164, 347-355. |
| 31 | Tyree MT, Dixon MA (1986). Water stress induced cavitation and embolism in some woody plants.Physiologia Plantarum, 66, 397-405. |
| 32 | Tyree MT, Ewers FW (1991). The hydraulic architecture of trees and other woody plants.New Phytologist, 119, 345-360. |
| 33 | Wang JL, Wen XF, Zhao FH, Fang QX, Yang XM (2012). Effects of doubled CO2 concentration on leaf photosynthesis, transpiration and water use efficiency of eight crop species.Chinese Journal of Plant Ecology, 36, 438-446. |
| (in Chinese with English abstract) [王建林, 温学发, 赵风华, 房全孝, 杨新民 (2012). CO2浓度倍增对8种作物叶片光合作用, 蒸腾作用和水分利用效率的影响. 植物生态学报, 36, 438-446.] | |
| 34 | Wang Y, Du ST, Li LL, Huang LD, Fang P, Lin XY, Zhang YS, Wang HL (2009). Effect of CO2 elevation on root growth and its relationship with indole acetic acid and ethylene in tomato seedlings.Pedosphere, 19, 570-576. |
| 35 | Wei X, Liu Y, Chen HB (2008). Anatomical and functional heterogeneity among different root orders of Phellodendron amurense. Journal of Plant Ecology (Chinese Version), 32, 1238-1247. |
| (in Chinese with English abstract) [卫星, 刘颖, 陈海波 (2008). 黄波罗不同根序的解剖结构及其功能异质性. 植物生态学报, 32, 1238-1247.] | |
| 36 | Woodward FI, Kelly CK (1995). The influence of CO2 concentration on stomatal density. New Phytologist, 131, 311-327. |
| 37 | Xu Y, Gu JC, Dong XY, Liu Y, Wang ZQ (2011). Fine root morphology, anatomy and tissue nitrogen and carbon contents of the first five orders in four tropical hardwood species in Hainan Island, China.Chinese Journal of Plant Ecology, 35, 955-964. |
| (in Chinese with English abstract) [许旸, 谷加存, 董雪云, 刘颖, 王政权 (2011). 海南岛4个热带阔叶树种前5级细根的形态、解剖结构和组织碳氮含量. 植物生态学报, 35, 955-964.] | |
| 38 | Zhou YM, Han SJ, Zheng JQ, Xin LH, Zhang HS (2007). Effects of elevated CO2 concentrations on soil microbial respiration and root/rhizosphere respiration in forest soil. Journal of Plant Ecology (Chinese Version), 31, 386-393. |
| (in Chinese with English abstract) [周玉梅, 韩士杰, 郑俊强, 辛丽花, 张海森 (2007). CO2浓度升高对森林土壤微生物呼吸与根(际)呼吸的影响. 植物生态学报, 31, 386-393.] | |
| 39 | Zuo WY, He JS, Han M, Ji CJ, Dan FBF, Fang JY (2005). Responses of plant stomata to elevated CO2 and temperature: Observations from 10 plant species grown in temperature and CO2 gradients.Acta Ecologica Sinica, 25, 565-574. |
| (in Chinese with English abstract) [左闻韵, 贺金生, 韩梅, 吉成均, Dan FBF, 方精云 (2005). 植物气孔对大气CO2浓度和温度升高的反应——基于在CO2浓度和温度梯度中生长的10种植物的观测. 生态学报, 25, 565-574.] |
| [1] | 李文博 孙龙 娄虎 于澄 韩宇 胡同欣. 火干扰对兴安落叶松种子萌发的影响[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [2] | 彭仲韬 金光泽 刘志理. 小兴安岭三种槭树叶性状随植株大小和林冠条件的变异[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [3] | 刘瑶 钟全林 徐朝斌 程栋梁 郑跃芳 邹宇星 张雪 郑新杰 周云若. 不同大小刨花楠细根功能性状与根际微环境关系[J]. 植物生态学报, 2024, 48(预发表): 0-0. |
| [4] | 陈科宇 邢森 唐玉 孙佳慧 任世杰 张静 纪宝明. 不同草地型土壤丛枝菌根真菌群落特征及其驱动因素[J]. 植物生态学报, 2024, 48(5): 660-674. |
| [5] | 徐子怡 金光泽. 阔叶红松林不同菌根类型幼苗细根功能性状的变异与权衡[J]. 植物生态学报, 2024, 48(5): 612-622. |
| [6] | 胡蝶 蒋欣琪 戴志聪 陈戴一 张雨 祁珊珊 杜道林. 丛枝菌根真菌提高入侵杂草南美蟛蜞菊对除草剂的耐受性[J]. 植物生态学报, 2024, 48(5): 651-659. |
| [7] | 臧妙涵, 王传宽, 梁逸娴, 刘逸潇, 上官虹玉, 全先奎. 基于纬度移栽的落叶松叶、枝、根生态化学计量特征对气候变暖的响应[J]. 植物生态学报, 2024, 48(4): 469-482. |
| [8] | 梁逸娴, 王传宽, 臧妙涵, 上官虹玉, 刘逸潇, 全先奎. 落叶松径向生长和生物量分配对气候变暖的响应[J]. 植物生态学报, 2024, 48(4): 459-468. |
| [9] | 付粱晨, 丁宗巨, 唐茂, 曾辉, 朱彪. 北京东灵山白桦和蒙古栎的根际效应及其季节动态[J]. 植物生态学报, 2024, 48(4): 508-522. |
| [10] | 曲泽坤, 朱丽琴, 姜琦, 王小红, 姚晓东, 蔡世锋, 罗素珍, 陈光水. 亚热带常绿阔叶林丛枝菌根树种养分觅食策略及其与细根形态间的关系[J]. 植物生态学报, 2024, 48(4): 416-427. |
| [11] | 萨其拉, 张霞, 朱琳, 康萨如拉. 长期不同放牧强度下荒漠草原优势种无芒隐子草叶片解剖结构变化[J]. 植物生态学报, 2024, 48(3): 331-340. |
| [12] | 范宏坤, 曾涛, 金光泽, 刘志理. 小兴安岭不同生长型阔叶植物叶性状变异及权衡[J]. 植物生态学报, 2024, 48(3): 364-376. |
| [13] | 吴君梅, 曾泉鑫, 梅孔灿, 林惠瑛, 谢欢, 刘苑苑, 徐建国, 陈岳民. 土壤磷有效性调控亚热带森林土壤酶活性和酶化学计量对凋落叶输入的响应[J]. 植物生态学报, 2024, 48(2): 242-253. |
| [14] | 程可心, 杜尧, 李凯航, 王浩臣, 杨艳, 金一, 何晓青. 玉米与叶际微生物组的互作遗传机制[J]. 植物生态学报, 2024, 48(2): 215-228. |
| [15] | 高敏, 缑倩倩, 王国华, 郭文婷, 张宇, 张妍. 低温胁迫对不同母树年龄柠条锦鸡儿种子萌发幼苗生理和生长的影响[J]. 植物生态学报, 2024, 48(2): 201-214. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
Copyright © 2022 版权所有 《植物生态学报》编辑部
地址: 北京香山南辛村20号, 邮编: 100093
Tel.: 010-62836134, 62836138; Fax: 010-82599431; E-mail: apes@ibcas.ac.cn, cjpe@ibcas.ac.cn
备案号: 京ICP备16067583号-19
